m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00206)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
CCND1
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Browse Drug
Fat mass and obesity-associated protein (FTO) [ERASER]
| Representative RNA-seq result indicating the expression of this target gene regulated by FTO | ||
| Cell Line | NB4 cell line | Homo sapiens |
|
Treatment: FTO inhibition NB4 cells
Control: NB4 cells
|
GSE103495 | |
| Regulation |
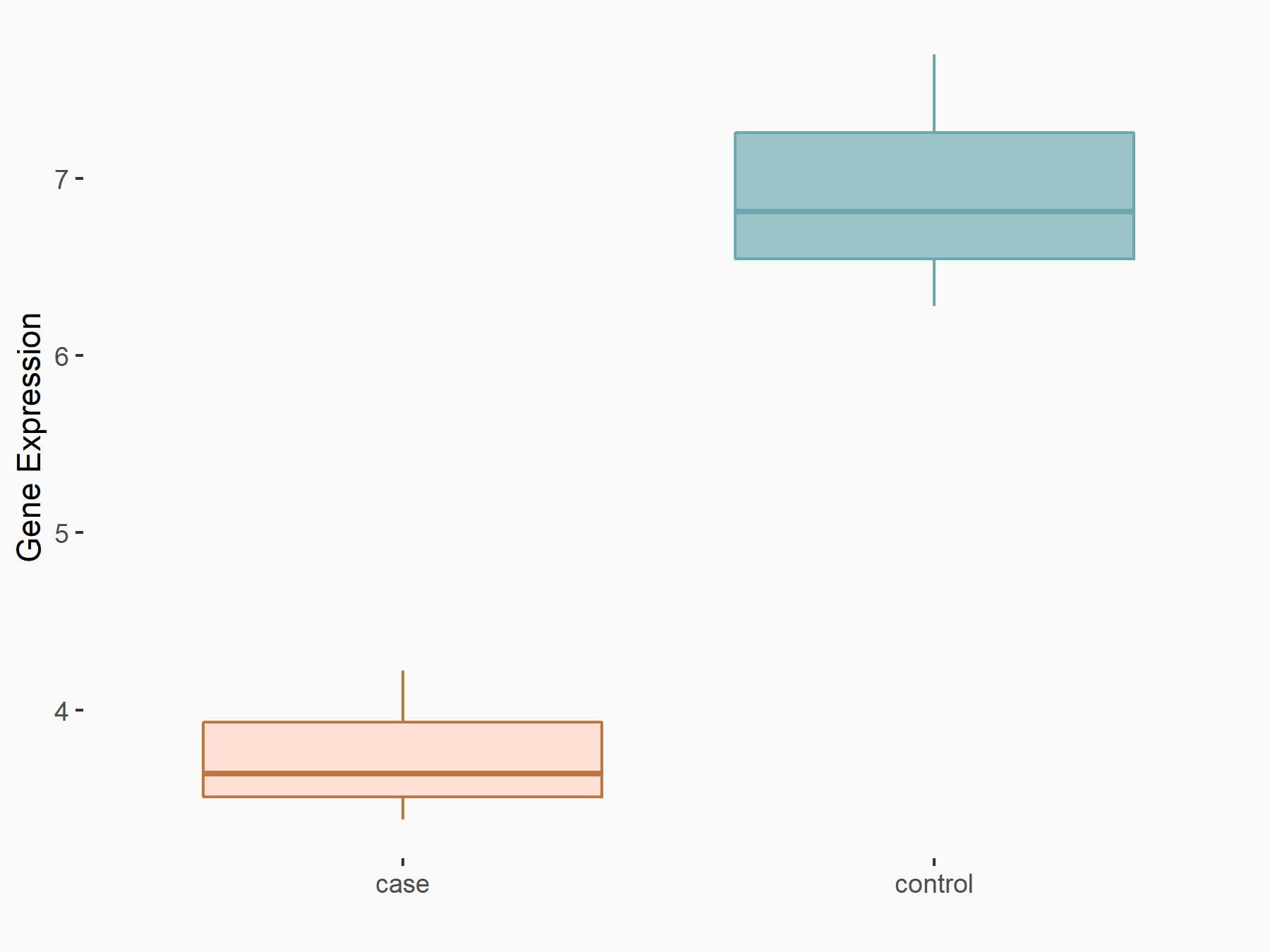  |
logFC: -7.40E-01 p-value: 1.34E-03 |
| More Results | Click to View More RNA-seq Results | |
| In total 2 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | FTO regulates myoblast proliferation by controlling G1/S-specific cyclin-D1 (CCND1) expression in an m6A-YTHDF2-dependent manner. | |||
| Target Regulation | Up regulation | |||
| Cell Process | Cell proliferation | |||
| Cell apoptosis | ||||
| In-vitro Model | GPM (Goat primary myoblasts) | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | Metformin could inhibit adipogenesis and combat obesity, metformin could inhibit protein expression of FTO, leading to increased m6A methylation levels of G1/S-specific cyclin-D1 (CCND1) and Cdk2(two crucial regulators in cell cycle). Ccnd1 and Cdk2 with increased m6A levels were recognised by YTHDF2, causing an YTHDF2-dependent decay and decreased protein expressions. | |||
| Responsed Disease | Obesity | ICD-11: 5B81 | ||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Cell cycle | |||
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | LNCaP cell line | Homo sapiens |
|
Treatment: shMETTL3 LNCaP cells
Control: shControl LNCaP cells
|
GSE147884 | |
| Regulation |
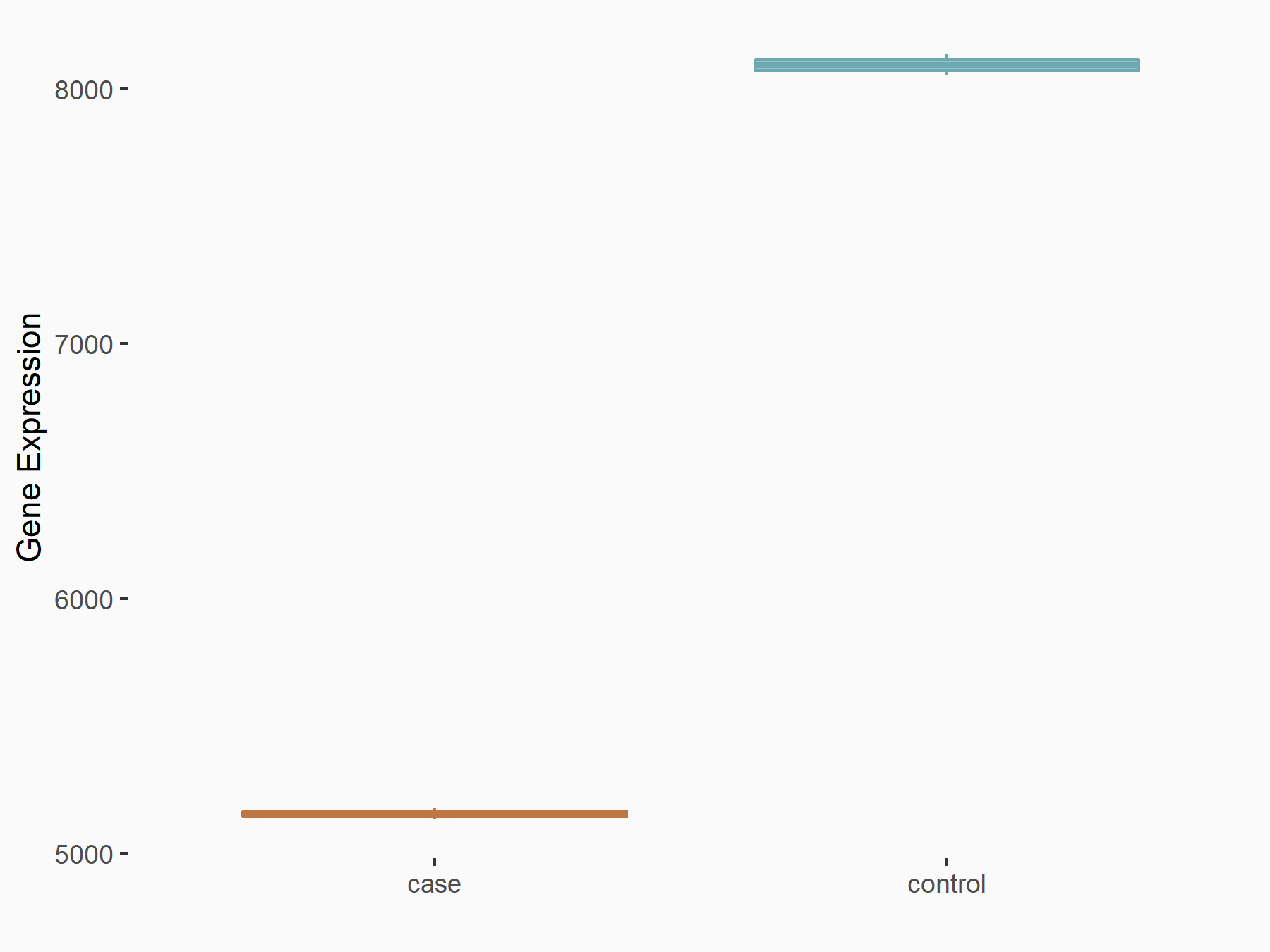  |
logFC: -6.51E-01 p-value: 1.46E-104 |
| More Results | Click to View More RNA-seq Results | |
| In total 4 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | Down-regulation of METTL3 inhibits the proliferation and mobility of human gastric cancer cells and leads to inactivation of the AKT signaling pathway, suggesting that METTL3 is a potential target for the treatment of human gastric cancer. METTL3 knockdown decreased Bcl2 and increased Bax and active Caspase-3 in gastric cancer cells, which suggested the apoptotic pathway was activated. METTL3 led to inactivation of the AKT signaling pathway in human gastric cancer cells, including decreased phosphorylation levels of AKT and expression of down-stream effectors p70S6K and G1/S-specific cyclin-D1 (CCND1). | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| In-vitro Model | AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [4] | |||
| Response Summary | METTL3 knockdown downregulated the phosphorylation levels of AKT and the expression of the downstream effector G1/S-specific cyclin-D1 (CCND1) in ovarian cancer. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Ovarian cancer | ICD-11: 2C73 | ||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Cell Process | Cell cycle | |||
| Cell apoptosis | ||||
| In-vitro Model | OVCAR-3 | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 |
| SK-OV-3 | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
| Experiment 3 Reporting the m6A Methylation Regulator of This Target Gene | [5] | |||
| Response Summary | METTL3 modulates miR-193b mature process in an m6A-dependent manner. Reintroduction of miR-193b profoundly inhibits tumorigenesis of cervical cancer cells both in vivo and in vitro through G1/S-specific cyclin-D1 (CCND1) targeting. | |||
| Target Regulation | Down regulation | |||
| Responsed Disease | Cervical cancer | ICD-11: 2C77 | ||
| In-vitro Model | SiHa | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| In-vivo Model | Mice were divided into two groups (n = 4/group) randomly. 3×106 cells suspended in 200 uL PBS were administered via subcutaneous injection over the right flank region of nude mice. After the development of palpable tumors (average volume, 50 mm3), intratumoral injection of synthetic miR-193b, or negative control complexed with siPORT Amine transfection reagent (Ambion, USA) was given 6 times at a 4-day interval. | |||
| Experiment 4 Reporting the m6A Methylation Regulator of This Target Gene | [6] | |||
| Response Summary | Obesity is becoming a global problem. ZFP217 knockdown-induced adipogenesis inhibition was caused by G1/S-specific cyclin-D1 (CCND1), which was mediated by METTL3 and YTHDF2 in an m6A-dependent manner. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Obesity | ICD-11: 5B81 | ||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Mitotic clonal | |||
| Prolonged G1/S transition | ||||
| In-vitro Model | 3T3-L1 | Normal | Mus musculus | CVCL_0123 |
YTH domain-containing family protein 2 (YTHDF2) [READER]
| Representative RNA-seq result indicating the expression of this target gene regulated by YTHDF2 | ||
| Cell Line | GSC11 cell line | Homo sapiens |
|
Treatment: siYTHDF2 GSC11 cells
Control: siControl GSC11 cells
|
GSE142825 | |
| Regulation |
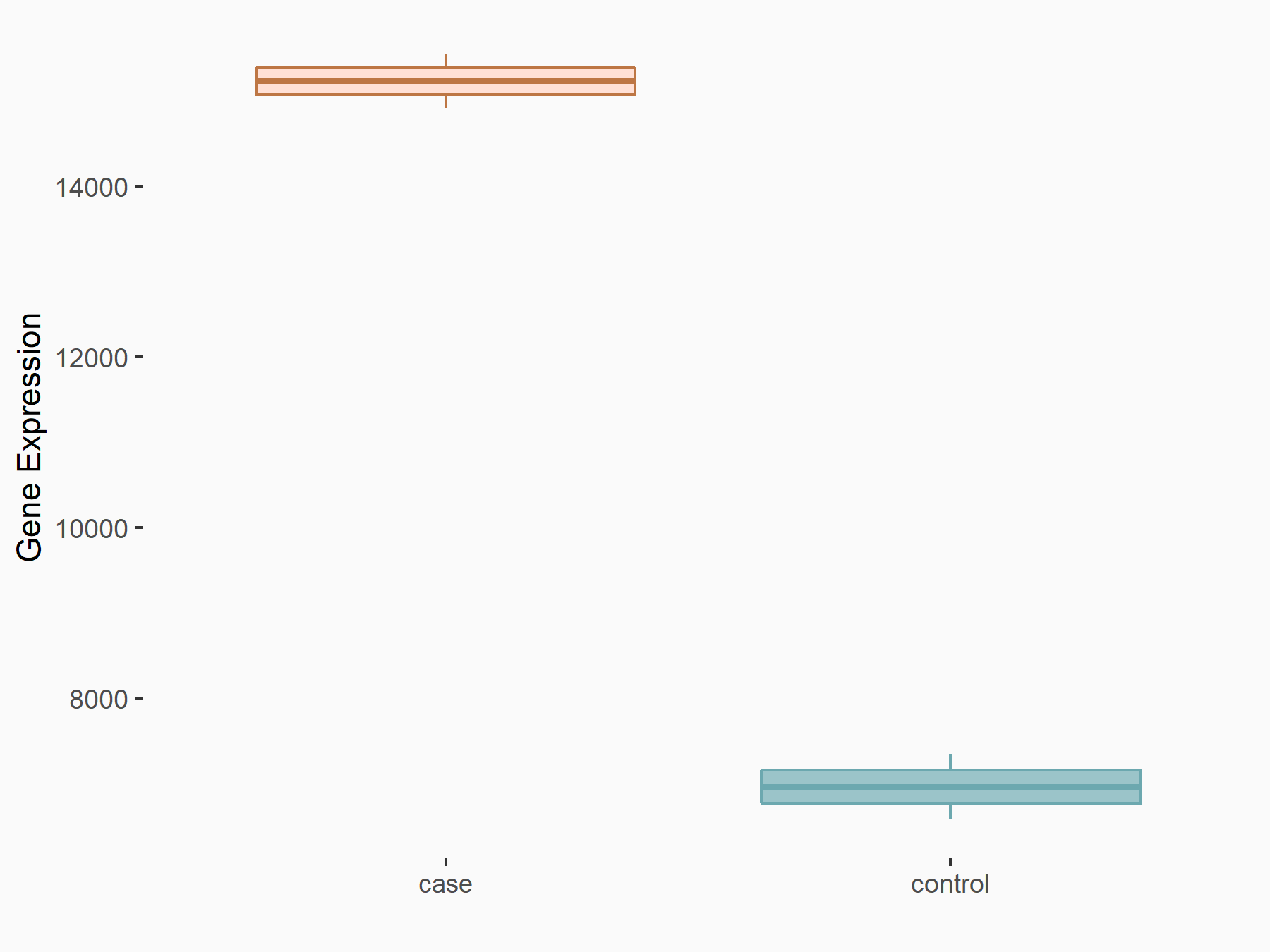  |
logFC: 1.13E+00 p-value: 1.41E-27 |
| More Results | Click to View More RNA-seq Results | |
| In total 3 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | FTO regulates myoblast proliferation by controlling G1/S-specific cyclin-D1 (CCND1) expression in an m6A-YTHDF2-dependent manner. | |||
| Target Regulation | Down regulation | |||
| Cell Process | Cell proliferation | |||
| Cell apoptosis | ||||
