m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00264)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
GADD45B
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Browse Drug
Fat mass and obesity-associated protein (FTO) [ERASER]
| Representative RNA-seq result indicating the expression of this target gene regulated by FTO | ||
| Cell Line | HEK293 cell line | Homo sapiens |
|
Treatment: FTO knockout HEK293 cells
Control: Wild type HEK293 cells
|
GSE79577 | |
| Regulation |
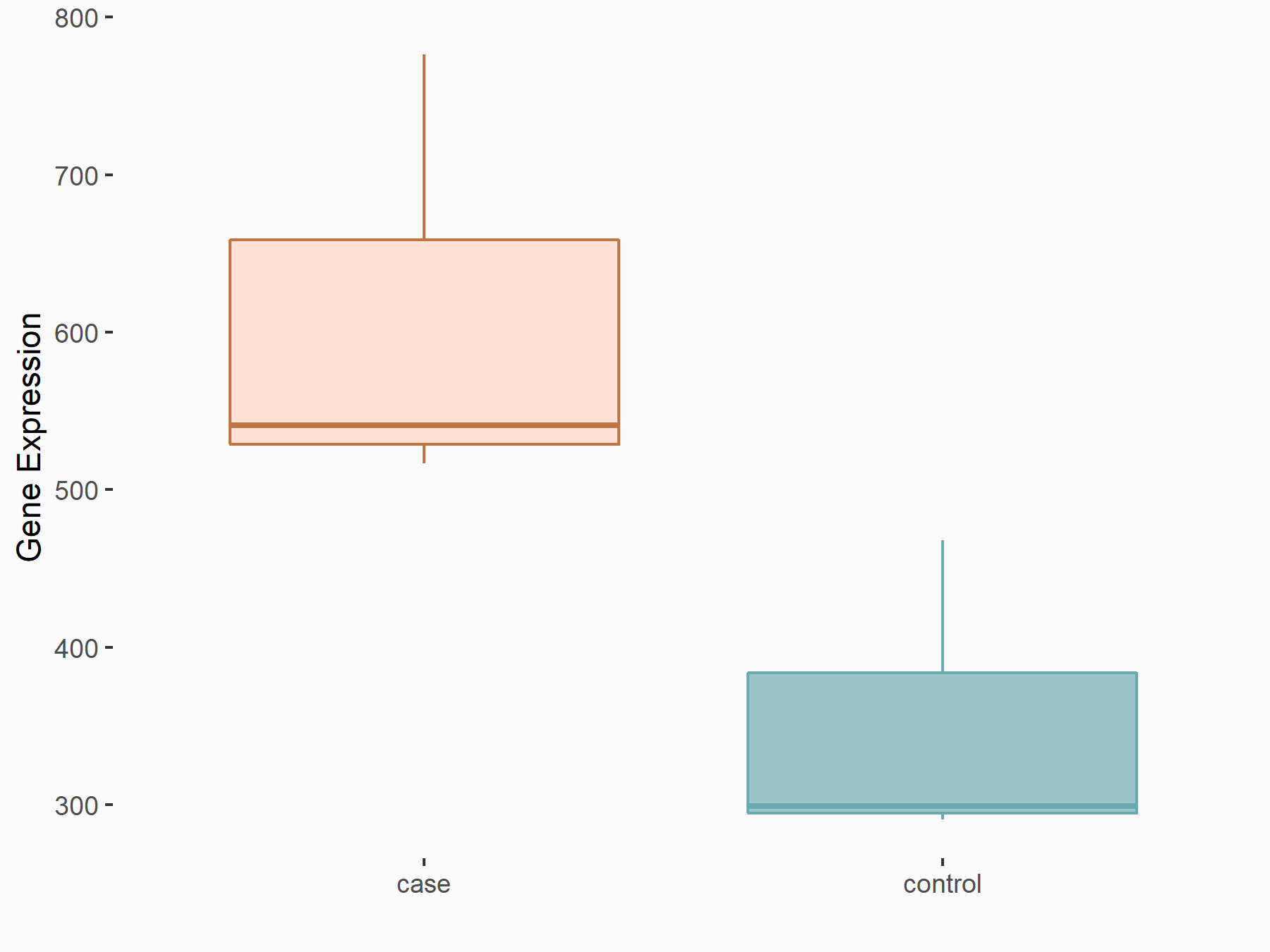 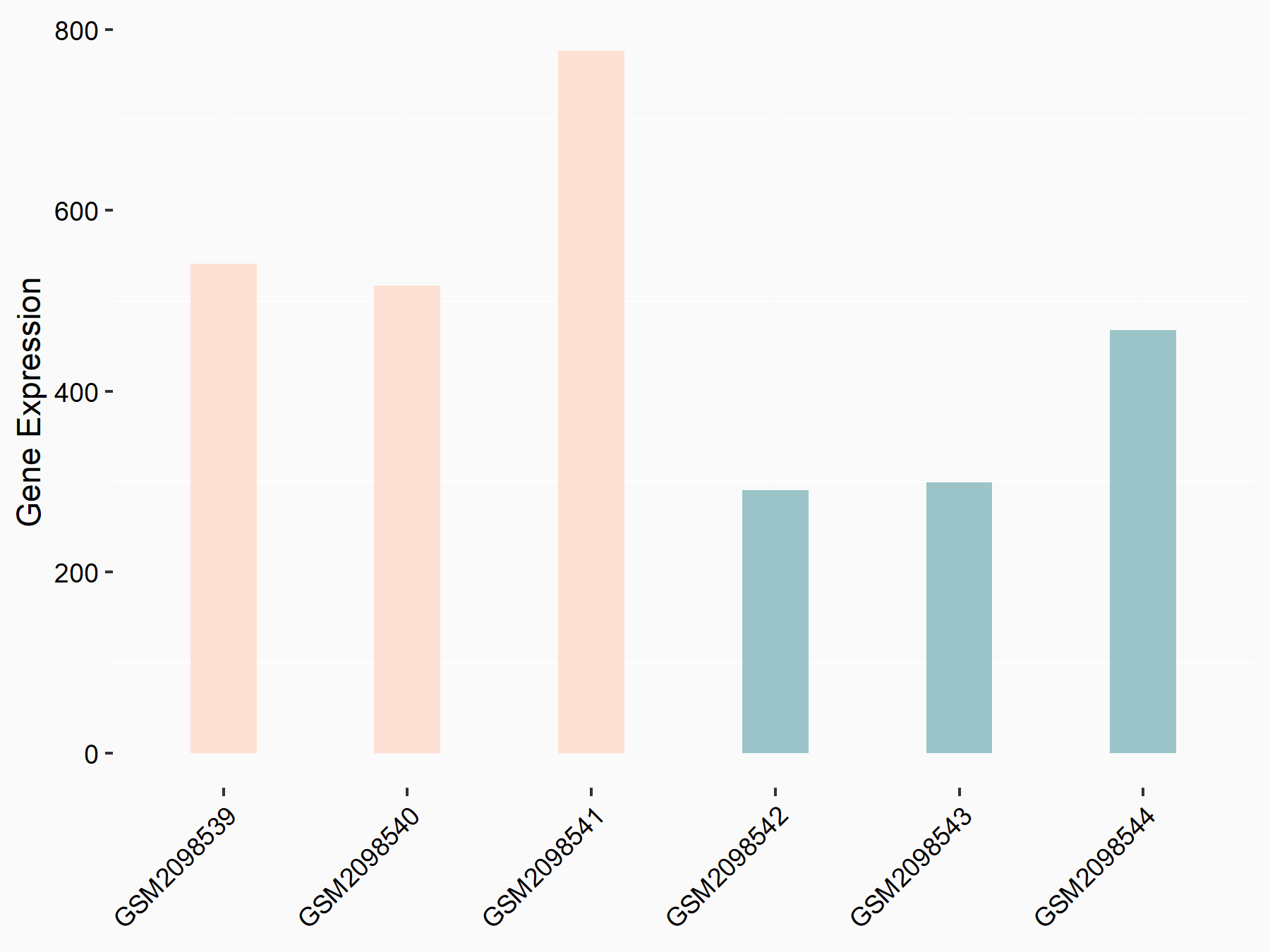 |
logFC: 8.04E-01 p-value: 5.27E-05 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | Negative growth regulatory protein MyD118 (GADD45B)-mediated m6A modification in Negative growth regulatory protein MyD118 (GADD45B) mRNA drives skeletal muscle differentiation by activating the p38 MAPK pathway, which provides a molecular mechanism for the regulation of myogenesis via RNA methylation. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Muscular dystrophies | ICD-11: 8C70 | ||
| Pathway Response | Nucleotide excision repair | hsa03420 | ||
| MAPK signaling pathway | hsa04010 | |||
| Cell Process | DNA repair | |||
| In-vitro Model | GPM (Goat primary myoblasts) | |||
| In-vivo Model | Sixteen female goats in good body condition and suitable for pregnancy were selected. All selected goats underwent estrus synchronization treatment and were naturally mated. After 75 days of gestation, four male fetuses were removed from five pregnant goats during abortion operations, and their longissimus muscle samples were collected. | |||
Protein virilizer homolog (VIRMA) [WRITER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and Negative growth regulatory protein MyD118 (GADD45B) levels) and downregulation of XLF and MRE11. | |||
| Target Regulation | Down regulation | |||
| Responsed Disease | Germ cell tumour of testis | ICD-11: 2C80.2 | ||
| Responsed Drug | Cisplatin | Approved | ||
| In-vitro Model | 2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
Testicular cancer [ICD-11: 2C80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and Negative growth regulatory protein MyD118 (GADD45B) levels) and downregulation of XLF and MRE11. | |||
| Responsed Disease | Germ cell tumour of testis [ICD-11: 2C80.2] | |||
| Target Regulator | Protein virilizer homolog (VIRMA) | WRITER | ||
| Target Regulation | Down regulation | |||
| Responsed Drug | Cisplatin | Approved | ||
| In-vitro Model | 2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
Muscular dystrophies [ICD-11: 8C70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | Negative growth regulatory protein MyD118 (GADD45B)-mediated m6A modification in Negative growth regulatory protein MyD118 (GADD45B) mRNA drives skeletal muscle differentiation by activating the p38 MAPK pathway, which provides a molecular mechanism for the regulation of myogenesis via RNA methylation. | |||
