m6A Regulator Information
General Information of the m6A Regulator (ID: REG00015)
| Regulator Name | Protein virilizer homolog (VIRMA) | ||||
|---|---|---|---|---|---|
| Synonyms |
KIAA1429; MSTP054
Click to Show/Hide
|
||||
| Gene Name | VIRMA | ||||
| Sequence |
MAVDSAMELLFLDTFKHPSAEQSSHIDVVRFPCVVYINEVRVIPPGVRAHSSLPDNRAYG
ETSPHTFQLDLFFNNVSKPSAPVFDRLGSLEYDENTSIIFRPNSKVNTDGLVLRGWYNCL TLAIYGSVDRVISHDRDSPPPPPPPPPPPQPQPSLKRNPKHADGEKEDQFNGSPPRPQPR GPRTPPGPPPPDDDEDDPVPLPVSGDKEEDAPHREDYFEPISPDRNSVPQEGQYSDEGEV EEEQQEEGEEDEDDVDVEEEEDEDEDDRRTVDSIPEEEEEDEEEEGEEDEEGEGDDGYEQ ISSDEDGIADLERETFKYPNFDVEYTAEDLASVPPMTYDPYDRELVPLLYFSCPYKTTFE IEISRMKDQGPDKENSGAIEASVKLTELLDLYREDRGAKWVTALEEIPSLIIKGLSYLQL KNTKQDSLGQLVDWTMQALNLQVALRQPIALNVRQLKAGTKLVSSLAECGAQGVTGLLQA GVISGLFELLFADHVSSSLKLNAFKALDSVISMTEGMEAFLRGRQNEKSGYQKLLELILL DQTVRVVTAGSAILQKCHFYEVLSEIKRLGDHLAEKTSSLPNHSEPDHDTDAGLERTNPE YENEVEASMDMDLLESSNISEGEIERLINLLEEVFHLMETAPHTMIQQPVKSFPTMARIT GPPERDDPYPVLFRYLHSHHFLELVTLLLSIPVTSAHPGVLQATKDVLKFLAQSQKGLLF FMSEYEATNLLIRALCHFYDQDEEEGLQSDGVIDDAFALWLQDSTQTLQCITELFSHFQR CTASEETDHSDLLGTLHNLYLITFNPVGRSAVGHVFSLEKNLQSLITLMEYYSKEALGDS KSKKSVAYNYACILILVVVQSSSDVQMLEQHAASLLKLCKADENNAKLQELGKWLEPLKN LRFEINCIPNLIEYVKQNIDNLMTPEGVGLTTALRVLCNVACPPPPVEGQQKDLKWNLAV IQLFSAEGMDTFIRVLQKLNSILTQPWRLHVNMGTTLHRVTTISMARCTLTLLKTMLTEL LRGGSFEFKDMRVPSALVTLHMLLCSIPLSGRLDSDEQKIQNDIIDILLTFTQGVNEKLT ISEETLANNTWSLMLKEVLSSILKVPEGFFSGLILLSELLPLPLPMQTTQVIEPHDISVA LNTRKLWSMHLHVQAKLLQEIVRSFSGTTCQPIQHMLRRICVQLCDLASPTALLIMRTVL DLIVEDLQSTSEDKEKQYTSQTTRLLALLDALASHKACKLAILHLINGTIKGDERYAEIF QDLLALVRSPGDSVIRQQCVEYVTSILQSLCDQDIALILPSSSEGSISELEQLSNSLPNK ELMTSICDCLLATLANSESSYNCLLTCVRTMMFLAEHDYGLFHLKSSLRKNSSALHSLLK RVVSTFSKDTGELASSFLEFMRQILNSDTIGCCGDDNGLMEVEGAHTSRTMSINAAELKQ LLQSKEESPENLFLELEKLVLEHSKDDDNLDSLLDSVVGLKQMLESSGDPLPLSDQDVEP VLSAPESLQNLFNNRTAYVLADVMDDQLKSMWFTPFQAEEIDTDLDLVKVDLIELSEKCC SDFDLHSELERSFLSEPSSPGRTKTTKGFKLGKHKHETFITSSGKSEYIEPAKRAHVVPP PRGRGRGGFGQGIRPHDIFRQRKQNTSRPPSMHVDDFVAAESKEVVPQDGIPPPKRPLKV SQKISSRGGFSGNRGGRGAFHSQNRFFTPPASKGNYSRREGTRGSSWSAQNTPRGNYNES RGGQSNFNRGPLPPLRPLSSTGYRPSPRDRASRGRGGLGPSWASANSGSGGSRGKFVSGG SGRGRHVRSFTR Click to Show/Hide
|
||||
| Family | vir family | ||||
| Function |
Associated component of the WMM complex, a complex that mediates N6-methyladenosine (m6A) methylation of RNAs, a modification that plays a role in the efficiency of mRNA splicing and RNA processing . Acts as a key regulator of m6A methylation by promoting m6A methylation of mRNAs in the 3'-UTR near the stop codon: recruits the catalytic core components METTL3 and METTL14, thereby guiding m6A methylation at specific sites. Required for mRNA polyadenylation via its role in selective m6A methylation: m6A methylation of mRNAs in the 3'-UTR near the stop codon correlating with alternative polyadenylation (APA).
Click to Show/Hide
|
||||
| Gene ID | 25962 | ||||
| Uniprot ID | |||||
| Regulator Type | WRITER ERASER READER | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
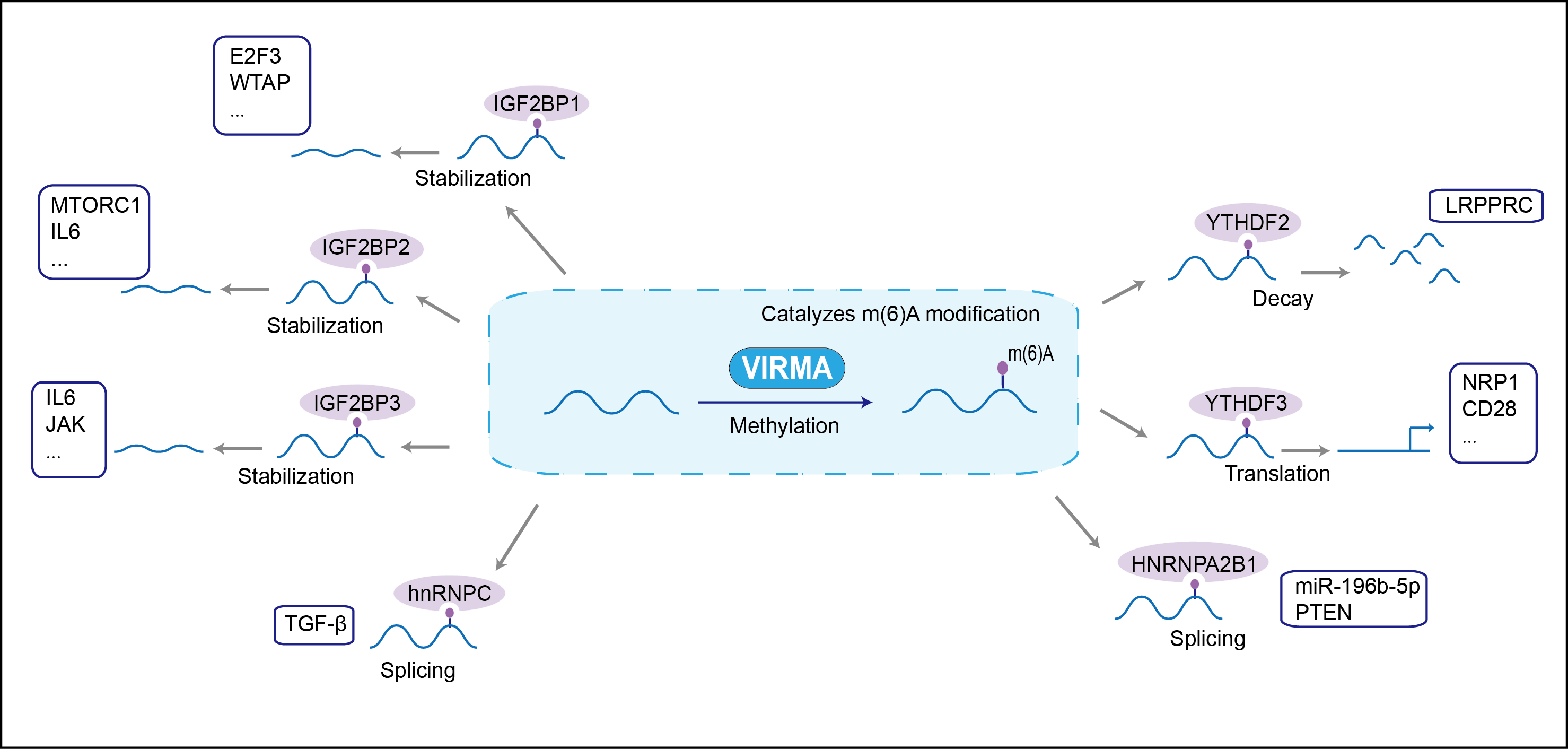
|
|||||
| Target Genes | Click to View Potential Target Genes of This Regulator | ||||
Full List of Target Gene(s) of This m6A Regulator and Corresponding Disease/Drug Response(s)
VIRMA can regulate the m6A methylation of following target genes, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulted from the regulation of certain target gene.