| In-vitro Model | GPM (Goat primary myoblasts) | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [6] | |||
| Response Summary | Obesity is becoming a global problem. ZFP217 knockdown-induced adipogenesis inhibition was caused by G1/S-specific cyclin-D1 (CCND1), which was mediated by METTL3 and YTHDF2 in an m6A-dependent manner. | |||
| Target Regulation | Down regulation | |||
| Responsed Disease | Obesity | ICD-11: 5B81 | ||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Mitotic clonal | |||
| Prolonged G1/S transition | ||||
| In-vitro Model | 3T3-L1 | Normal | Mus musculus | CVCL_0123 |
| Experiment 3 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | Metformin could inhibit adipogenesis and combat obesity, metformin could inhibit protein expression of FTO, leading to increased m6A methylation levels of G1/S-specific cyclin-D1 (CCND1) and Cdk2(two crucial regulators in cell cycle). Ccnd1 and Cdk2 with increased m6A levels were recognised by YTHDF2, causing an YTHDF2-dependent decay and decreased protein expressions. | |||
| Responsed Disease | Obesity | ICD-11: 5B81 | ||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Cell cycle | |||
Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) [READER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [11] | |||
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, G1/S-specific cyclin-D1 (CCND1), CDK4, EGR2, YBX1, and TLX, which were associated with lung cancers. | |||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | ||
| Cell Process | Cell apoptosis | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
Methyltransferase-like 16 (METTL16) [WRITER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [14] | |||
| Response Summary | METTL16-mediated m6A methylation promotes proliferation of gastric cancer cells through enhancing G1/S-specific cyclin-D1 (CCND1) expression. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | ||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | G1/S blocking | |||
| In-vitro Model | SNU-719 | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_5086 |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MKN28 | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1416 | |
| MGC-803 | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | |
| HGC-27 | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| GES-1 | Normal | Homo sapiens | CVCL_EQ22 | |
| AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| In-vivo Model | Xenograft mouse model was used to verify the tumorigenic effect of METTL16 in vivo. BALB/c nude mice (4 weeks old) were injected with METTL16 gene knock-down stable MGC803 GC cells (3 × 106 cells/mice, subcutaneous injection) or shNC control cells (3 × 106, subcutaneous injection), and the dose was 100 uL, with PBS as solvent. The tumour size was measured every 3-5 days. At the end of feeding (6 weeks after subcutaneous injection), the mice were killed and the tumours were extracted for histological analysis. | |||
Protein virilizer homolog (VIRMA) [WRITER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [11] | |||
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, G1/S-specific cyclin-D1 (CCND1), CDK4, EGR2, YBX1, and TLX, which were associated with lung cancers. | |||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | ||
| Cell Process | Cell apoptosis | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
YTH domain-containing family protein 1 (YTHDF1) [READER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [16] | |||
| Response Summary | YTHDF1 deficiency inhibits Non-small cell lung cancer cell proliferation and xenograft tumor formation through regulating the translational efficiency of CDK2, CDK4, p27, and G1/S-specific cyclin-D1 (CCND1), and that YTHDF1 depletion restrains de novo lung adenocarcinomas (ADC) progression. Mechanistic studies identified the Keap1-Nrf2-AKR1C1 axis as the downstream mediator of YTHDF1. YTHDF1 high expression correlates with better clinical outcome, with its depletion rendering cancerous cells resistant to cisplatin (DDP) treatment. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Chemical carcinogenesis - reactive oxygen species | hsa05208 | ||
| Cell cycle | hsa04110 | |||
| Cell Process | Biological regulation | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| A549-DDP (Human lung adenocarcinoma is resistant to cisplatin) | ||||
| GLC-82 | Endocervical adenocarcinoma | Homo sapiens | CVCL_3371 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| NCI-H1650 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1483 | |
| NCI-H838 | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | |
| SPC-A1 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | |
| In-vivo Model | Mice were treated via nasal inhalation of adenovirus carrying Cre recombinase (5 × 106 p.f.u for Ad-Cre, Biowit Inc., Shenzhen, Guangdong), and were then killed at indicated times for gross inspection and histopathological examination. | |||
YTH domain-containing family protein 3 (YTHDF3) [READER]
| In total 2 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [19] | |||
| Response Summary | Dysfunction of Ythdf3 and Mettl3 results in the translational defect of G1/S-specific cyclin-D1 (CCND1). Ythdf3 and Mettl3 regulates HSCs by transmitting m6A RNA methylation on the 5'UTR of Ccnd1. | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [19] | |||
| Response Summary | Dysfunction of Ythdf3 and Mettl3 results in the translational defect of G1/S-specific cyclin-D1 (CCND1). Ythdf3 and Mettl3 regulates HSCs by transmitting m6A RNA methylation on the 5'UTR of Ccnd1. | |||