| Responsed Disease | Muscular dystrophies [ICD-11: 8C70] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Nucleotide excision repair | hsa03420 | ||
| MAPK signaling pathway | hsa04010 | |||
| Cell Process | DNA repair | |||
| In-vitro Model | GPM (Goat primary myoblasts) | |||
| In-vivo Model | Sixteen female goats in good body condition and suitable for pregnancy were selected. All selected goats underwent estrus synchronization treatment and were naturally mated. After 75 days of gestation, four male fetuses were removed from five pregnant goats during abortion operations, and their longissimus muscle samples were collected. | |||
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [2] | |||
| Response Summary | VIRMA has an oncogenic role in germ cell tumor confirming our previous tissue-based study and is further involved in response to cisplatin by interfering with DNA repair. Enhanced response to cisplatin after VIRMA knockdown was related to significant increase in DNA damage (with higher Gamma-H2AX and Negative growth regulatory protein MyD118 (GADD45B) levels) and downregulation of XLF and MRE11. | |||
| Target Regulator | Protein virilizer homolog (VIRMA) | WRITER | ||
| Target Regulation | Down regulation | |||
| Responsed Disease | Germ cell tumour of testis | ICD-11: 2C80.2 | ||
| In-vitro Model | 2102EP | Embryonal carcinoma | Homo sapiens | CVCL_C522 |
| NCC-IT | Testicular embryonal carcinoma | Homo sapiens | CVCL_1451 | |
| NT2 | Malignant neoplasms | Mus musculus | CVCL_JA57 | |
| TCam-2 | Testicular seminoma | Homo sapiens | CVCL_T012 | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00264)
| In total 39 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE038066 | Click to Show/Hide the Full List | ||
| mod site | chr19:2476144-2476145:+ | [3] | |
| Sequence | TCAGATCGCCGAAGCGTCGGACTACCGTTGGTTTCCGCAAC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; GM12878; Huh7; Jurkat; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1; ENST00000586759.1 | ||
| External Link | RMBase: m6A_site_409633 | ||
| mod ID: M6ASITE038067 | Click to Show/Hide the Full List | ||
| mod site | chr19:2476188-2476189:+ | [3] | |
| Sequence | CTGGATTATCCTCGCCAAGGACTTTGCAATATATTTTTCCG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; GM12878; MM6; Huh7; Jurkat; CD4T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1; ENST00000587345.1; ENST00000586759.1; ENST00000585359.1 | ||
| External Link | RMBase: m6A_site_409634 | ||
| mod ID: M6ASITE038068 | Click to Show/Hide the Full List | ||
| mod site | chr19:2476317-2476318:+ | [3] | |
| Sequence | TTTGCAATTTCTCCCTGGGGACTGCCGTGGAGCCGCATCCA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; GM12878; MM6; Huh7; Jurkat; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000585359.1; ENST00000215631.9; ENST00000592937.1; ENST00000586759.1; ENST00000587345.1 | ||