Browse Target Gene related Disease
Browse Target Gene related Drug
Cyclin-dependent kinase 1 (CDK1)
| Representative RNA-seq result indicating the expression of this target gene regulated by VIRMA | ||
| Cell Line | Human umbilical vein endothelial cells | Homo sapiens |
|
Treatment: siVIRMA HUVECs
Control: siControl HUVECs
|
GSE167067 | |
| Regulation |
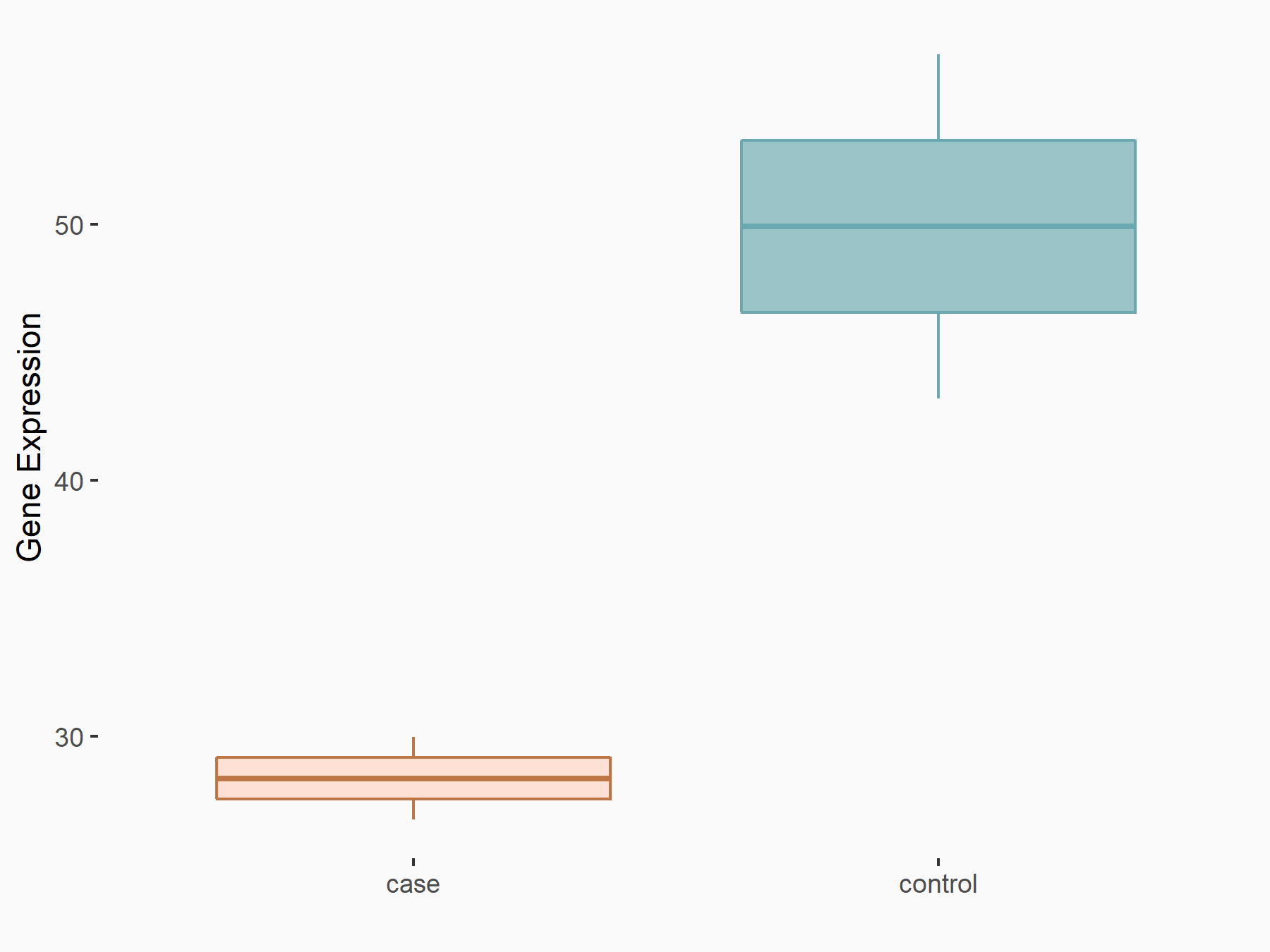  |
logFC: -7.84E-01 p-value: 3.08E-02 |
| More Results | Click to View More RNA-seq Results | |
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [1] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Responsed Drug | Fluorouracil | Approved | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Cell proliferation and metastasis | |||
| Response Summary | KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6-methyladenosine-independent manner.5'-fluorouracil was found to be very effective in reducing the expression of KIAA1429 and Cyclin-dependent kinase 1 (CDK1) in breast cancer. | |||
Death-associated protein kinase 3 (DAPK3)
| Representative RNA-seq result indicating the expression of this target gene regulated by VIRMA | ||
| Cell Line | Human umbilical vein endothelial cells | Homo sapiens |
|
Treatment: siVIRMA HUVECs
Control: siControl HUVECs
|
GSE167067 | |
| Regulation |
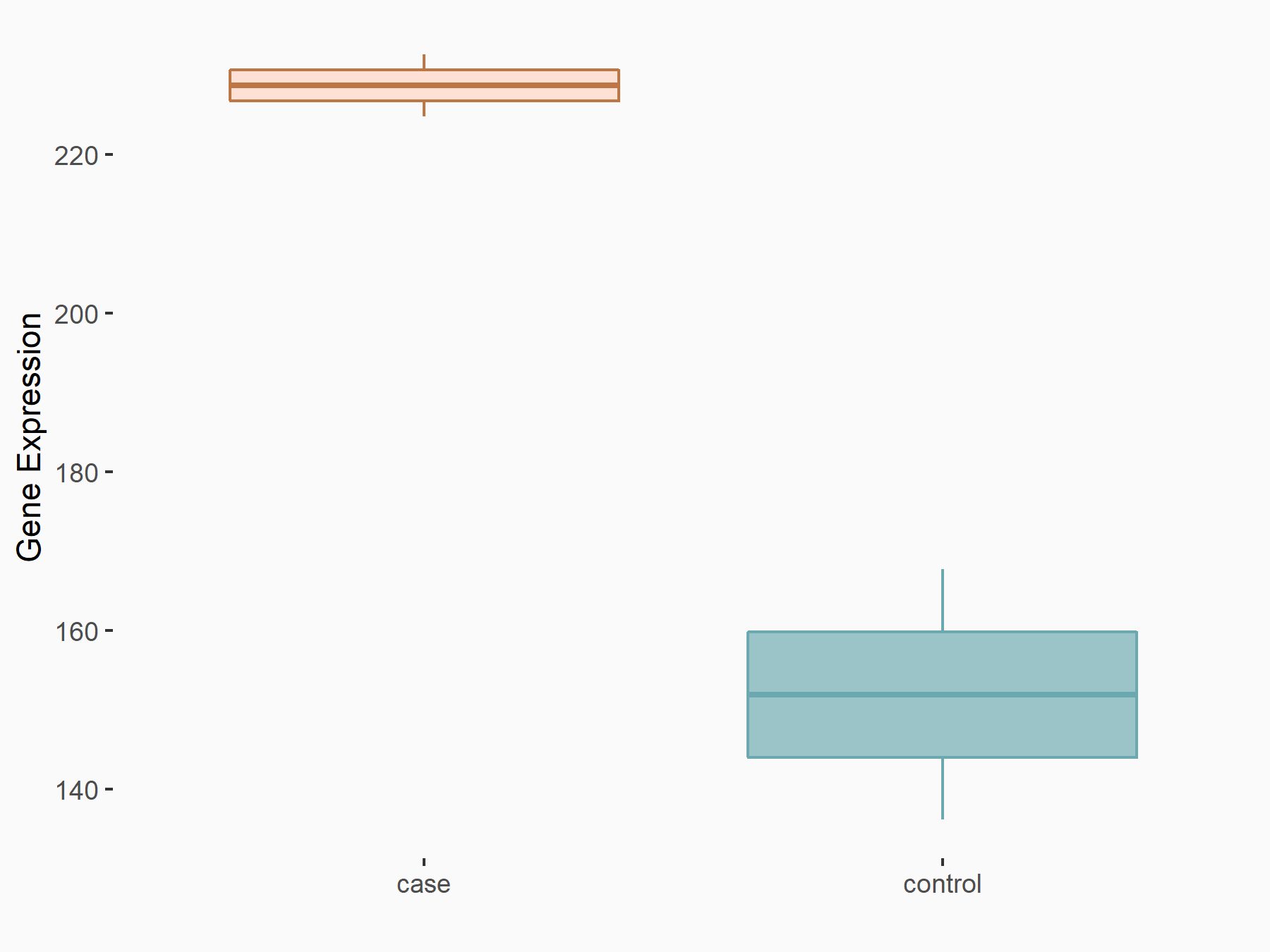 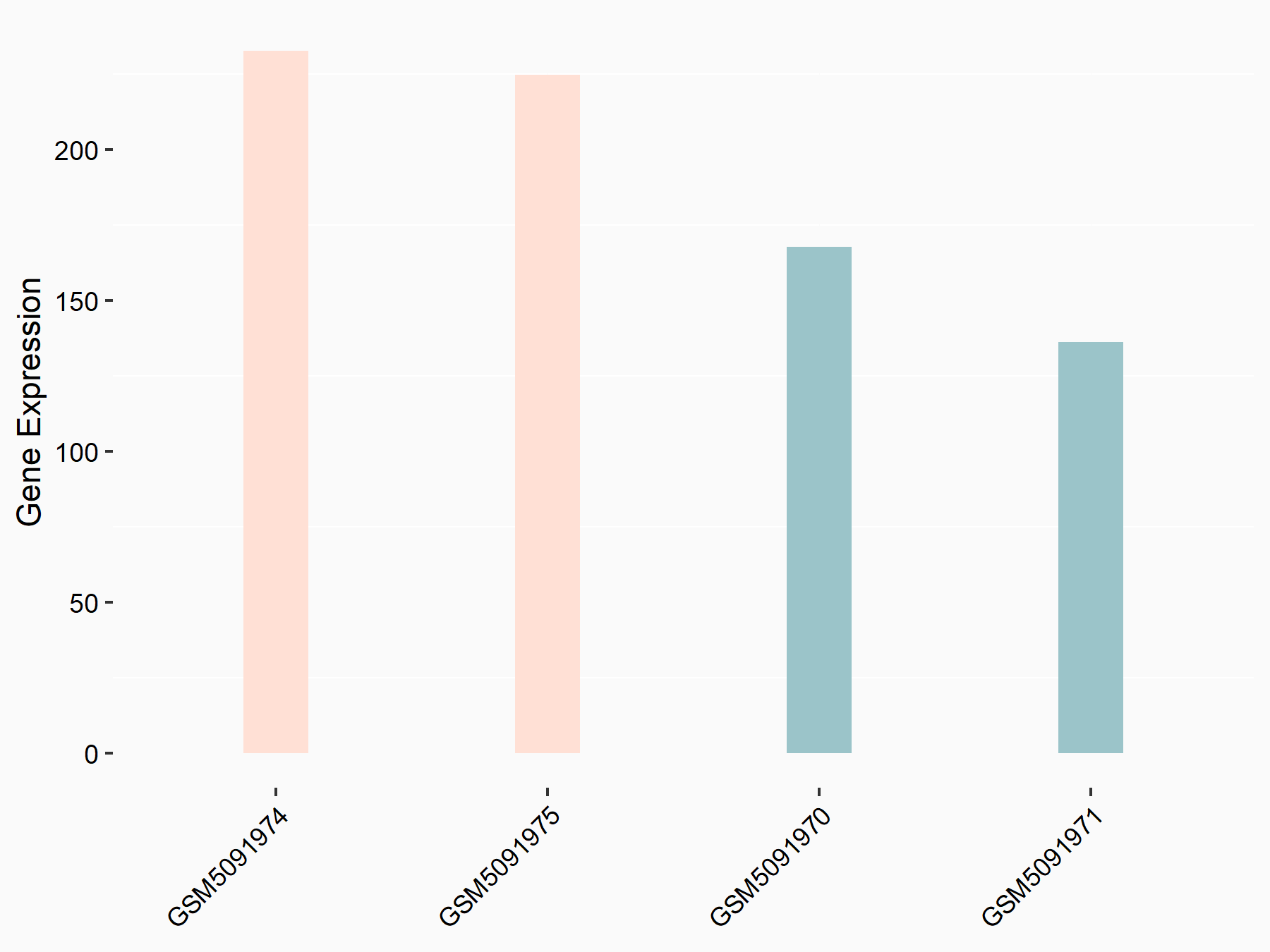 |
logFC: 5.95E-01 p-value: 2.81E-02 |
| More Results | Click to View More RNA-seq Results | |
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [2] | |||
| Responsed Disease | Non-small-cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Target Regulation | Down regulation | |||
In-vitro Model |
PC-9 | Lung adenocarcinoma | Homo sapiens | CVCL_B260 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| Response Summary | High expression of KIAA1429 was testified in patients with non-small cell lung cancer and predicted worse prognosis in patients. KIAA1429-guided m6A modifications promoted NSCLC progression via m6A-dependent degradation of Death-associated protein kinase 3 (DAPK3) mRNA. | |||
Histone H2AX (H2AX)
| Representative RNA-seq result indicating the expression of this target gene regulated by VIRMA | ||
| Cell Line | Human umbilical vein endothelial cells | Homo sapiens |
|
Treatment: siVIRMA HUVECs
Control: siControl HUVECs
|
GSE167067 | |
| Regulation |
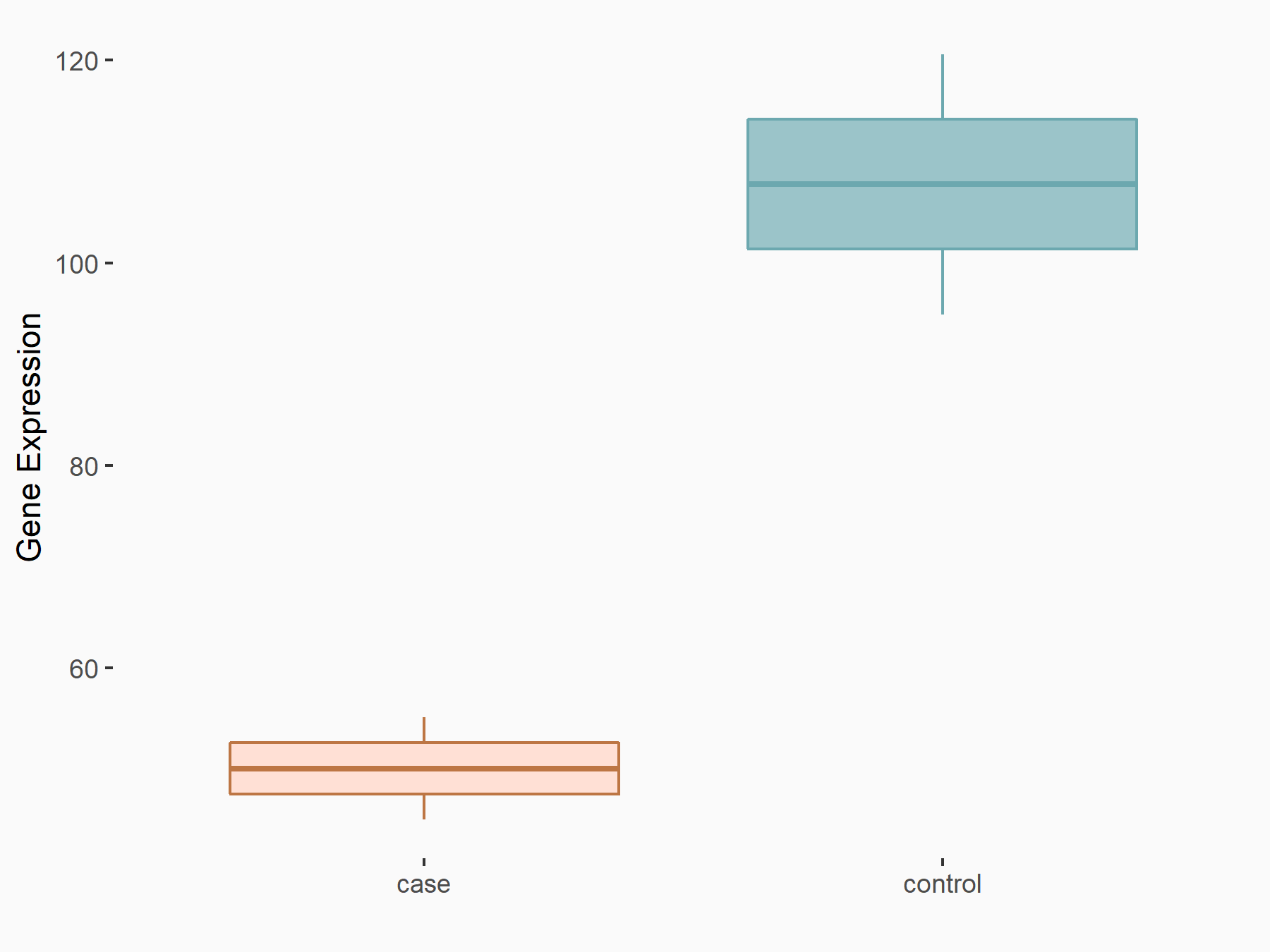 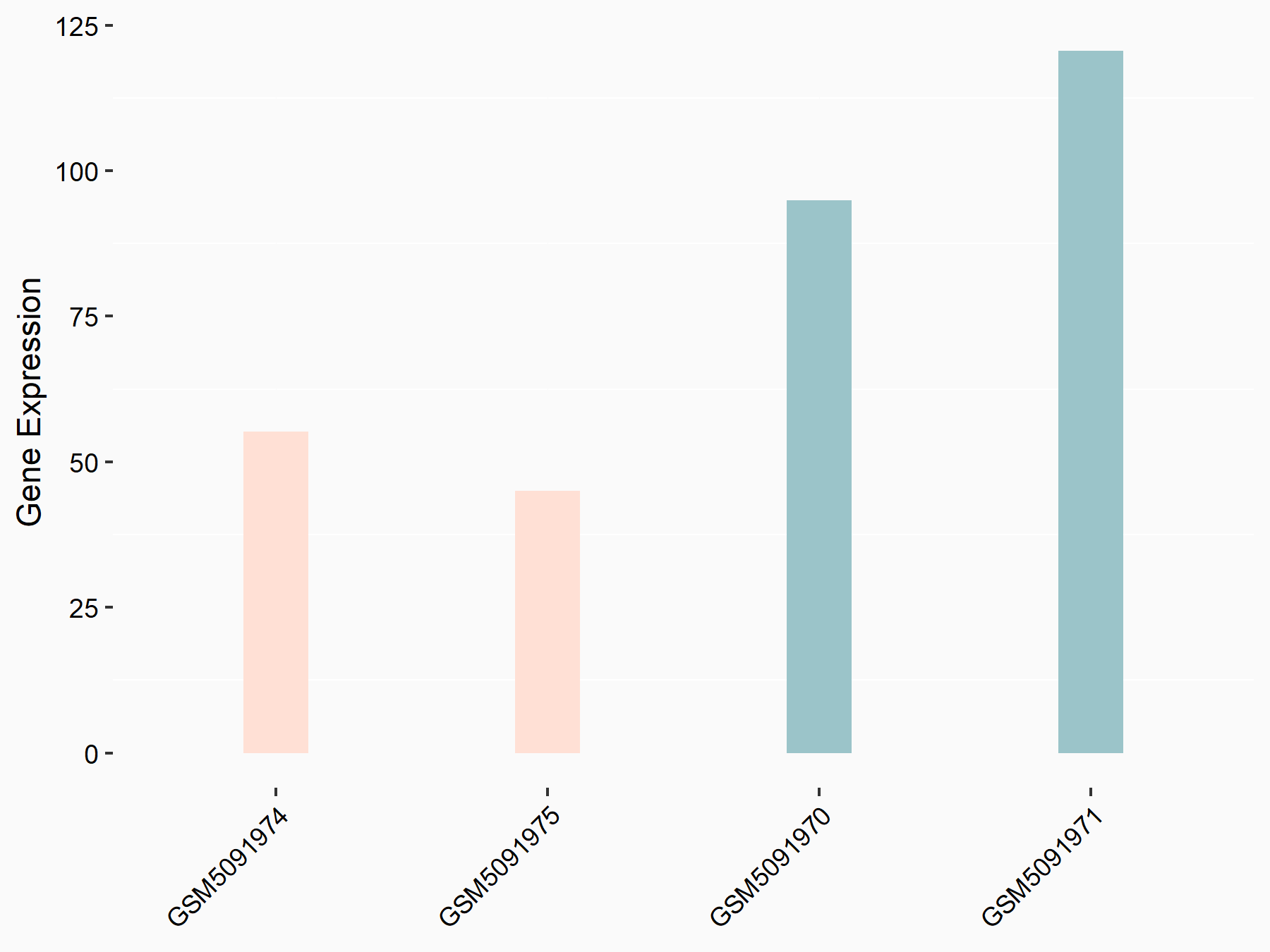 |
logFC: -1.09E+00 p-value: 1.68E-02 |
| More Results | Click to View More RNA-seq Results | |
Testicular cancer [ICD-11: 2C80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [3] | |||
| Responsed Disease | Germ cell tumour of testis [ICD-11: 2C80.2] | |||
| Responsed Drug | Cisplatin | Approved | ||
| Target Regulation | Down regulation | |||
In-vitro Model |
2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Histone H2AX (H2AX) and GADD45B levels) and downregulation of XLF and MRE11. | |||
SMC protein 1A (SMC1A)
| Representative RIP-seq result supporting the interaction between the target gene and VIRMA | ||
| Cell Line | HeLa | Homo sapiens |
| Regulation | logFC: 1.58E+00 | GSE102493 |
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [4] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | Adherens junction | hsa04520 | ||
| Cell Process | Epithelial-mesenchymal transition | |||
| Cell migration | ||||
| Cell invasion | ||||
In-vitro Model |
ZR-75-1 | Invasive breast carcinoma | Homo sapiens | CVCL_0588 |
| SUM1315MO2 | Invasive breast carcinoma of no special type | Homo sapiens | CVCL_5589 | |
| In-vivo Model | For the mouse lung metastasis model, SUM-1315 cells (2 × 106/0.2 mL) expressing NC, shKIAA1429, SNAIL, or shKIAA1429+SNAIL were injected into the nude mice through the tail vein. | |||
| Response Summary | KIAA1429 could significantly promote the migration and invasion of breast cancer cells. KIAA1429 could bind to the motif in the 3' UTR of SMC protein 1A (SMC1A) mRNA directly and enhance SMC1A mRNA stability. In conclusion, the study revealed a novel mechanism of the KIAA1429/SMC1A/SNAIL axis in the regulation of metastasis of breast cancer. | |||
Transcription factor Jun (c-Jun/JUN)
| Representative RIP-seq result supporting the interaction between the target gene and VIRMA | ||
| Cell Line | HeLa | Homo sapiens |
| Regulation | logFC: 1.80E+00 | GSE102493 |
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [5] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Arrest cell cycle at the S phase | |||
In-vitro Model |
MGC-803 | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| In-vivo Model | Gastric cancer cell line MGC803 (shRNA-NC or shKIAA1429; 1 × 107) was injected subcutaneously into the armpit of BALB/c nude mice (5-week-old, male, n = 4 for each group). Tumor growth was monitored at 3-day intervals. | |||
| Response Summary | KIAA1429 played a key role in promoting gastric cancer by regulating Transcription factor Jun (c-Jun/JUN) expression in an m6A independent manner. | |||
Alpha-enolase (ENO1)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [6] | |||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
ES-2 | Ovarian clear cell adenocarcinoma | Homo sapiens | CVCL_3509 |
| HEY | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0297 | |
| OVCAR-3 | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 | |
| SK-OV-3 | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| A2780 | Ovarian endometrioid adenocarcinoma | Homo sapiens | CVCL_0134 | |
|
IOSE-80
|
N.A. | Homo sapiens | CVCL_5546 | |
Cyclin-dependent kinase 4 (CDK4)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Cell Process | Cell apoptosis | |||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, CCND1, Cyclin-dependent kinase 4 (CDK4), EGR2, YBX1, and TLX, which were associated with lung cancers. | |||
Cystine/glutamate transporter (SLC7A11)
Liver cancer [ICD-11: 2C12]
| In total 3 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [8] | |||
| Responsed Disease | Liver hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Responsed Drug | Cycloleucine | Investigative | ||
In-vitro Model |
Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Experiment 2 Reporting the m6A-centered Disease Response of This Target Gene | [8] | |||
| Responsed Disease | Liver hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Responsed Drug | Ferrostatin-1 | Investigative | ||
In-vitro Model |
Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Experiment 3 Reporting the m6A-centered Disease Response of This Target Gene | [8] | |||
| Responsed Disease | Liver hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Responsed Drug | Liproxstatin-1 | Investigative | ||
In-vitro Model |
Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
DNA-binding protein inhibitor ID-2 (ID2)
Liver cancer [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [9] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | RNA degradation | hsa03018 | ||
| Cell Process | Cell migration and invasion | |||
In-vitro Model |
Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Hep-G2/2.2.15 | Hepatoblastoma | Homo sapiens | CVCL_L855 | |
| L-02 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Response Summary | KIAA1429 facilitated migration and invasion of Hepatocellular carcinoma cells by inhibiting DNA-binding protein inhibitor ID-2 (ID2) via upregulating m6A modification of ID2 mRNA. | |||
Double-strand break repair protein MRE11 (MRE11)
Testicular cancer [ICD-11: 2C80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [3] | |||
| Responsed Disease | Germ cell tumour of testis [ICD-11: 2C80.2] | |||
| Responsed Drug | Cisplatin | Approved | ||
| Target Regulation | Up regulation | |||
In-vitro Model |
2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and GADD45B levels) and downregulation of XLF and Double-strand break repair protein MRE11 (MRE11). | |||
E3 SUMO-protein ligase EGR2 (EGR2)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Cell Process | Cell apoptosis | |||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, CCND1, CDK4, E3 SUMO-protein ligase EGR2 (EGR2), YBX1, and TLX, which were associated with lung cancers. | |||
E3 ubiquitin-protein ligase Hakai (CBLL1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [10] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulation | Up regulation | |||
| Cell Process | Cell apoptosis | |||
| Response Summary | KIAA1429 had the highest mutation frequency. Higher E3 ubiquitin-protein ligase Hakai (CBLL1) expression was associated with a better prognosis in breast cancer than lower CBLL1 expression. | |||
Forkhead box protein M1 (FOXM1)
Multiple myeloma [ICD-11: 2A83]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [11] | |||
| Responsed Disease | Multiple myeloma [ICD-11: 2A83.1] | |||
In-vitro Model |
NCI-H929 | Plasma cell myeloma | Homo sapiens | CVCL_1600 |