Gastric cancer [ICD-11: 2B72]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [14] | |||
| Response Summary | METTL16-mediated m6A methylation promotes proliferation of gastric cancer cells through enhancing G1/S-specific cyclin-D1 (CCND1) expression. | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Target Regulator | Methyltransferase-like 16 (METTL16) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | G1/S blocking | |||
| In-vitro Model | SNU-719 | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_5086 |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| MKN28 | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1416 | |
| MGC-803 | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 | |
| HGC-27 | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| GES-1 | Normal | Homo sapiens | CVCL_EQ22 | |
| AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| In-vivo Model | Xenograft mouse model was used to verify the tumorigenic effect of METTL16 in vivo. BALB/c nude mice (4 weeks old) were injected with METTL16 gene knock-down stable MGC803 GC cells (3 × 106 cells/mice, subcutaneous injection) or shNC control cells (3 × 106, subcutaneous injection), and the dose was 100 uL, with PBS as solvent. The tumour size was measured every 3-5 days. At the end of feeding (6 weeks after subcutaneous injection), the mice were killed and the tumours were extracted for histological analysis. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [3] | |||
| Response Summary | Down-regulation of METTL3 inhibits the proliferation and mobility of human gastric cancer cells and leads to inactivation of the AKT signaling pathway, suggesting that METTL3 is a potential target for the treatment of human gastric cancer. METTL3 knockdown decreased Bcl2 and increased Bax and active Caspase-3 in gastric cancer cells, which suggested the apoptotic pathway was activated. METTL3 led to inactivation of the AKT signaling pathway in human gastric cancer cells, including decreased phosphorylation levels of AKT and expression of down-stream effectors p70S6K and G1/S-specific cyclin-D1 (CCND1). | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Cell Process | Cell proliferation | |||
| Cell migration | ||||
| Cell invasion | ||||
| In-vitro Model | AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
Lung cancer [ICD-11: 2C25]
| In total 3 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [11] | |||
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, G1/S-specific cyclin-D1 (CCND1), CDK4, EGR2, YBX1, and TLX, which were associated with lung cancers. | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Target Regulator | Insulin-like growth factor 2 mRNA-binding protein 1 (IGF2BP1) | READER | ||
| Cell Process | Cell apoptosis | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Experiment 2 Reporting the m6A-centered Disease Response | [11] | |||
| Response Summary | GSEA revealed that KIAA1429, METTL3, and IGF2BP1 were significantly related to multiple biological behaviors, including proliferation, apoptosis, metastasis, energy metabolism, drug resistance, and recurrence, and that KIAA1429 and IGF2BP1 had potential target genes, including E2F3, WTAP, G1/S-specific cyclin-D1 (CCND1), CDK4, EGR2, YBX1, and TLX, which were associated with lung cancers. | |||
| Responsed Disease | Lung cancer [ICD-11: 2C25] | |||
| Target Regulator | Protein virilizer homolog (VIRMA) | WRITER | ||
| Cell Process | Cell apoptosis | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| HBE (Human bronchial epithelial cell line) | ||||
| LTEP-a2 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6929 | |
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | |
| Experiment 3 Reporting the m6A-centered Disease Response | [16] | |||
| Response Summary | YTHDF1 deficiency inhibits Non-small cell lung cancer cell proliferation and xenograft tumor formation through regulating the translational efficiency of CDK2, CDK4, p27, and G1/S-specific cyclin-D1 (CCND1), and that YTHDF1 depletion restrains de novo lung adenocarcinomas (ADC) progression. Mechanistic studies identified the Keap1-Nrf2-AKR1C1 axis as the downstream mediator of YTHDF1. YTHDF1 high expression correlates with better clinical outcome, with its depletion rendering cancerous cells resistant to cisplatin (DDP) treatment. | |||
| Responsed Disease | Non-small-cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Target Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Chemical carcinogenesis - reactive oxygen species | hsa05208 | ||
| Cell cycle | hsa04110 | |||
| Cell Process | Biological regulation | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| A549-DDP (Human lung adenocarcinoma is resistant to cisplatin) | ||||
| GLC-82 | Endocervical adenocarcinoma | Homo sapiens | CVCL_3371 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| NCI-H1650 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1483 | |
| NCI-H838 | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | |
| SPC-A1 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | |
| In-vivo Model | Mice were treated via nasal inhalation of adenovirus carrying Cre recombinase (5 × 106 p.f.u for Ad-Cre, Biowit Inc., Shenzhen, Guangdong), and were then killed at indicated times for gross inspection and histopathological examination. | |||
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [4] | |||