| External Link | RMBase: m6A_site_409635 | ||
| mod ID: M6ASITE038069 | Click to Show/Hide the Full List | ||
| mod site | chr19:2476639-2476640:+ | [3] | |
| Sequence | TTGATGAATGTGTGAGTCAGACCCCCTTCCCGGGCTGGGCG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; MM6; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000585359.1; ENST00000587887.5; ENST00000587345.1; ENST00000593043.1; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409636 | ||
| mod ID: M6ASITE038070 | Click to Show/Hide the Full List | ||
| mod site | chr19:2476673-2476674:+ | [4] | |
| Sequence | CTGGGCGCGGGTGGGACGGGACCTCCCCTCCGCTCTGGACG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD34; MM6; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000593043.1; ENST00000592937.1; ENST00000587345.1; ENST00000587887.5; ENST00000585359.1 | ||
| External Link | RMBase: m6A_site_409637 | ||
| mod ID: M6ASITE038071 | Click to Show/Hide the Full List | ||
| mod site | chr19:2476815-2476816:+ | [5] | |
| Sequence | TCCTGATGTATCGTCTGCAAACACCCCTCCCGCCGTGGGCC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | MT4 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000593043.1; ENST00000592937.1; ENST00000587345.1; ENST00000587887.5; ENST00000585359.1 | ||
| External Link | RMBase: m6A_site_409638 | ||
| mod ID: M6ASITE038072 | Click to Show/Hide the Full List | ||
| mod site | chr19:2476861-2476862:+ | [5] | |
| Sequence | CCCCCTACCCCATACTTTGAACCGTGTGCCCTCCCCTCCCC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | MT4 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1; ENST00000587887.5; ENST00000593043.1; ENST00000587345.1; ENST00000585359.1 | ||
| External Link | RMBase: m6A_site_409639 | ||
| mod ID: M6ASITE038073 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477030-2477031:+ | [3] | |
| Sequence | ACCCTTTGGCCCCCTCAGGGACCCAGACAGCGTGGTCCTCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000587887.5; ENST00000593043.1; ENST00000592937.1; ENST00000585359.1; ENST00000587345.1 | ||
| External Link | RMBase: m6A_site_409640 | ||
| mod ID: M6ASITE038074 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477036-2477037:+ | [3] | |
| Sequence | TGGCCCCCTCAGGGACCCAGACAGCGTGGTCCTCTGCCTCT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; liver; endometrial | ||
| Seq Type List | m6A-seq; m6A-REF-seq | ||
| Transcript ID List | ENST00000587345.1; ENST00000592937.1; ENST00000215631.9; ENST00000585359.1; ENST00000593043.1; ENST00000587887.5 | ||
| External Link | RMBase: m6A_site_409641 | ||
| mod ID: M6ASITE038075 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477084-2477085:+ | [6] | |
| Sequence | TGACGAGGAGGAGGAGGATGACATCGCCCTGCAAATCCACT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000587345.1; ENST00000215631.9; ENST00000587887.5; ENST00000593043.1; ENST00000592937.1; ENST00000585359.1 | ||