| MM1.S | Plasma cell myeloma | Homo sapiens | CVCL_8792 | |
| U266B1 | Plasma cell myeloma | Homo sapiens | CVCL_0566 | |
| RPMI-8226 | Plasma cell myeloma | Homo sapiens | CVCL_0014 | |
| CAG | Plasma cell myeloma | Homo sapiens | CVCL_D569 | |
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [12] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Responsed Drug | Oxaliplatin | Approved | ||
| Target Regulation | Up regulation | |||
In-vitro Model |
GES-1 | Normal | Homo sapiens | CVCL_EQ22 |
| AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| HGC-27 | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
G1/S-specific cyclin-D1 (CCND1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Cell Process | Cell apoptosis | |||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, G1/S-specific cyclin-D1 (CCND1), CDK4, EGR2, YBX1, and TLX, which were associated with lung cancers. | |||
Glycogen synthase kinase-3 beta (GSK3Beta/GSK3B)
Structural developmental anomalies of large intestine [ICD-11: LB16]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [13] | |||
| Responsed Disease | Structural developmental anomalies of large intestine [ICD-11: LB16.1] | |||
In-vitro Model |
HEK293T | Normal | Homo sapiens | CVCL_0063 |
| SH-SY5Y | Neuroblastoma | Homo sapiens | CVCL_0019 | |
Hexokinase-2 (HK2)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [14] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | Metabolic pathways | hsa01100 | ||
| Glycolysis / Gluconeogenesis | hsa00010 | |||
| Cell Process | Glycolysis | |||
In-vitro Model |
SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 |
| HT29 | Colon cancer | Mus musculus | CVCL_A8EZ | |
| FHC | Normal | Homo sapiens | CVCL_3688 | |
| Response Summary | KIAA1429 has the potential to promote CRC carcinogenesis by targeting Hexokinase-2 (HK2) via m6A-independent manner, providing insight into the critical roles of m6A in CRC. | |||
Homeobox protein Hox-A1 (HOXA1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [15] | |||
| Responsed Disease | Non-small-cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Responsed Drug | Gefitinib | Approved | ||
| Target Regulation | Up regulation | |||
In-vitro Model |
Gefitinib-resistant cell line PC9GR (Gefitinib-resistant PC9 cell line) | |||
| PC-9 | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | |
| NHBE (Normal bronchial epithelial cells) | ||||
| In-vivo Model | PC9-GR cells stably infected with KIAA1429-targeting shRNA and control were suspended in 100 uL of PBS with Matrigel matrix (BD Biosciences). Then, cells were injected into one of the flanks of BALB/c nude mice. | |||
| Response Summary | m6A methyltransferase KIAA1429 was highly expressed in gefitinib-resistant NSCLC cells (PC9-GR), tissues, and closely related to unfavorable survival. KIAA1429 plays essential oncogenic roles in NSCLC gefitinib resistance, which provided a feasible therapeutic target for NSCLC. | |||
Mucin-3A (MUC3A)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [16] | |||
| Responsed Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Target Regulation | Up regulation | |||
| Cell Process | Cell migration | |||
| celCl proliferation | ||||
| Cell invasion | ||||
In-vitro Model |
NCI-H460 | Lung large cell carcinoma | Homo sapiens | CVCL_0459 |
| HBE (Human bronchial epithelial cell line) | ||||
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| PLA-801D | Lung giant cell carcinoma | Homo sapiens | CVCL_7110 | |
| Response Summary | KIAA1429 regulates Mucin-3A (MUC3A) expression through m6A modification to modulate LUAD cells to proliferate, migrate, invade, and induce cell cycle arrest. | |||
NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [17] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 |
| SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | |
| NCM460 | Normal | Homo sapiens | CVCL_0460 | |
| LoVo | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | |
| HT29 | Colon cancer | Mus musculus | CVCL_A8EZ | |
| HCT 8 | Colon adenocarcinoma | Homo sapiens | CVCL_2478 | |
| HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| In-vivo Model | 5.0 × 106 SW480 cells (infected with scr or KIAA1429 shRNA) that suspended in 50 ul PBS and mixed with an equal volume of matrigel were subcutaneously injected in a 6-weeks-old male NOD/SCID (The Jackson Laboratory, Stock No: 001303) mice flank. We started measuring tumor size at the indicated times one week after injection. | |||
| Response Summary | KIAA1429 increased the expression of NAD-dependent protein deacetylase sirtuin-1 (SIRT1) via regulating its mRNA stability in an m6A-dependent manner. More importantly, in vivo experiment showed that depletion of KIAA1429 significantly inhibited colorectal tumor growth. | |||
Negative growth regulatory protein MyD118 (GADD45B)
Testicular cancer [ICD-11: 2C80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [3] | |||
| Responsed Disease | Germ cell tumour of testis [ICD-11: 2C80.2] | |||
| Responsed Drug | Cisplatin | Approved | ||
| Target Regulation | Down regulation | |||
In-vitro Model |
2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and Negative growth regulatory protein MyD118 (GADD45B) levels) and downregulation of XLF and MRE11. | |||
Non-homologous end-joining factor 1 (NHEJ1/XLF)
Testicular cancer [ICD-11: 2C80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [3] | |||
| Responsed Disease | Germ cell tumour of testis [ICD-11: 2C80.2] | |||
| Responsed Drug | Cisplatin | Approved | ||
| Target Regulation | Up regulation | |||
In-vitro Model |
2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and GADD45B levels) and downregulation of Non-homologous end-joining factor 1 (NHEJ1/XLF) and MRE11. | |||
Nuclear receptor subfamily 2 group E member 1 (TLX/NR2E1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Cell Process | Cell apoptosis | |||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, CCND1, CDK4, EGR2, YBX1, and Nuclear receptor subfamily 2 group E member 1 (TLX/NR2E1), which were associated with lung cancers. | |||
Pre-mRNA-splicing regulator WTAP (WTAP)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Cell Process | Cell apoptosis | |||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, Pre-mRNA-splicing regulator WTAP (WTAP), CCND1, CDK4, EGR2, YBX1, and TLX, which were associated with lung cancers. | |||
Protein BTG2 (BTG2)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [18] | |||
| Responsed Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| SPC-A1 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| HBE (Human bronchial epithelial cell line) | ||||
| In-vivo Model | For the in vivo cell proliferation assay, A549 and SPCA1 cells were stably transfected with sh-Ctrl and sh-KIAA1429 using lentivirus (GeneChem, Shanghai, China). The cells were subcutaneously injected into either side of the posterior flanks of the mouse. The tumor volume was measured every few days (length×width2×0.5). | |||
| Response Summary | Knockdown of KIAA1429 significantly decreased the m6A levels of Protein BTG2 (BTG2) mRNA, leading to enhanced YTHDF2-dependent BTG2 mRNA stability and promoted the expression of BTG2; thus, participating in the tumorigenesis of LUAD. | |||
Trans-acting T-cell-specific transcription factor GATA-3 (GATA3)
Liver cancer [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [19] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Target Regulation | Down regulation | |||
| Cell Process | Cell proliferation and metastasis | |||
In-vitro Model |
HCCLM3 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_6832 |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| Hep 3B2.1-7 | Childhood hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| SNU-182 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0090 | |
| SNU-449 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 | |
| Response Summary | KIAA1429 induced m6A methylation on the 3' UTR of Trans-acting T-cell-specific transcription factor GATA-3 (GATA3) pre-mRNA, leading to the separation of the RNA-binding protein HuR and the degradation of GATA3 pre-mRNA. KIAA1429 was considerably upregulated in Hepatocellular carcinoma tissues. | |||
Transcription factor AP-2-alpha (TFAP2A)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [20] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
EO771 | Malignant neoplasms of the mouse mammary gland | Mus musculus | CVCL_GR23 |
| EMT6 | Malignant neoplasms of the mouse mammary gland | Mus musculus | CVCL_1923 | |
| 4T1.2 | Malignant neoplasms of the mouse mammary gland | Mus musculus | CVCL_GR32 | |
|
NMuMG
|
N.A. | Mus musculus | CVCL_0075 | |
Transcription factor E2F3 (E2F3)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Cell Process | Cell apoptosis | |||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including Transcription factor E2F3 (E2F3), WTAP, CCND1, CDK4, EGR2, YBX1, and TLX, which were associated with lung cancers. | |||