| Response Summary | METTL3 knockdown downregulated the phosphorylation levels of AKT and the expression of the downstream effector G1/S-specific cyclin-D1 (CCND1) in ovarian cancer. | |||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | ||
| Cell Process | Cell cycle | |||
| Cell apoptosis | ||||
| In-vitro Model | OVCAR-3 | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0465 |
| SK-OV-3 | Ovarian serous cystadenocarcinoma | Homo sapiens | CVCL_0532 | |
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [5] | |||
| Response Summary | METTL3 modulates miR-193b mature process in an m6A-dependent manner. Reintroduction of miR-193b profoundly inhibits tumorigenesis of cervical cancer cells both in vivo and in vitro through G1/S-specific cyclin-D1 (CCND1) targeting. | |||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Down regulation | |||
| In-vitro Model | SiHa | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| In-vivo Model | Mice were divided into two groups (n = 4/group) randomly. 3×106 cells suspended in 200 uL PBS were administered via subcutaneous injection over the right flank region of nude mice. After the development of palpable tumors (average volume, 50 mm3), intratumoral injection of synthetic miR-193b, or negative control complexed with siPORT Amine transfection reagent (Ambion, USA) was given 6 times at a 4-day interval. | |||
Obesity [ICD-11: 5B81]
| In total 4 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | Metformin could inhibit adipogenesis and combat obesity, metformin could inhibit protein expression of FTO, leading to increased m6A methylation levels of G1/S-specific cyclin-D1 (CCND1) and Cdk2(two crucial regulators in cell cycle). Ccnd1 and Cdk2 with increased m6A levels were recognised by YTHDF2, causing an YTHDF2-dependent decay and decreased protein expressions. | |||
| Responsed Disease | Obesity [ICD-11: 5B81] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Cell cycle | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [6] | |||
| Response Summary | Obesity is becoming a global problem. ZFP217 knockdown-induced adipogenesis inhibition was caused by G1/S-specific cyclin-D1 (CCND1), which was mediated by METTL3 and YTHDF2 in an m6A-dependent manner. | |||
| Responsed Disease | Obesity [ICD-11: 5B81] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Mitotic clonal | |||
| Prolonged G1/S transition | ||||
| In-vitro Model | 3T3-L1 | Normal | Mus musculus | CVCL_0123 |
| Experiment 3 Reporting the m6A-centered Disease Response | [6] | |||
| Response Summary | Obesity is becoming a global problem. ZFP217 knockdown-induced adipogenesis inhibition was caused by G1/S-specific cyclin-D1 (CCND1), which was mediated by METTL3 and YTHDF2 in an m6A-dependent manner. | |||
| Responsed Disease | Obesity [ICD-11: 5B81] | |||
| Target Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | ||
| Target Regulation | Down regulation | |||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Mitotic clonal | |||
| Prolonged G1/S transition | ||||
| In-vitro Model | 3T3-L1 | Normal | Mus musculus | CVCL_0123 |
| Experiment 4 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | Metformin could inhibit adipogenesis and combat obesity, metformin could inhibit protein expression of FTO, leading to increased m6A methylation levels of G1/S-specific cyclin-D1 (CCND1) and Cdk2(two crucial regulators in cell cycle). Ccnd1 and Cdk2 with increased m6A levels were recognised by YTHDF2, causing an YTHDF2-dependent decay and decreased protein expressions. | |||
| Responsed Disease | Obesity [ICD-11: 5B81] | |||
| Target Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | ||
| Pathway Response | Cell cycle | hsa04110 | ||
| Cell Process | Cell cycle | |||
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [16] | |||
| Response Summary | YTHDF1 deficiency inhibits Non-small cell lung cancer cell proliferation and xenograft tumor formation through regulating the translational efficiency of CDK2, CDK4, p27, and G1/S-specific cyclin-D1 (CCND1), and that YTHDF1 depletion restrains de novo lung adenocarcinomas (ADC) progression. Mechanistic studies identified the Keap1-Nrf2-AKR1C1 axis as the downstream mediator of YTHDF1. YTHDF1 high expression correlates with better clinical outcome, with its depletion rendering cancerous cells resistant to cisplatin (DDP) treatment. | |||
| Target Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Pathway Response | Chemical carcinogenesis - reactive oxygen species | hsa05208 | ||
| Cell cycle | hsa04110 | |||
| Cell Process | Biological regulation | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| A549-DDP (Human lung adenocarcinoma is resistant to cisplatin) | ||||
| GLC-82 | Endocervical adenocarcinoma | Homo sapiens | CVCL_3371 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| NCI-H1650 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1483 | |
| NCI-H838 | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | |
| SPC-A1 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | |
| In-vivo Model | Mice were treated via nasal inhalation of adenovirus carrying Cre recombinase (5 × 106 p.f.u for Ad-Cre, Biowit Inc., Shenzhen, Guangdong), and were then killed at indicated times for gross inspection and histopathological examination. | |||
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
Histone modification
m6A Regulator: Methyltransferase-like 3 (METTL3)
| In total 3 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT03509 | ||
| Epigenetic Regulator | Histone acetyltransferase p300 (P300) | |