| External Link | RMBase: m6A_site_409642 | ||
| mod ID: M6ASITE038076 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477203-2477204:+ | [3] | |
| Sequence | TCCTGGGAGAGCCGGCCGAGACCCAGGGCACCACCGAGGCC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9; ENST00000585359.1; ENST00000587345.1 | ||
| External Link | RMBase: m6A_site_409643 | ||
| mod ID: M6ASITE038077 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477228-2477229:+ | [3] | |
| Sequence | GGGCACCACCGAGGCCCGAGACCTGCATTGTCTCCTGGTCA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000587345.1; ENST00000215631.9; ENST00000592937.1; ENST00000585359.1 | ||
| External Link | RMBase: m6A_site_409644 | ||
| mod ID: M6ASITE038078 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477464-2477465:+ | [3] | |
| Sequence | GGAGAGGGAGGCTCCACTAAACCCCTTCTTTTCCCTCCTAC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000585359.1; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409645 | ||
| mod ID: M6ASITE038079 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477488-2477489:+ | [3] | |
| Sequence | CTTCTTTTCCCTCCTACAGAACCCTCACACGGACGCCTGGA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; MT4; MM6; peripheral-blood; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000585359.1; ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409646 | ||
| mod ID: M6ASITE038080 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477494-2477495:+ | [7] | |
| Sequence | TTCCCTCCTACAGAACCCTCACACGGACGCCTGGAAGAGCC | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000585359.1; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409647 | ||
| mod ID: M6ASITE038081 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477560-2477561:+ | [7] | |
| Sequence | CTGCGAAGAAAGCCGGGGCAACAACCAGTGGGTCCCCTACA | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000585359.1; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409648 | ||
| mod ID: M6ASITE038082 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477578-2477579:+ | [7] | |
| Sequence | CAACAACCAGTGGGTCCCCTACATCTCTCTTCAGGAACGCT | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000585359.1; ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409649 | ||
| mod ID: M6ASITE038083 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477638-2477639:+ | [7] | |
| Sequence | GAATCTGTTGAGTTGCTGCCACAAACAAAAAATACAATAAA | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000585359.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409650 | ||
| mod ID: M6ASITE038084 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477642-2477643:+ | [3] | |
| Sequence | CTGTTGAGTTGCTGCCACAAACAAAAAATACAATAAATATT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1A; GM12878; LCLs; CD8T; MT4; H1299; MM6; Huh7; CD4T; peripheral-blood; HEK293A-TOA; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000592937.1; ENST00000585359.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409651 | ||