Wee1-like protein kinase (WEE1)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [21] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Target Regulation | Down regulation | |||
| Response Summary | KIAA1429 plays an oncogenic role in CRC cells by inhibiting the expression of Wee1-like protein kinase (WEE1) in an m6A-independent manner and is associated with poor survival in CRC patients. | |||
Y-box-binding protein 1 (YBX1)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Cell Process | Cell apoptosis | |||
In-vitro Model |
A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, CCND1, CDK4, EGR2, Y-box-binding protein 1 (YBX1), and TLX, which were associated with lung cancers. | |||
Zinc finger protein SNAI1 (SNAI1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [4] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | Adherens junction | hsa04520 | ||
| Cell Process | Epithelial-mesenchymal transition | |||
| Cell migration | ||||
| Cell invasion | ||||
In-vitro Model |
ZR-75-1 | Invasive breast carcinoma | Homo sapiens | CVCL_0588 |
| SUM1315MO2 | Invasive breast carcinoma of no special type | Homo sapiens | CVCL_5589 | |
| In-vivo Model | For the mouse lung metastasis model, SUM-1315 cells (2 × 106/0.2 mL) expressing NC, shKIAA1429, SNAIL, or shKIAA1429+SNAIL were injected into the nude mice through the tail vein. | |||
| Response Summary | KIAA1429 could significantly promote the migration and invasion of breast cancer cells. KIAA1429 could bind to the motif in the 3' UTR of SMC1A mRNA directly and enhance SMC1A mRNA stability. In conclusion, the study revealed a novel mechanism of the KIAA1429/SMC1A/Zinc finger protein SNAI1 (SNAI1) axis in the regulation of metastasis of breast cancer. | |||
Colon cancer associated transcript 1 (CCAT1)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [22] | |||
| Responsed Disease | Prostate cancer [ICD-11: 2C82] | |||
| Target Regulation | Up regulation | |||
| Cell Process | RNA stability | |||
In-vitro Model |
22Rv1 | Prostate carcinoma | Homo sapiens | CVCL_1045 |
| DU145 | Prostate carcinoma | Homo sapiens | CVCL_0105 | |
| LNCaP | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| PC-3 | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| VCaP | Prostate carcinoma | Homo sapiens | CVCL_2235 | |
| Response Summary | VIRMA downregulation attenuates the aggressive phenotype of prostate cancer by overall reduction of m6A-levels decreasing stability and abundance of oncogenic lncRNAs. VIRMA depletion and m6A reduction decreased the stability and abundance of Colon cancer associated transcript 1 (CCAT1) transcripts. | |||
Colon cancer associated transcript 2 (CCAT2)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [22] | |||
| Responsed Disease | Prostate cancer [ICD-11: 2C82] | |||
| Target Regulation | Up regulation | |||
| Cell Process | RNA stability | |||
In-vitro Model |
22Rv1 | Prostate carcinoma | Homo sapiens | CVCL_1045 |
| DU145 | Prostate carcinoma | Homo sapiens | CVCL_0105 | |
| LNCaP | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| PC-3 | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| VCaP | Prostate carcinoma | Homo sapiens | CVCL_2235 | |
| Response Summary | VIRMA downregulation attenuates the aggressive phenotype of prostate cancer by overall reduction of m6A-levels decreasing stability and abundance of oncogenic lncRNAs. VIRMA depletion and m6A reduction decreased the stability and abundance of Colon cancer associated transcript 2 (CCAT2) transcripts. | |||
Long intergenic non-protein coding RNA 958 (LINC00958)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [23] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Target Regulation | Up regulation | |||
| Cell Process | Glycolysis | |||
In-vitro Model |
SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| GES-1 | Normal | Homo sapiens | CVCL_EQ22 | |
| BGC-823 | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| In-vivo Model | Ten four-week-old BALB/c nude mice were injected with LINC00958-overexpressing or vector-transfected cells. Briefly, 5 × 106 cells were subcutaneously injected in the flank of mice. Four weeks after injection, the mice were sacrificed and examined by weighting. | |||
| Response Summary | Long intergenic non-protein coding RNA 958 (LINC00958) accelerated the aerobic glycolysis of GC cells. Mechanistically, KIAA1429 interacted with the m6A modification site and promoted the enrichment of LINC00958, and LINC00958 subsequently cooperated with GLUT1 mRNA to enhance its mRNA stability. | |||
Nuclear paraspeckle assembly transcript 1 (NEAT1)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [24] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
SK-BR-3 | Breast adenocarcinoma | Homo sapiens | CVCL_0033 |
| MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MDA-MB-453 | Breast adenocarcinoma | Homo sapiens | CVCL_0418 | |
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| AU565 | Breast adenocarcinoma | Homo sapiens | CVCL_1074 | |
| Hs 578T | Invasive breast carcinoma | Homo sapiens | CVCL_0332 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| In-vivo Model | Mice were acclimatised at the Centenary Institute Animal Facility for a minimum of 7 days after initial arrival. Prior to tumour injection, mice were anaesthetised by ketamine/xylazine by intraperitoneal injection. Subsequently, the fur surrounding the 4th mammary fat pad on the right was removed using the hair removal cream. MDA-MB-231 cells (5 × 106) in 100 μL of 1:1 HBSS:Matrigel were then injected subcutaneously into the 4th right mammary fat pad using 27 g insulin needles. Mice were injected intraperitoneally with the reversal atipamezole to improve the recovery from anaesthesia. Mice were monitored twice weekly by assessing body condition, measuring body weights and tumour sizes. The frequency of monitoring was increased to daily when tumours reached > 500 mm3 in size. Mice were killed when tumours reached > 1000 mm3 in size and the relevant organs were harvested for analysis. | |||
hsa-miR-143-3p
Aortic aneurysm or dissection [ICD-11: BD50]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [25] | |||
| Responsed Disease | Aortic aneurysm or dissection [ICD-11: BD50] | |||
| Target Regulation | Down regulation | |||
| Pathway Response | RNA degradation | hsa03018 | ||
| Cell Process | Cell apoptosis | |||
In-vitro Model |
IM-HAEC | Normal | Homo sapiens | CVCL_B5WL |
| In-vivo Model | Osmotic mini-pumps containing AngII (1 ug/kg/min, Enzo Bioche) were implanted in 7-week-old male mice. To interfere with the expression of KIAA1429, ALKBH5, or DDX6 in vivo, adeno-associated virus 9 (AAV9) vectors carrying a variety of overexpression plasmids or interfering RNA were randomly injected through the tail vein to C57BL/6N mice. | |||
| Response Summary | KIAA1429 is downregulated while ALKBH5 is upregulated in aortic tissues from aortic dissection patients. KIAA1429/ALKBH5-mediated m6A modifications can regulate the processing of hsa-miR-143-3p through interacting with the microprocessor protein DGCR8. KIAA1429 and ALKBH5 can oppositely regulate HASMC proliferation, HAEC apoptosis, and AD progression in AngII-infused mice via the miR-143-3p/DDX6 pathway. | |||
Carbohydrate sulfotransferase 11 (CHST11)
Diffuse large B-cell lymphomas [ICD-11: 2A81]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [26] | |||
| Responsed Disease | Diffuse large B-cell lymphomas [ICD-11: 2A81] | |||
| Target Regulation | Down regulation | |||
In-vitro Model |
OCI-Ly1 | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1879 |
| OCI-Ly8 | Diffuse large B-cell lymphoma germinal center B-cell type | Homo sapiens | CVCL_8803 | |
| OCI-Ly3 | Diffuse large B-cell lymphoma activated B-cell type | Homo sapiens | CVCL_8800 | |
| VAL | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1819 | |
| U-2932 | Diffuse large B-cell lymphoma | Homo sapiens | CVCL_1896 | |
| In-vivo Model | A total of 1 × 107 KIAA1429 stable knockdown OCI-LY1 cells or CHST11 stable knockdown OCI-LY1 cells were injected subcutaneously into the right armpit of mice. Two investigators who were blinded to the mice allocation observed the general condition of mice and tumor growth every 2 days, measuring tumor size with a vernier caliper upon the tumor size was higher than the skin surface and recording it. | |||
CCN family member 2 (CTGF)
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [27] | |||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
SV40 MES 13
|
N.A. | Mus musculus | CVCL_5368 |
| NRK-52E | Normal | Rattus norvegicus | CVCL_0468 | |
Circ_DLC1