| Regulated Target | Histone H3 lysine 27 acetylation (H3K27ac) | |
| Crosstalk relationship | Histone modification → m6A | |
| Disease | Cervical cancer | |
| Crosstalk ID: M6ACROT03522 | ||
| Epigenetic Regulator | WD repeat-containing protein 5 (WDR5) | |
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | |
| Crosstalk relationship | Histone modification → m6A | |
| Disease | Cervical cancer | |
| Crosstalk ID: M6ACROT03639 | ||
| Epigenetic Regulator | Histone-lysine N-methyltransferase EZH2 (EZH2) | |
| Regulated Target | Histone H3 lysine 27 trimethylation (H3K27me3) | |
| Crosstalk relationship | Histone modification → m6A | |
| Disease | Gastric cancer | |
Non-coding RNA
m6A Regulator: Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05098 | ||
| Epigenetic Regulator | HNF1A antisense RNA 1 (HNF1A-AS1) | |
| Regulated Target | Insulin like growth factor 2 mRNA binding protein 2 (IGF2BP2) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Colorectal cancer | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00206)
| In total 31 m6A sequence/site(s) in this target gene | |||
| mod ID: M5CSITE005290 | Click to Show/Hide the Full List | ||
| mod site | chr11:69641514-69641515:+ | [21] | |
| Sequence | CCACCTGGATGCTGGAGGTGCGGGGCTTCGGGCGGCTCTCT | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000535993.1; ENST00000227507.2; ENST00000539241.1 | ||
| External Link | RMBase: m5C_site_8369 | ||
| mod ID: M5CSITE005291 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642439-69642440:+ | ||
| Sequence | CGCCCAGGGGGAAGGGGGGGCCCCGGAGTTTGAATTCCTGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000539241.1; ENST00000535993.1; ENST00000227507.2 | ||
| External Link | RMBase: m5C_site_8370 | ||
| mod ID: M5CSITE005292 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642440-69642441:+ | ||
| Sequence | GCCCAGGGGGAAGGGGGGGCCCCGGAGTTTGAATTCCTGGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000539241.1; ENST00000227507.2; ENST00000535993.1 | ||
| External Link | RMBase: m5C_site_8371 | ||
| mod ID: M5CSITE005293 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642441-69642442:+ | ||
| Sequence | CCCAGGGGGAAGGGGGGGCCCCGGAGTTTGAATTCCTGGGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000539241.1; ENST00000535993.1 | ||
| External Link | RMBase: m5C_site_8372 | ||
| mod ID: M5CSITE005294 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642442-69642443:+ | ||
| Sequence | CCAGGGGGAAGGGGGGGCCCCGGAGTTTGAATTCCTGGGGC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000539241.1; ENST00000535993.1 | ||
| External Link | RMBase: m5C_site_8373 | ||
| mod ID: M5CSITE005295 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642462-69642463:+ | ||
| Sequence | CGGAGTTTGAATTCCTGGGGCTCCCCCCGGAGCCTGTAACG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000535993.1; ENST00000539241.1; ENST00000227507.2 | ||
| External Link | RMBase: m5C_site_8374 | ||
| mod ID: M5CSITE005296 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642464-69642465:+ | ||
| Sequence | GAGTTTGAATTCCTGGGGCTCCCCCCGGAGCCTGTAACGAA | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000535993.1; ENST00000539241.1; ENST00000536559.1; ENST00000227507.2 | ||
| External Link | RMBase: m5C_site_8375 | ||
| mod ID: M5CSITE005297 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642465-69642466:+ | ||
| Sequence | AGTTTGAATTCCTGGGGCTCCCCCCGGAGCCTGTAACGAAC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000535993.1; ENST00000227507.2; ENST00000539241.1; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8376 | ||
| mod ID: M5CSITE005298 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642466-69642467:+ | ||
| Sequence | GTTTGAATTCCTGGGGCTCCCCCCGGAGCCTGTAACGAACT | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000535993.1; ENST00000227507.2; ENST00000539241.1; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8377 | ||
| mod ID: M5CSITE005299 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642467-69642468:+ | ||
| Sequence | TTTGAATTCCTGGGGCTCCCCCCGGAGCCTGTAACGAACTC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000539241.1; ENST00000227507.2; ENST00000535993.1 | ||
| External Link | RMBase: m5C_site_8378 | ||
| mod ID: M5CSITE005300 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642468-69642469:+ | ||
| Sequence | TTGAATTCCTGGGGCTCCCCCCGGAGCCTGTAACGAACTCC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000535993.1; ENST00000539241.1 | ||
| External Link | RMBase: m5C_site_8379 | ||
| mod ID: M5CSITE005301 | Click to Show/Hide the Full List | ||
| mod site | chr11:69642469-69642470:+ | ||
| Sequence | TGAATTCCTGGGGCTCCCCCCGGAGCCTGTAACGAACTCCC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000535993.1; ENST00000539241.1; ENST00000227507.2; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8380 | ||
| mod ID: M5CSITE005302 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645552-69645553:+ | ||
| Sequence | GAGTCCACTCCAGGGTGGGTCCCGAGGGAGGGGCAGGAGAC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000545484.1; ENST00000227507.2; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8381 | ||
| mod ID: M5CSITE005303 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645553-69645554:+ | ||
| Sequence | AGTCCACTCCAGGGTGGGTCCCGAGGGAGGGGCAGGAGACC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8382 | ||
| mod ID: M5CSITE005304 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645554-69645555:+ | ||
| Sequence | GTCCACTCCAGGGTGGGTCCCGAGGGAGGGGCAGGAGACCA | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8383 | ||