| mod ID: M6ASITE038085 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477666-2477667:+ | [3] | |
| Sequence | AAAATACAATAAATATTTGAACCCCCTCCCCCCCAGCACAA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; GM12878; LCLs; MT4; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409652 | ||
| mod ID: M6ASITE038086 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477697-2477698:+ | [3] | |
| Sequence | CCCAGCACAACCCCCCCAAAACAACCCAACCCACGAGGACC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1A; H1B; hNPCs; GM12878; LCLs; MT4; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000592937.1; rmsk_4914468; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409653 | ||
| mod ID: M6ASITE038087 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477715-2477716:+ | [3] | |
| Sequence | AAACAACCCAACCCACGAGGACCATCGGGGGCAGAGTCGTT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; GM12878; LCLs; MT4; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409654 | ||
| mod ID: M6ASITE038088 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477740-2477741:+ | [3] | |
| Sequence | CGGGGGCAGAGTCGTTGGAGACTGAAGAGGAAGAGGAGGAG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; fibroblasts; GM12878; LCLs; MT4; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409655 | ||
| mod ID: M6ASITE038089 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477837-2477838:+ | [3] | |
| Sequence | GATCCGATGGAGAAGGGGGGACCCAGGCCAGCAGGAGACAG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; fibroblasts; GM12878; LCLs; MT4; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409656 | ||
| mod ID: M6ASITE038090 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477854-2477855:+ | [3] | |
| Sequence | GGGACCCAGGCCAGCAGGAGACAGGACCCCCGAAGCTGAGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; fibroblasts; GM12878; LCLs; MT4; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409657 | ||
| mod ID: M6ASITE038091 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477859-2477860:+ | [3] | |
| Sequence | CCAGGCCAGCAGGAGACAGGACCCCCGAAGCTGAGGCCTTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; fibroblasts; GM12878; LCLs; MT4; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409658 | ||
| mod ID: M6ASITE038092 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477942-2477943:+ | [3] | |
| Sequence | CCCCATCACGGAGGGTCCAGACTGTCCACTCGGGGGTGGAG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409659 | ||
| mod ID: M6ASITE038093 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477967-2477968:+ | [3] | |
| Sequence | CCACTCGGGGGTGGAGTGAGACTGACTGCAAGCCCCACCCT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409660 | ||
| mod ID: M6ASITE038094 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477971-2477972:+ | [8] | |