Liver cancer [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [28] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Target Regulation | Down regulation | |||
| Pathway Response | RNA degradation | hsa03018 | ||
| Cell Process | RNA stability | |||
In-vitro Model |
SNU-449 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0454 |
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hep 3B2.1-7 | Childhood hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| In-vivo Model | 5 × 105 cells were injected subcutaneously into the right axilla of mice. Tumor volume was measured by a caliper weekly and calculated as length × width2 × 0.52. For the liver orthotopic-implanted models, each liver of mice was injected with 1 × 106 cells. | |||
| Response Summary | Circ_DLC1, a downstream target of KIAA1429, is a promising prognostic marker for HCC patients, and the circDLC1-HuR-MMP1 axis serve as a potential therapeutic target for HCC treatment. | |||
endogenous Bornavirus like nucleoprotein 3, pseudogene (EBLN3P)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [29] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
NCM460 | Normal | Homo sapiens | CVCL_0460 |
| HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | |
| LoVo | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | |
Hyaluronan synthase 2 (HAS2)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [30] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 |
| MDA-MB-157 | Breast carcinoma | Homo sapiens | CVCL_0618 | |
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | |
Kinesin-like protein KIF15 (KIF15)
Triple-negative breast cancer [ICD-11: 2C6Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [31] | |||
| Responsed Disease | Triple-negative breast cancer [ICD-11: 2C6Z] | |||
| Target Regulation | Up regulation | |||
long intergenic non-protein coding RNA 1106 (LINC01106)
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [32] | |||
| Responsed Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
long intergenic non-protein coding RNA 667 (LINC00667)
Breast cancer [ICD-11: 2C60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [33] | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulation | Up regulation | |||
long intergenic non-protein coding RNA 839 (LINC00839)
Nasopharyngeal carcinoma [ICD-11: 2B6B]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [34] | |||
| Responsed Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
NP69SV40T
|
N.A. | Homo sapiens | CVCL_F755 |
| N2Tert (The human immortalized nasopharyngeal epithelial cell lines) | ||||
| HONE-1 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_8706 | |
| SUNE1 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_6946 | |
| C666 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_M597 | |
| HNE-1 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_0308 | |
| 5-8F | Nasopharyngeal carcinoma | Homo sapiens | CVCL_C528 | |
| 6-10B | Nasopharyngeal carcinoma | Homo sapiens | CVCL_C529 | |
| S18 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_B0U9 | |
| S26 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_B0UB | |
| CNE-1 | Normal | Homo sapiens | CVCL_6888 | |
| CNE-2 | Nasopharyngeal carcinoma | Homo sapiens | CVCL_6889 | |
| HK-1 | Lung large cell carcinoma | Homo sapiens | CVCL_7047 | |
| In-vivo Model | For xenograft growth model, 1 × 106 SUNE-1 cells stably expressing scrambled or sh-LINC00839 were inoculated subcutaneously into the axillas of nude mice. The tumor volumes were measured every 3 days. After 21 days, the mice were sacrificed. Simultaneously, the subcutaneous tumors were excised and weighed. For the lung metastatic colonization model, 1 × 106 SUNE-1 cells stably expressing scrambled or sh-LINC00839 were injected into the tail veins of nude mice. After 5 weeks, the mice were sacrificed, with their lung tissues dissected. All subcutaneous tumors and lung tissues were paraffin embedded and sectioned for subsequent analyses. | |||
Microfibril-associated glycoprotein 4 (MFAP4)
Photoaging [ICD-11: EJ20]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [35] | |||
| Responsed Disease | Photoaging [ICD-11: EJ20] | |||
| Target Regulation | Up regulation | |||
| In-vivo Model | A total of 30, six-week-old male C57/BL6 mice were purchased from Orient Bio Inc. (Beijing, China), and they were fed according to standard procedures. After one week of adaptive feeding, the mice were randomly divided into two groups, namely, the control group (n = 7) and the UVR group (n = 7). All mice were shaved once a week. The UVR mice were irradiated with a mixed source of UVA (315 nm ~ 400nm, 0.60 mW/cm2) and UVB (290nm ~ 315nm, 3.5 mW/cm2) ray every other day for 12 weeks. The initial irradiation time was 15 min for the first week base on the minimal erythema dose (MED), followed by a graduated increase until it reached 80 min. The total irradiated dose was approximately 151 J/cm2 for UVA and 23 J/cm2for UVB, respectively. | |||
Phosphoglycerate kinase 1 (PGK1)
Head and neck squamous carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [36] | |||
| Responsed Disease | Oral squamous cell carcinoma [ICD-11: 2B6E.0] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
HOK | Normal | Hexagrammos otakii | CVCL_YE19 |
| SCC-9 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1685 | |
| CAL-27 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1107 | |
POU6F2 antisense RNA 1 (POU6F2-AS1)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [37] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 |
| NCM460 | Normal | Homo sapiens | CVCL_0460 | |
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | |
Ras-related protein Rab-27B (RAB27B)
Malignant haematopoietic neoplasm [ICD-11: 2B33]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [38] | |||
| Responsed Disease | Chronic myeloid leukaemia [ICD-11: 2B33.2] | |||
| Responsed Drug | Rucaparib | Approved | ||
| Target Regulation | Up regulation | |||
In-vitro Model |
K-562 | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 |
| KCL-22 | Chronic myelogenous leukemia | Homo sapiens | CVCL_2091 | |
| In-vivo Model | K562 cells (1 × 106) were suspended in 100 μL of normal saline and the suspension was mixed with an equal volume of Matrigel. This mixture was subcutaneously injected into the right armpit of 4-week-old mice. Tumor size measurements were initiated on the day of inoculation. The tumor size was calculated using the formula: 0.5 × (long diameter) × (short diameter).2 When the tumor volume reached 100 mm3 (± 20%), rucaparib was given via intragastric administration at 50 mg/kg/d. | |||
| Experiment 2 Reporting the m6A-centered Disease Response of This Target Gene | [38] | |||
| Responsed Disease | Chronic myeloid leukaemia [ICD-11: 2B33.2] | |||
| Responsed Drug | Imatinib | Approved | ||
| Target Regulation | Up regulation | |||
In-vitro Model |
K-562 | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 |
| KCL-22 | Chronic myelogenous leukemia | Homo sapiens | CVCL_2091 | |
| In-vivo Model | K562 cells (1 × 106) were suspended in 100 μL of normal saline and the suspension was mixed with an equal volume of Matrigel. This mixture was subcutaneously injected into the right armpit of 4-week-old mice. Tumor size measurements were initiated on the day of inoculation. The tumor size was calculated using the formula: 0.5 × (long diameter) × (short diameter).2 When the tumor volume reached 100 mm3 (± 20%), rucaparib was given via intragastric administration at 50 mg/kg/d. | |||
Rho-associated protein kinase 2 (ROCK2)
Atherosclerosis [ICD-11: BD40]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [39] | |||
| Responsed Disease | Atherosclerosis [ICD-11: BD40.Z] | |||
| Target Regulation | Up regulation | |||
Serine protease hepsin (HPN)
Liver cancer [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [40] | |||
| Responsed Disease | Liver hepatocellular carcinoma [ICD-11: 2C12.02] | |||
In-vitro Model |
Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| HLF | Adult hepatocellular carcinoma | Homo sapiens | CVCL_2947 | |
| SNU-423 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0366 | |
| Li-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_3840 | |
| L-02 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6926 | |
Serine/threonine-protein kinase 10 (STK10)
Injuries of spine or trunk [ICD-11: ND51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [41] | |||
| Responsed Disease | Spinal cord injury [ICD-11: ND51.2] | |||
| Target Regulation | Down regulation | |||
In-vitro Model |
PC12 | Rat adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
Transcription factor E2F7 (E2F7)
Nasopharyngeal carcinoma [ICD-11: 2B6B]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [42] | |||
| Responsed Disease | Nasopharyngeal carcinoma [ICD-11: 2B6B] | |||
| Target Regulation | Up regulation | |||