| mod ID: M5CSITE005305 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645580-69645581:+ | ||
| Sequence | AGGGGCAGGAGACCAGGGGACCCACCCCTGCAAAGTGCTCC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000536559.1; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8384 | ||
| mod ID: M5CSITE005306 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645581-69645582:+ | ||
| Sequence | GGGGCAGGAGACCAGGGGACCCACCCCTGCAAAGTGCTCCG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000545484.1; ENST00000227507.2; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8385 | ||
| mod ID: M5CSITE005307 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645582-69645583:+ | ||
| Sequence | GGGCAGGAGACCAGGGGACCCACCCCTGCAAAGTGCTCCGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8386 | ||
| mod ID: M5CSITE005308 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645584-69645585:+ | ||
| Sequence | GCAGGAGACCAGGGGACCCACCCCTGCAAAGTGCTCCGGGT | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000545484.1; ENST00000227507.2 | ||
| External Link | RMBase: m5C_site_8387 | ||
| mod ID: M5CSITE005309 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645585-69645586:+ | ||
| Sequence | CAGGAGACCAGGGGACCCACCCCTGCAAAGTGCTCCGGGTC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000536559.1; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8388 | ||
| mod ID: M5CSITE005310 | Click to Show/Hide the Full List | ||
| mod site | chr11:69645586-69645587:+ | ||
| Sequence | AGGAGACCAGGGGACCCACCCCTGCAAAGTGCTCCGGGTCC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000545484.1; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8389 | ||
| mod ID: M5CSITE005311 | Click to Show/Hide the Full List | ||
| mod site | chr11:69646452-69646453:+ | ||
| Sequence | GGGATGAAGTCTCGGATGGGCCGCCACACCCCTGGCGGCCC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000545484.1; ENST00000227507.2; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8390 | ||
| mod ID: M5CSITE005312 | Click to Show/Hide the Full List | ||
| mod site | chr11:69646453-69646454:+ | ||
| Sequence | GGATGAAGTCTCGGATGGGCCGCCACACCCCTGGCGGCCCG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000545484.1; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8391 | ||
| mod ID: M5CSITE005313 | Click to Show/Hide the Full List | ||
| mod site | chr11:69646455-69646456:+ | ||
| Sequence | ATGAAGTCTCGGATGGGCCGCCACACCCCTGGCGGCCCGTG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000536559.1; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8392 | ||
| mod ID: M5CSITE005314 | Click to Show/Hide the Full List | ||
| mod site | chr11:69646456-69646457:+ | ||
| Sequence | TGAAGTCTCGGATGGGCCGCCACACCCCTGGCGGCCCGTGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000545484.1; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8393 | ||
| mod ID: M5CSITE005315 | Click to Show/Hide the Full List | ||
| mod site | chr11:69646467-69646468:+ | ||
| Sequence | ATGGGCCGCCACACCCCTGGCGGCCCGTGGGGGCCCCTCTC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000536559.1; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8394 | ||
| mod ID: M5CSITE005316 | Click to Show/Hide the Full List | ||
| mod site | chr11:69646470-69646471:+ | ||
| Sequence | GGCCGCCACACCCCTGGCGGCCCGTGGGGGCCCCTCTCCCT | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000545484.1; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8395 | ||
| mod ID: M5CSITE005317 | Click to Show/Hide the Full List | ||
| mod site | chr11:69646471-69646472:+ | ||
| Sequence | GCCGCCACACCCCTGGCGGCCCGTGGGGGCCCCTCTCCCTT | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000536559.1; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8396 | ||
| mod ID: M5CSITE005318 | Click to Show/Hide the Full List | ||
| mod site | chr11:69646472-69646473:+ | ||
| Sequence | CCGCCACACCCCTGGCGGCCCGTGGGGGCCCCTCTCCCTTT | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000545484.1; ENST00000227507.2; ENST00000536559.1 | ||
| External Link | RMBase: m5C_site_8397 | ||
| mod ID: M5CSITE005319 | Click to Show/Hide the Full List | ||
| mod site | chr11:69648117-69648118:+ | [21] | |
| Sequence | TACCGCCTCACACGCTTCCTCTCCAGAGTGATCAAGTGTGA | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000542367.1; ENST00000227507.2; ENST00000536559.1; ENST00000545484.1 | ||
| External Link | RMBase: m5C_site_8398 | ||
| mod ID: M5CSITE005320 | Click to Show/Hide the Full List | ||
| mod site | chr11:69651359-69651360:+ | [21] | |
| Sequence | GTGCTCCCCTGACAGTCCCTCCTCTCCGGAGCATTTTGATA | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000542367.1; ENST00000227507.2 | ||
| External Link | RMBase: m5C_site_8399 | ||
N6-methyladenosine (m6A)
| In total 67 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE007770 | Click to Show/Hide the Full List | ||
| mod site | chr11:69641111-69641112:+ | [22] | |
| Sequence | ACAACAGTAACGTCACACGGACTACAGGGGAGTTTTGTTGA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; H1B; fibroblasts; A549; HEK293A-TOA; MSC; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152838 | ||
| mod ID: M6ASITE007771 | Click to Show/Hide the Full List | ||
| mod site | chr11:69641262-69641263:+ | [22] | |
| Sequence | GAAGCGAGAGCCGAGCGCGGACCCAGCCAGGACCCACAGCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; A549; H1A; H1B; fibroblasts; H1299; CD4T; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000535993.1; ENST00000539241.1 | ||
| External Link | RMBase: m6A_site_152839 | ||
| mod ID: M6ASITE007772 | Click to Show/Hide the Full List | ||