| Sequence | TCGGGGGTGGAGTGAGACTGACTGCAAGCCCCACCCTCCTT | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | CD8T; AML | ||
| Seq Type List | m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409661 | ||
| mod ID: M6ASITE038095 | Click to Show/Hide the Full List | ||
| mod site | chr19:2477995-2477996:+ | [3] | |
| Sequence | CAAGCCCCACCCTCCTTGAGACTGGAGCTGGCGTCTGCATA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409662 | ||
| mod ID: M6ASITE038096 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478022-2478023:+ | [3] | |
| Sequence | CTGGCGTCTGCATACGAGAGACTTGGTTGAACTTGGTTGGT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409663 | ||
| mod ID: M6ASITE038097 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478032-2478033:+ | [3] | |
| Sequence | CATACGAGAGACTTGGTTGAACTTGGTTGGTCCTTGTCTGC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409664 | ||
| mod ID: M6ASITE038098 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478060-2478061:+ | [7] | |
| Sequence | GGTCCTTGTCTGCACCCTCGACAAGACCACACTTTGGGACT | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | hESC-HEK293T; A549 | ||
| Seq Type List | MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409665 | ||
| mod ID: M6ASITE038099 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478065-2478066:+ | [3] | |
| Sequence | TTGTCTGCACCCTCGACAAGACCACACTTTGGGACTTGGGA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409666 | ||
| mod ID: M6ASITE038100 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478068-2478069:+ | [6] | |
| Sequence | TCTGCACCCTCGACAAGACCACACTTTGGGACTTGGGAGCT | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409667 | ||
| mod ID: M6ASITE038101 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478078-2478079:+ | [3] | |
| Sequence | CGACAAGACCACACTTTGGGACTTGGGAGCTGGGGCTGAAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409668 | ||
| mod ID: M6ASITE038102 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478116-2478117:+ | [3] | |
| Sequence | AAGTTGCTCTGTACCCATGAACTCCCAGTTTGCGAATTATA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409669 | ||
| mod ID: M6ASITE038103 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478140-2478141:+ | [3] | |
| Sequence | CCAGTTTGCGAATTATAGAGACAATCTATTTTGTTACTTGC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; kidney; liver; A549; hESC-HEK293T; BGC823; U2OS; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-REF-seq; MAZTER-seq; miCLIP | ||
| Transcript ID List | ENST00000215631.9; ENST00000592937.1 | ||
| External Link | RMBase: m6A_site_409670 | ||
| mod ID: M6ASITE038104 | Click to Show/Hide the Full List | ||
| mod site | chr19:2478174-2478175:+ | [3] | |
| Sequence | TACTTGCACTTGTTATTCGAACCACTGAGAGCGAGATGGGA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; U2OS; H1A; H1B; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000592937.1; ENST00000215631.9 | ||
| External Link | RMBase: m6A_site_409671 | ||
References