| In-vivo Model | For the tumor growth model, 1 × 106 SUNE-1 cells stably expressing scrambled or sh-VIRMA were injected subcutaneously into the axilla of mice, and the tumor size was measured every 4 days. After 32 days, the mice were sacrificed, and the tumors were retrieved. For the tumor inguinal lymph node metastasis model, 1 × 106 scrambled or sh-VIRMA SUNE-1 cells were injected into the footpads of mice. After 6 weeks, the mice were euthanized. The footpad tumors and inguinal lymph nodes were excised. Tumors and lymph nodes were subjected to subsequent in situ hybridization and immunohistochemistry analysis. | |||
Transcription factor SOX-8 (SOX8)
Colorectal cancer [ICD-11: 2B91]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [43] | |||
| Responsed Disease | Colorectal cancer [ICD-11: 2B91] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
FHC | Normal | Homo sapiens | CVCL_3688 |
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | |
| HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| HCT 8 | Colon adenocarcinoma | Homo sapiens | CVCL_2478 | |
| RKO | Colon carcinoma | Homo sapiens | CVCL_0504 | |
| In-vivo Model | SW620 cells were seeded in 6-well plates and infected with USP29-knockdown lentivirus (sh-USP29) or the negative control (sh-NC), respectively, and cells were treated with 4 μg/mL of puromycin (Invitrogen) for two weeks to screen stable knockout cells. Afterward, each mouse was subcutaneously injected via the axilla with stable infected SW620 cells (N = 5 × 106 cells). | |||
Unspecific Target Gene
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [44] | |||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| Responsed Drug | Gemcitabine | Approved | ||
| Pathway Response | Adipocytokine signaling pathway | hsa04920 | ||
| Cell Process | Epithelial-mesenchymal transition | |||
In-vitro Model |
BxPC-3 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 |
| HDE-CT cell line (A normal human pancreatic cell line) | ||||
| MIA PaCa-2 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | |
| Response Summary | Lasso regression identified a six-m6A-regulator-signature prognostic model (KIAA1429, HNRNPC, METTL3, YTHDF1, IGF2BP2, and IGF2BP3). Gene set enrichment analysis revealed m6A regulators (KIAA1429, HNRNPC, and IGF2BP2) were related to multiple biological behaviors in pancreatic cancer, including adipocytokine signaling, the well vs. poorly differentiated tumor pathway, tumor metastasis pathway, epithelial mesenchymal transition pathway, gemcitabine resistance pathway, and stemness pathway. | |||
Liver cancer [ICD-11: 2C12]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [45] | |||
| Responsed Disease | Liver hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Responsed Drug | Sorafenib | Approved | ||
In-vitro Model |
Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Sora | Canine transitional cell carcinoma | Canis lupus familiaris | CVCL_WH26 | |
| EA.hy 926 | Normal | Homo sapiens | CVCL_3901 | |
| In-vivo Model | Six-week-old BALB/c-nu mice (n = 10) were purchased from Hunan STA Laboratory Animal Co., Ltd. Nude mice were adaptively fed in a specific pathogen free (SPF) environment for 7 days. The study protocol was ethically approved by the Kunming Yan'an Hospital Experimental Animal Ethics Committee (Kunming, China; approval no. 2020004). Mice were randomly divided into a control group (HepG2) and an experimental group (HepG2/Sora) with 5 mice in each group. A cell suspension (4 × 106 cells per mouse) was injected into the right lateral thighs of mice after light anesthesia using 37.5 mg/kg pelltobarbitalum natricum (cat.no. P-010; Sigma-Aldrich LLC.). The drug treatment was carried out when the tumor size was approximately 100 mm3. Sorafenib was prepared with 0.4% DMSO+PBS solution and administered to mice by intraperitoneal injection at a dose of 100 mg/kg after light anesthesia using 37.5 mg/kg pelltobarbitalum natricum. Sorafenib was administered once a day for 5 consecutive days. The physical state of the nude mice was observed and recorded every day. Mice in poor condition were terminated in time and euthanized immediately. All the mice were sacrificed using intraperitoneal injection of 200 mg/kg pelltobarbitalum natricum 5 days after sorafenib administration. Before euthanasia, the mice were given oral administration of ibuprofen (40 mg/kg; cat.no.14883; Sigma-Aldrich LLC.) with water to relieve pain. The tumors were removed surgically and photographed using a camera. | |||
| Experiment 2 Reporting the m6A-centered Disease Response of This Target Gene | [46] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Responsed Drug | Teniposide | Approved | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Nucleotide excision repair | hsa03420 | ||
| mTOR signaling pathway | hsa04150 | |||
| Cell Process | DNA repair | |||
In-vitro Model |
Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| In-vivo Model | To establish a tumour model, C57BL/6 mice were intraperitoneal injected with 25 mg/kg diethylnitrosamine at 2 weeks of age. | |||
| Response Summary | The m6A model includes LRPPRC, YTHDF2, KIAA14219, and RBM15B, classified A-hepatocellular carcinoma patients into high/low-risk subtypes. The expression of Immunosuppressive cytokines DNMT1/EZH2 was up-regulated in A-hepatocellular carcinoma patients, and teniposide can be a potential therapeutic drug for A-hepatocellular carcinoma. | |||
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [47] | |||
| Responsed Disease | Lung adenocarcinoma [ICD-11: 2C25.0] | |||
| Responsed Drug | Gefitinib | Approved | ||
In-vitro Model |
NCI-H1573 | Lung adenocarcinoma | Homo sapiens | CVCL_1478 |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
Testicular cancer [ICD-11: 2C80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [48] | |||
| Responsed Disease | Testicular cancer [ICD-11: 2C80] | |||
| Target Regulation | Up regulation | |||
| Cell Process | Transcription | |||
| Response Summary | Abundance of m6A and expression of VIRMA/YTHDF3 were different among Testicular Germ Cell Tumors subtypes, with higher levels in SEs, suggesting a contribution to SE phenotype maintenance. | |||
Cyclin-dependent kinase 1 (CDK1)
| Representative RNA-seq result indicating the expression of this target gene regulated by VIRMA | ||
| Cell Line | Human umbilical vein endothelial cells | Homo sapiens |
|
Treatment: siVIRMA HUVECs
Control: siControl HUVECs
|
GSE167067 | |
| Regulation |
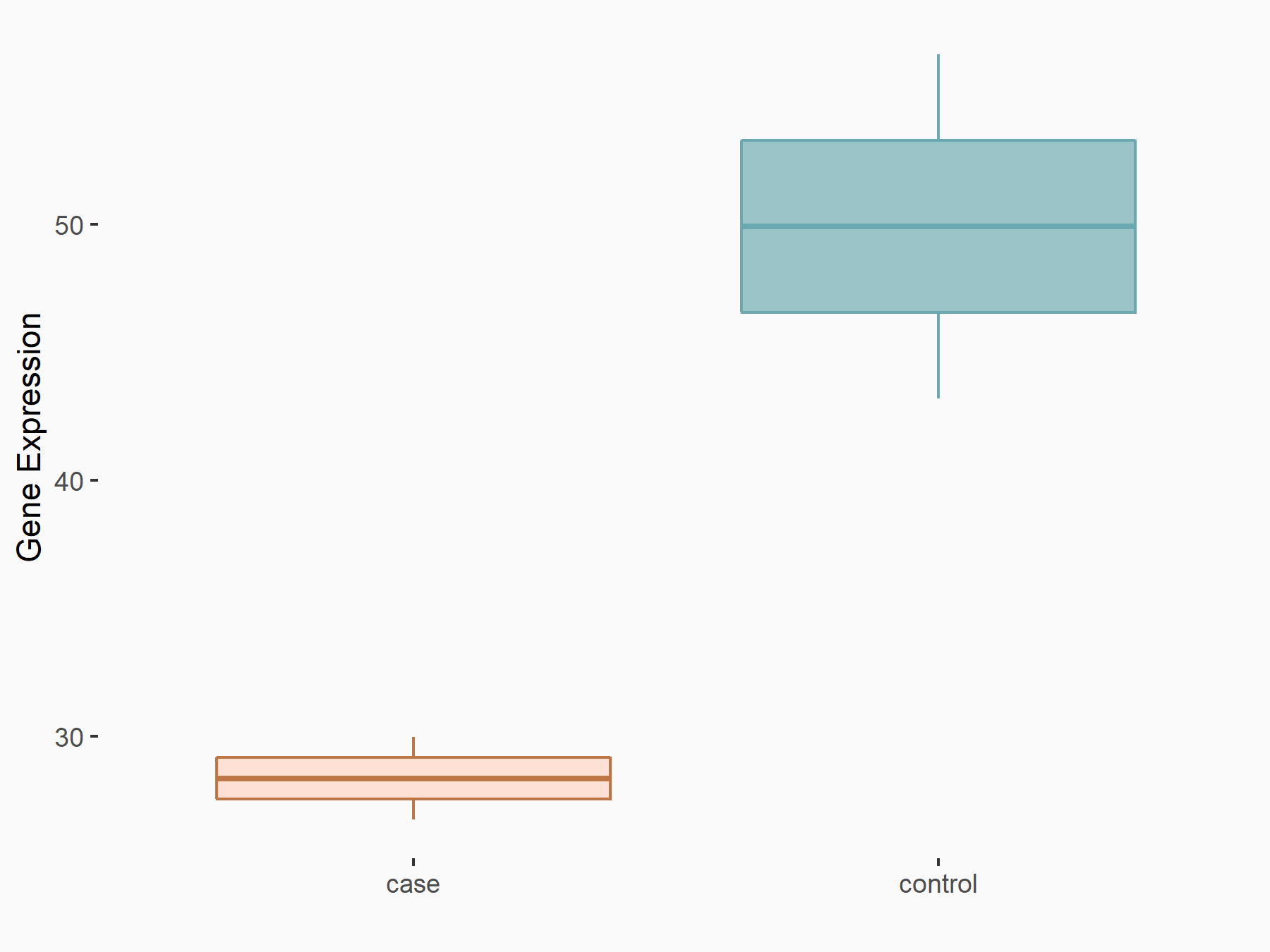 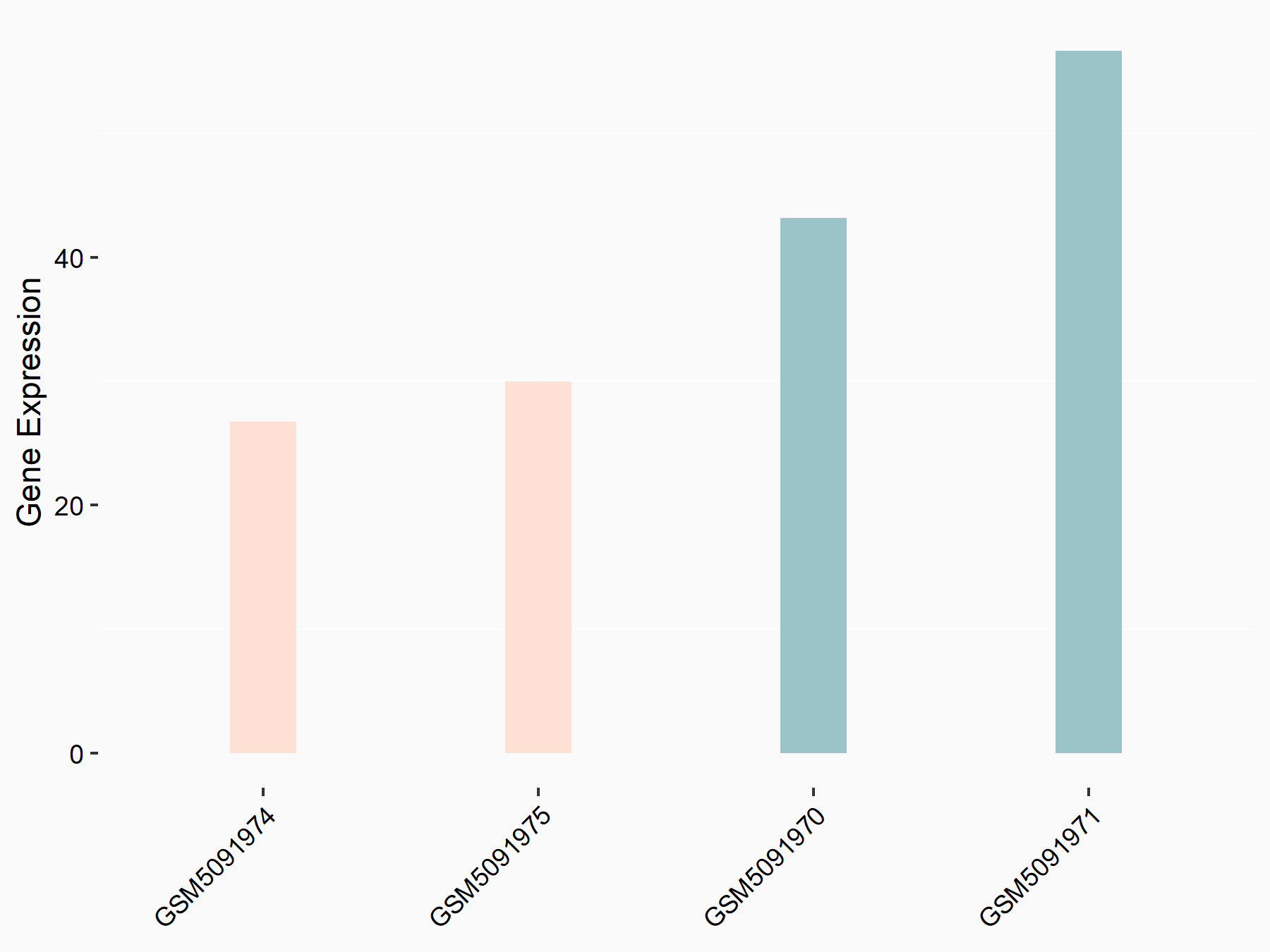 |
logFC: -7.84E-01 p-value: 3.08E-02 |
| More Results | Click to View More RNA-seq Results | |
Fluorouracil
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [1] | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Cell proliferation and metastasis | |||
| Response Summary | KIAA1429 acts as an oncogenic factor in breast cancer by regulating CDK1 in an N6-methyladenosine-independent manner.5'-fluorouracil was found to be very effective in reducing the expression of KIAA1429 and Cyclin-dependent kinase 1 (CDK1) in breast cancer. | |||
Histone H2AX (H2AX)
| Representative RNA-seq result indicating the expression of this target gene regulated by VIRMA | ||
| Cell Line | Human umbilical vein endothelial cells | Homo sapiens |
|
Treatment: siVIRMA HUVECs
Control: siControl HUVECs
|
GSE167067 | |
| Regulation |
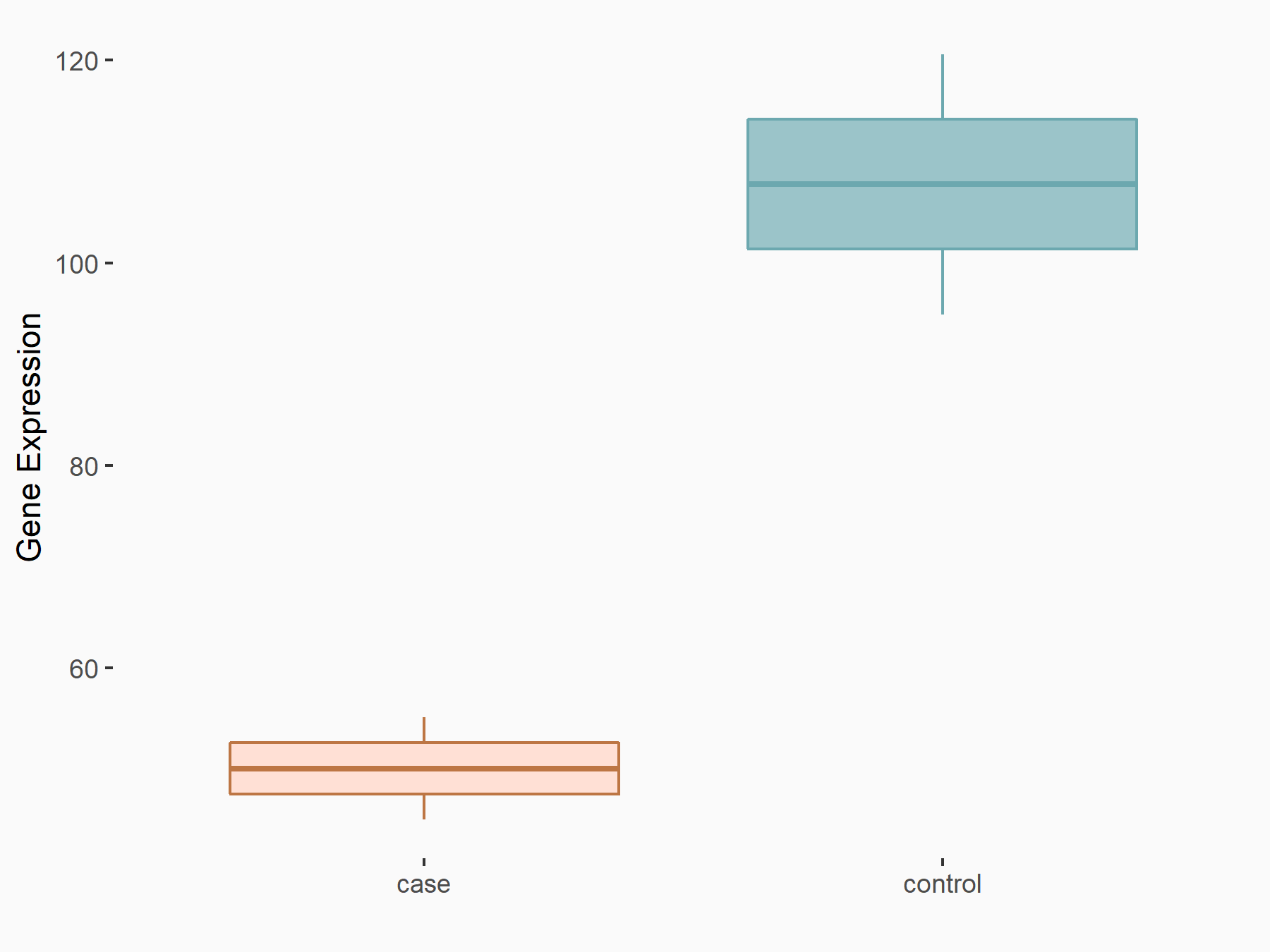 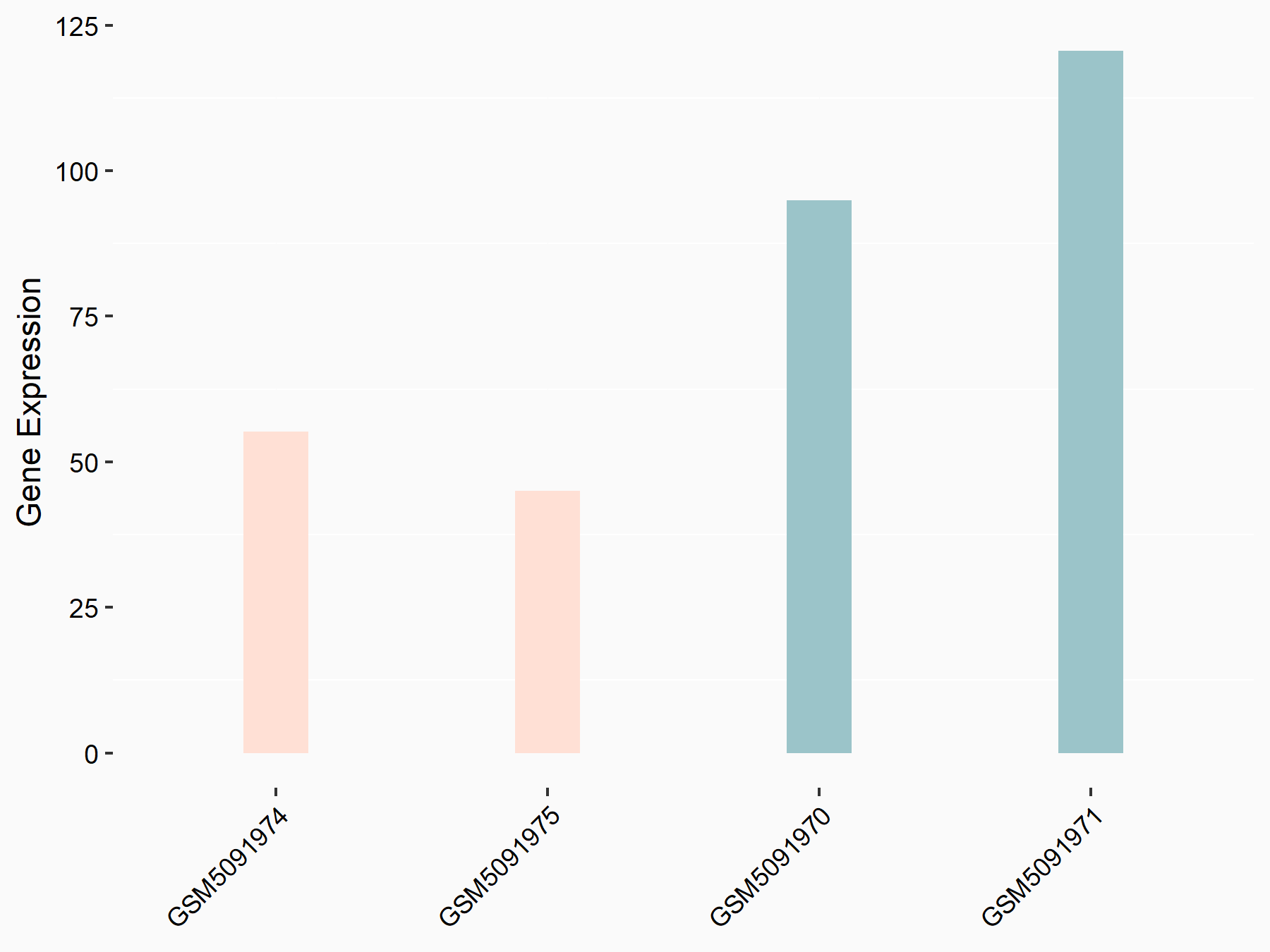 |
logFC: -1.09E+00 p-value: 1.68E-02 |
| More Results | Click to View More RNA-seq Results | |
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [3] | |||
| Responsed Disease | Germ cell tumour of testis | ICD-11: 2C80.2 | ||
| Target Regulation | Down regulation | |||
| In-vitro Model | 2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Histone H2AX (H2AX) and GADD45B levels) and downregulation of XLF and MRE11. | |||
Cystine/glutamate transporter (SLC7A11)
Cycloleucine
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [8] | |||
| Responsed Disease | Liver hepatocellular carcinoma | ICD-11: 2C12.02 | ||
| In-vitro Model | Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
Ferrostatin-1
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [8] | |||
| Responsed Disease | Liver hepatocellular carcinoma | ICD-11: 2C12.02 | ||
| In-vitro Model | Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
Liproxstatin-1
[Investigative]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [8] | |||
| Responsed Disease | Liver hepatocellular carcinoma | ICD-11: 2C12.02 | ||
| In-vitro Model | Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 |
| SK-HEP-1 | Liver and intrahepatic bile duct epithelial neoplasm | Homo sapiens | CVCL_0525 | |
Double-strand break repair protein MRE11 (MRE11)
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [3] | |||