| mod site | chr11:69641273-69641274:+ | [22] | |
| Sequence | CGAGCGCGGACCCAGCCAGGACCCACAGCCCTCCCCAGCTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; A549; H1A; H1B; fibroblasts; MT4; H1299; CD4T; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000539241.1; ENST00000227507.2; ENST00000536559.1; ENST00000535993.1 | ||
| External Link | RMBase: m6A_site_152840 | ||
| mod ID: M6ASITE007773 | Click to Show/Hide the Full List | ||
| mod site | chr11:69641318-69641319:+ | [22] | |
| Sequence | GGAAGAGCCCCAGCCATGGAACACCAGCTCCTGTGCTGCGA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; A549; H1A; H1B; H1299; Huh7; CD4T; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000539241.1; ENST00000536559.1; ENST00000535993.1; ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152841 | ||
| mod ID: M6ASITE007774 | Click to Show/Hide the Full List | ||
| mod site | chr11:69641346-69641347:+ | [22] | |
| Sequence | TCCTGTGCTGCGAAGTGGAAACCATCCGCCGCGCGTACCCC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; A549; H1B; H1A; H1299; Huh7; CD4T; GSC-11; HEK293T; HEK293A-TOA; iSLK; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000539241.1; ENST00000535993.1; ENST00000227507.2; ENST00000536559.1 | ||
| External Link | RMBase: m6A_site_152842 | ||
| mod ID: M6ASITE007775 | Click to Show/Hide the Full List | ||
| mod site | chr11:69641421-69641422:+ | [22] | |
| Sequence | CCATGCTGAAGGCGGAGGAGACCTGCGCGCCCTCGGTGTCC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; A549; H1299; Huh7; CD4T; GSC-11; HEK293T; iSLK; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000535993.1; ENST00000539241.1; ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152843 | ||
| mod ID: M6ASITE007776 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643041-69643042:+ | [22] | |
| Sequence | CCTCCGTAGGTCTGCGAGGAACAGAAGTGCGAGGAGGAGGT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; A549; Huh7; CD4T; GSC-11; HEK293T; MSC; TIME; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000539241.1; ENST00000535993.1; ENST00000536559.1 | ||
| External Link | RMBase: m6A_site_152844 | ||
| mod ID: M6ASITE007777 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643079-69643080:+ | [22] | |
| Sequence | GGTCTTCCCGCTGGCCATGAACTACCTGGACCGCTTCCTGT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; A549; Huh7; GSC-11; HEK293T; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000535993.1; ENST00000539241.1; ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152845 | ||
| mod ID: M6ASITE007778 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643088-69643089:+ | [22] | |
| Sequence | GCTGGCCATGAACTACCTGGACCGCTTCCTGTCGCTGGAGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; A549; Huh7; GSC-11; HEK293T; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000539241.1; ENST00000227507.2; ENST00000536559.1; ENST00000535993.1 | ||
| External Link | RMBase: m6A_site_152846 | ||
| mod ID: M6ASITE007779 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643177-69643178:+ | [22] | |
| Sequence | TGGCCTCTAAGATGAAGGAGACCATCCCCCTGACGGCCGAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; A549; Huh7; GSC-11; HEK293T; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000535993.1; ENST00000539241.1; ENST00000227507.2; ENST00000536559.1 | ||
| External Link | RMBase: m6A_site_152847 | ||
| mod ID: M6ASITE007780 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643211-69643212:+ | [23] | |
| Sequence | GGCCGAGAAGCTGTGCATCTACACCGACAACTCCATCCGGC | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000535993.1; ENST00000539241.1; ENST00000536559.1 | ||
| External Link | RMBase: m6A_site_152848 | ||
| mod ID: M6ASITE007781 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643217-69643218:+ | [23] | |
| Sequence | GAAGCTGTGCATCTACACCGACAACTCCATCCGGCCCGAGG | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000535993.1; ENST00000539241.1; ENST00000536559.1; ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152849 | ||
| mod ID: M6ASITE007782 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643257-69643258:+ | [22] | |
| Sequence | GAGCTGCTGGTAACCACTGGACCCCGCCGCCCCCCGCCCCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; A549; Huh7; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000539241.1; ENST00000535993.1; ENST00000536559.1 | ||
| External Link | RMBase: m6A_site_152850 | ||
| mod ID: M6ASITE007783 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643295-69643296:+ | [22] | |
| Sequence | CCCCGCGAGCCGCACGCAGGACCACGGGGCCGGGGAAGGTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; A549; Huh7; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000539241.1; ENST00000535993.1; ENST00000536559.1; ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152851 | ||
| mod ID: M6ASITE007784 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643853-69643854:+ | [22] | |
| Sequence | AATGGAGCTGCTCCTGGTGAACAAGCTCAAGTGGAACCTGG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; hESC-HEK293T; H1A; hESCs; A549; Huh7; GSC-11; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MAZTER-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000545484.1; ENST00000539241.1; ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152852 | ||
| mod ID: M6ASITE007785 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643868-69643869:+ | [22] | |
| Sequence | GGTGAACAAGCTCAAGTGGAACCTGGCCGCAATGACCCCGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2; H1A; A549; Huh7; GSC-11; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000536559.1; ENST00000539241.1; ENST00000545484.1 | ||