| Responsed Disease | Germ cell tumour of testis | ICD-11: 2C80.2 | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | 2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and GADD45B levels) and downregulation of XLF and Double-strand break repair protein MRE11 (MRE11). | |||
Forkhead box protein M1 (FOXM1)
Oxaliplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [12] | |||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | GES-1 | Normal | Homo sapiens | CVCL_EQ22 |
| AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| HGC-27 | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
Homeobox protein Hox-A1 (HOXA1)
Gefitinib
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [15] | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | Gefitinib-resistant cell line PC9GR (Gefitinib-resistant PC9 cell line) | |||
| PC-9 | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | |
| NHBE (Normal bronchial epithelial cells) | ||||
| In-vivo Model | PC9-GR cells stably infected with KIAA1429-targeting shRNA and control were suspended in 100 uL of PBS with Matrigel matrix (BD Biosciences). Then, cells were injected into one of the flanks of BALB/c nude mice. | |||
| Response Summary | m6A methyltransferase KIAA1429 was highly expressed in gefitinib-resistant NSCLC cells (PC9-GR), tissues, and closely related to unfavorable survival. KIAA1429 plays essential oncogenic roles in NSCLC gefitinib resistance, which provided a feasible therapeutic target for NSCLC. | |||
Negative growth regulatory protein MyD118 (GADD45B)
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [3] | |||
| Responsed Disease | Germ cell tumour of testis | ICD-11: 2C80.2 | ||
| Target Regulation | Down regulation | |||
| In-vitro Model | 2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and Negative growth regulatory protein MyD118 (GADD45B) levels) and downregulation of XLF and MRE11. | |||
Non-homologous end-joining factor 1 (NHEJ1/XLF)
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [3] | |||
| Responsed Disease | Germ cell tumour of testis | ICD-11: 2C80.2 | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | 2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and GADD45B levels) and downregulation of Non-homologous end-joining factor 1 (NHEJ1/XLF) and MRE11. | |||
Ras-related protein Rab-27B (RAB27B)
Rucaparib
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [38] | |||
| Responsed Disease | Chronic myeloid leukaemia | ICD-11: 2B33.2 | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | K-562 | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 |
| KCL-22 | Chronic myelogenous leukemia | Homo sapiens | CVCL_2091 | |
| In-vivo Model | K562 cells (1 × 106) were suspended in 100 μL of normal saline and the suspension was mixed with an equal volume of Matrigel. This mixture was subcutaneously injected into the right armpit of 4-week-old mice. Tumor size measurements were initiated on the day of inoculation. The tumor size was calculated using the formula: 0.5 × (long diameter) × (short diameter).2 When the tumor volume reached 100 mm3 (± 20%), rucaparib was given via intragastric administration at 50 mg/kg/d. | |||
Imatinib
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [38] | |||
| Responsed Disease | Chronic myeloid leukaemia | ICD-11: 2B33.2 | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | K-562 | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 |
| KCL-22 | Chronic myelogenous leukemia | Homo sapiens | CVCL_2091 | |
| In-vivo Model | K562 cells (1 × 106) were suspended in 100 μL of normal saline and the suspension was mixed with an equal volume of Matrigel. This mixture was subcutaneously injected into the right armpit of 4-week-old mice. Tumor size measurements were initiated on the day of inoculation. The tumor size was calculated using the formula: 0.5 × (long diameter) × (short diameter).2 When the tumor volume reached 100 mm3 (± 20%), rucaparib was given via intragastric administration at 50 mg/kg/d. | |||
Unspecific Target Gene
Gemcitabine
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [44] | |||
| Responsed Disease | Pancreatic cancer | ICD-11: 2C10 | ||
| Pathway Response | Adipocytokine signaling pathway | hsa04920 | ||
| Cell Process | Epithelial-mesenchymal transition | |||
| In-vitro Model | BxPC-3 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 |
| HDE-CT cell line (A normal human pancreatic cell line) | ||||
| MIA PaCa-2 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | |
| Response Summary | Lasso regression identified a six-m6A-regulator-signature prognostic model (KIAA1429, HNRNPC, METTL3, YTHDF1, IGF2BP2, and IGF2BP3). Gene set enrichment analysis revealed m6A regulators (KIAA1429, HNRNPC, and IGF2BP2) were related to multiple biological behaviors in pancreatic cancer, including adipocytokine signaling, the well vs. poorly differentiated tumor pathway, tumor metastasis pathway, epithelial mesenchymal transition pathway, gemcitabine resistance pathway, and stemness pathway. | |||
Sorafenib
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [45] | |||
| Responsed Disease | Liver hepatocellular carcinoma | ICD-11: 2C12.02 | ||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Sora | Canine transitional cell carcinoma | Canis lupus familiaris | CVCL_WH26 | |
| EA.hy 926 | Normal | Homo sapiens | CVCL_3901 | |
| In-vivo Model | Six-week-old BALB/c-nu mice (n = 10) were purchased from Hunan STA Laboratory Animal Co., Ltd. Nude mice were adaptively fed in a specific pathogen free (SPF) environment for 7 days. The study protocol was ethically approved by the Kunming Yan'an Hospital Experimental Animal Ethics Committee (Kunming, China; approval no. 2020004). Mice were randomly divided into a control group (HepG2) and an experimental group (HepG2/Sora) with 5 mice in each group. A cell suspension (4 × 106 cells per mouse) was injected into the right lateral thighs of mice after light anesthesia using 37.5 mg/kg pelltobarbitalum natricum (cat.no. P-010; Sigma-Aldrich LLC.). The drug treatment was carried out when the tumor size was approximately 100 mm3. Sorafenib was prepared with 0.4% DMSO+PBS solution and administered to mice by intraperitoneal injection at a dose of 100 mg/kg after light anesthesia using 37.5 mg/kg pelltobarbitalum natricum. Sorafenib was administered once a day for 5 consecutive days. The physical state of the nude mice was observed and recorded every day. Mice in poor condition were terminated in time and euthanized immediately. All the mice were sacrificed using intraperitoneal injection of 200 mg/kg pelltobarbitalum natricum 5 days after sorafenib administration. Before euthanasia, the mice were given oral administration of ibuprofen (40 mg/kg; cat.no.14883; Sigma-Aldrich LLC.) with water to relieve pain. The tumors were removed surgically and photographed using a camera. | |||
Teniposide
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [46] | |||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12.02 | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Nucleotide excision repair | hsa03420 | ||
| mTOR signaling pathway | hsa04150 | |||
| Cell Process | DNA repair | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| In-vivo Model | To establish a tumour model, C57BL/6 mice were intraperitoneal injected with 25 mg/kg diethylnitrosamine at 2 weeks of age. | |||
| Response Summary | The m6A model includes LRPPRC, YTHDF2, KIAA14219, and RBM15B, classified A-hepatocellular carcinoma patients into high/low-risk subtypes. The expression of Immunosuppressive cytokines DNMT1/EZH2 was up-regulated in A-hepatocellular carcinoma patients, and teniposide can be a potential therapeutic drug for A-hepatocellular carcinoma. | |||
Gefitinib
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [47] | |||
| Responsed Disease | Lung adenocarcinoma | ICD-11: 2C25.0 | ||
| In-vitro Model | NCI-H1573 | Lung adenocarcinoma | Homo sapiens | CVCL_1478 |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
RNA modification
m6A Target: DNA-binding protein inhibitor ID-2 (ID2)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00532 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 19a (MIR19A) | |
| Crosstalk relationship | A-to-I → m6A | |
m6A Target: pri-miR-143
| In total 5 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00567 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | Signal transducer and activator of transcription 3 (STAT3) | |
| Crosstalk relationship | m6A → A-to-I | |
| Crosstalk ID: M6ACROT00570 | ||
| Epigenetic Regulator | Fat mass and obesity-associated protein (FTO) | |
| Regulated Target | Signal transducer and activator of transcription 3 (STAT3) | |
| Crosstalk relationship | m6A → m6Am | |
| Crosstalk ID: M6ACROT00573 | ||
| Epigenetic Regulator | Y-box-binding protein 1 (YBX1) | |
| Regulated Target | Hypoxia-inducible factor 1-alpha (HIF-1-Alpha/HIF1A) | |
| Crosstalk relationship | m6A → m5C | |
| Crosstalk ID: M6ACROT00576 | ||
| Epigenetic Regulator | H/ACA ribonucleoprotein complex subunit DKC1 (DKC1) | |
| Regulated Target | Hypoxia-inducible factor 1-alpha (HIF-1-Alpha/HIF1A) | |
| Crosstalk relationship | m6A → Pseudouridine | |
| Crosstalk ID: M6ACROT00579 | ||
| Epigenetic Regulator | RNA cytosine C(5)-methyltransferase NSUN2 (NSUN2) | |
| Regulated Target | H19 imprinted maternally expressed transcript (H19) | |
| Crosstalk relationship | m5C → m6A | |
Non-coding RNA
m6A Target: Trans-acting T-cell-specific transcription factor GATA-3 (GATA3)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05057 | ||
| Epigenetic Regulator | GATA3 antisense RNA 1 (GATA3-AS1) | |
| Regulated Target | Protein virilizer homolog (VIRMA) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Liver cancer | |
m6A Target: long intergenic non-protein coding RNA 667 (LINC00667)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05093 | ||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 667 (LINC00667) | |
| Regulated Target | hsa-miR-556-5p | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Breast cancer | |
| Crosstalk ID: M6ACROT05094 | ||
| Epigenetic Regulator | hsa-miR-556-5p | |
| Regulated Target | Protein virilizer homolog (VIRMA) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Breast cancer | |
m6A Target: endogenous Bornavirus like nucleoprotein 3, pseudogene (EBLN3P)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05100 | ||
| Epigenetic Regulator | hsa-miR-153-3p | |
| Regulated Target | Protein virilizer homolog (VIRMA) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Colorectal cancer | |
| Crosstalk ID: M6ACROT05101 | ||
| Epigenetic Regulator | Endogenous Bornavirus like nucleoprotein 3, pseudogene (EBLN3P) | |
| Regulated Target | hsa-miR-153-3p | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Colorectal cancer | |
m6A Target: CCN family member 2 (CTGF)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05187 | ||
| Epigenetic Regulator | AI662270 | |
| Regulated Target | Protein virilizer homolog (VIRMA) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Chronic kidney disease | |
m6A Target: Colon cancer associated transcript 1 (CCAT1)
| In total 3 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05427 | ||
| Epigenetic Regulator | Colon cancer associated transcript 1 (CCAT1) | |
| Regulated Target | Myc proto-oncogene protein (MYC) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Prostate cancer | |
| Drug | Actinomycin D | |
| Crosstalk ID: M6ACROT05964 | ||
| Epigenetic Regulator | Colon cancer associated transcript 1 (CCAT1) | |
| Regulated Target | MicroRNA let-7a-1 (Let-7A) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Prostate cancer | |
| Drug | Actinomycin D | |
| Crosstalk ID: M6ACROT05965 | ||
| Epigenetic Regulator | MicroRNA let-7a-1 (Let-7A) | |
| Regulated Target | Myc proto-oncogene protein (MYC) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Prostate cancer | |
| Drug | Actinomycin D | |
m6A Target: Colon cancer associated transcript 2 (CCAT2)
| In total 3 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05428 | ||
| Epigenetic Regulator | Colon cancer associated transcript 2 (CCAT2) | |
| Regulated Target | Myc proto-oncogene protein (MYC) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Prostate cancer | |
| Drug | Actinomycin D | |
| Crosstalk ID: M6ACROT05966 | ||
| Epigenetic Regulator | Colon cancer associated transcript 2 (CCAT2) | |
| Regulated Target | MicroRNA 145 (MIR145) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Prostate cancer | |
| Drug | Actinomycin D | |
| Crosstalk ID: M6ACROT05967 | ||
| Epigenetic Regulator | MicroRNA 145 (MIR145) | |
| Regulated Target | Myc proto-oncogene protein (MYC) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Prostate cancer | |
| Drug | Actinomycin D | |
m6A Target: hsa-miR-143-3p
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05512 | ||
| Epigenetic Regulator | pri-miR-143 | |
| Regulated Target | DEAD-box helicase 6 (DDX6) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Aortic aneurysm or dissection | |
m6A Target: Circ_DLC1
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05575 | ||
| Epigenetic Regulator | Circ_DLC1 | |
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Liver cancer | |
m6A Target: Long intergenic non-protein coding RNA 958 (LINC00958)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05614 | ||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 958 (LINC00958) | |
| Regulated Target | Solute carrier family 2 member 1 (SLC2A1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Gastric cancer | |
m6A Target: POU6F2 antisense RNA 1 (POU6F2-AS1)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05670 | ||
| Epigenetic Regulator | POU6F2 antisense RNA 1 (POU6F2-AS1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Colorectal cancer | |
m6A Target: Nuclear paraspeckle assembly transcript 1 (NEAT1)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05679 | ||
| Epigenetic Regulator | Nuclear paraspeckle assembly transcript 1 (NEAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Breast cancer | |
m6A Target: Long intergenic non-protein coding RNA 839 (LINC00839)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05740 | ||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 839 (LINC00839) | |
| Regulated Target | TATA-box binding protein associated factor 15 (TAF15) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Nasopharyngeal carcinoma | |
m6A Target: Long intergenic non-protein coding RNA 1106 (LINC01106)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05826 | ||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 1106 (LINC01106) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Lung cancer | |
Xenobiotics Compound(s) Regulating the m6A Methylation Regulator
| Compound Name | 5-FU | Approved |
|---|---|---|
| Synonyms |
5-Fluorouracil; 51-21-8; fluorouracil; 5-FU; Fluoroplex; Adrucil; Efudex; Carac; Fluracil; Fluoroblastin; 5-fluoropyrimidine-2,4(1H,3H)-dione; Kecimeton; Timazin; Carzonal; Efudix; Arumel; Fluril; Queroplex; Fluracilum; Ulup; 5-Fluoracil; Phthoruracil; Fluro Uracil; 5-Fluoro-2,4(1H,3H)-pyrimidinedione; Ftoruracil; Fluorouracilum; Efurix; Fluri; 5 Fluorouracil; Effluderm (free base); 5-fluoro-1H-pyrimidine-2,4-dione; Fluorouracilo; Fluroblastin; Phtoruracil; 2,4-Dihydroxy-5-fluoropyrimidine; 2,4(1H,3H)-Pyrimidinedione, 5-fluoro-; Adrucil; Effluderm; Fluorouracile; Fluoruracil; Fluracedyl; Flurodex; Neofluor; Onkofluor; Ribofluor; Tetratogen; URF; Allergan Brand of Fluorouracil; Biosyn Brand of Fluorouracil; CSP Brand of Fluorouracil; Cinco FU; Dakota Brand of Fluorouracil; Dermatech Brand of Fluorouracil; Dermik Brandof Fluorouracil; Ferrer Brand of Fluorouracil; Fluoro Uracile ICN; Fluorouracil GRY; Fluorouracil Mononitrate; Fluorouracil Monopotassium Salt; Fluorouracil Monosodium Salt; Fluorouracil Potassium Salt; Fluorouracil Teva Brand; Fluorouracile Dakota; Fluorouracile [DCIT]; Fluorouracilo Ferrer Far; Gry Brand of Fluorouracil; Haemato Brand of Fluorouracil; Haemato fu; Hexal Brand of Fluorouracil; ICN Brand of Fluorouracil; Inhibits thymilidate synthetase; Medac Brand of Fluorouracil; Neocorp Brand of Fluorouracil; Onkoworks Brand of Fluorouracil; Ribosepharm Brand of Fluorouracil; Riemser Brand of Fluorouracil; Roche Brand of Fluorouracil; Teva Brand of Fluorouracil; F 6627; F0151; IN1335; U 8953; Adrucil (TN); Carac (TN); Dakota, Fluorouracile; Efudex (TN); Fluoro-Uracile ICN; Fluoro-uracile; Fluoro-uracilo; Fluoroplex (TN); Fluorouracil-GRY; Fluorouracilo [INN-Spanish]; Fluorouracilum [INN-Latin]; Haemato-fu; Ro 2-9757; U-8953; Ro-2-9757; Fluorouracil (JP15/USP/INN); Fluorouracil [USAN:INN:BAN:JAN]; 1-fluoro-1h-pyrimidine-2,4-dione; 2,4-Dioxo-5-fluoropryimidine; 2,4-Dioxo-5-fluoropyrimidine; 5 FU Lederle; 5 FU medac; 5 Fluorouracil biosyn; 5 HU Hexal; 5-FU (TN); 5-FU Lederle; 5-FU medac; 5-Faracil; 5-Fluor-2,4(1H,3H)-pyrimidindion; 5-Fluor-2,4(1H,3H)-pyrimidindion [Czech]; 5-Fluor-2,4-dihydroxypyrimidin; 5-Fluor-2,4-dihydroxypyrimidin [Czech]; 5-Fluor-2,4-pyrimidindiol; 5-Fluor-2,4-pyrimidindiol [Czech]; 5-Fluoracil [German]; 5-Fluoracyl; 5-Fluoro-2,4-pyrimidinedione; 5-Fluoropyrimidin-2,4-diol; 5-Fluoropyrimidine-2,4-dione; 5-Fluorouracil-biosyn; 5-Fluoruracil; 5-Fluoruracil [German]; 5-Ftouracyl; 5-HU Hexal; 5-fluoro uracil; 5FU
Click to Show/Hide
|
|
| External link | ||
| Description |
KIAA1429 acts as an oncogenic factor inbreast cancer by regulating CDK1 in an N6-methyladenosine-independent manner.5'-fluorouracil was found to be very effective in reducing the expression of KIAA1429 andCDK1 in breast cancer.
|
[1] |
| Compound Name | . | Investigative |
References