| External Link | RMBase: m6A_site_152853 | ||
| mod ID: M6ASITE007786 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643902-69643903:+ | [22] | |
| Sequence | ACCCCGCACGATTTCATTGAACACTTCCTCTCCAAAATGCC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; hESC-HEK293T; H1A; H1B; A549; Huh7; GSC-11; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MAZTER-seq; m6A-CLIP/IP; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000545484.1; ENST00000536559.1; ENST00000539241.1 | ||
| External Link | RMBase: m6A_site_152854 | ||
| mod ID: M6ASITE007787 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643937-69643938:+ | [22] | |
| Sequence | AATGCCAGAGGCGGAGGAGAACAAACAGATCATCCGCAAAC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; H1A; H1B; A549; Huh7; GSC-11; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000539241.1; ENST00000227507.2; ENST00000536559.1; ENST00000545484.1 | ||
| External Link | RMBase: m6A_site_152855 | ||
| mod ID: M6ASITE007788 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643941-69643942:+ | [23] | |
| Sequence | CCAGAGGCGGAGGAGAACAAACAGATCATCCGCAAACACGC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | hESC-HEK293T; A549 | ||
| Seq Type List | MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000536559.1; ENST00000545484.1; ENST00000539241.1; ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152856 | ||
| mod ID: M6ASITE007789 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643956-69643957:+ | [22] | |
| Sequence | AACAAACAGATCATCCGCAAACACGCGCAGACCTTCGTTGC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2; H1A; H1B; A549; Huh7; GSC-11; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000536559.1; ENST00000545484.1; ENST00000539241.1 | ||
| External Link | RMBase: m6A_site_152857 | ||
| mod ID: M6ASITE007790 | Click to Show/Hide the Full List | ||
| mod site | chr11:69643966-69643967:+ | [22] | |
| Sequence | TCATCCGCAAACACGCGCAGACCTTCGTTGCCCTCTGTGCC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; H1A; H1B; A549; GSC-11; HEK293A-TOA; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000545484.1; ENST00000539241.1 | ||
| External Link | RMBase: m6A_site_152858 | ||
| mod ID: M6ASITE007791 | Click to Show/Hide the Full List | ||
| mod site | chr11:69644100-69644101:+ | [22] | |
| Sequence | GTCTGGGAAGATGTCCCCAGACCCCCTCCTGCGCTGGAGAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; GSC-11; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000539241.1; ENST00000545484.1; ENST00000227507.2; ENST00000536559.1 | ||
| External Link | RMBase: m6A_site_152859 | ||
| mod ID: M6ASITE007792 | Click to Show/Hide the Full List | ||
| mod site | chr11:69648065-69648066:+ | [22] | |
| Sequence | GGCCGCAGTGCAAGGCCTGAACCTGAGGAGCCCCAACAACT | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; GSC-11; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000545484.1; ENST00000542367.1 | ||
| External Link | RMBase: m6A_site_152860 | ||
| mod ID: M6ASITE007793 | Click to Show/Hide the Full List | ||
| mod site | chr11:69648106-69648107:+ | [23] | |
| Sequence | TCCTGTCCTACTACCGCCTCACACGCTTCCTCTCCAGAGTG | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000536559.1; ENST00000227507.2; ENST00000542367.1; ENST00000545484.1 | ||
| External Link | RMBase: m6A_site_152861 | ||
| mod ID: M6ASITE007794 | Click to Show/Hide the Full List | ||
| mod site | chr11:69648192-69648193:+ | [22] | |
| Sequence | GCCGGGGCTTACAGGGGGAGACACCTAGTGCCACGGAAATG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; H1299; Huh7; GSC-11; HEK293T; MSC; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000542367.1; ENST00000536559.1 | ||
| External Link | RMBase: m6A_site_152862 | ||
| mod ID: M6ASITE007795 | Click to Show/Hide the Full List | ||
| mod site | chr11:69651187-69651188:+ | [22] | |
| Sequence | CCTGCGCCAGGCCCAGCAGAACATGGACCCCAAGGCCGCCG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; A549; hESC-HEK293T; H1A; H1B; hNPCs; hESCs; fibroblasts; H1299; Huh7; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000542367.1 | ||
| External Link | RMBase: m6A_site_152863 | ||
| mod ID: M6ASITE007796 | Click to Show/Hide the Full List | ||
| mod site | chr11:69651193-69651194:+ | [22] | |
| Sequence | CCAGGCCCAGCAGAACATGGACCCCAAGGCCGCCGAGGAGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; H1299; Huh7; CD4T; peripheral-blood; GSC-11; HEK293T; HEK293A-TOA; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000542367.1 | ||
| External Link | RMBase: m6A_site_152864 | ||
| mod ID: M6ASITE007797 | Click to Show/Hide the Full List | ||
| mod site | chr11:69651238-69651239:+ | [22] | |
| Sequence | AGAGGAGGAGGAGGAGGTGGACCTGGCTTGCACACCCACCG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; A549; H1A; H1B; hNPCs; hESCs; HEK293T; fibroblasts; H1299; Huh7; CD4T; GSC-11; HEK293A-TOA; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000542367.1; rmsk_3579100 | ||
| External Link | RMBase: m6A_site_152865 | ||
| mod ID: M6ASITE007798 | Click to Show/Hide the Full List | ||
| mod site | chr11:69651274-69651275:+ | [22] | |
| Sequence | CACCGACGTGCGGGACGTGGACATCTGAGGGCGCCAGGCAG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; H1299; Huh7; CD4T; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2; ENST00000542367.1 | ||
| External Link | RMBase: m6A_site_152866 | ||
| mod ID: M6ASITE007799 | Click to Show/Hide the Full List | ||
| mod site | chr11:69651451-69651452:+ | [24] | |
| Sequence | CTTTCCCCCTTCCATCTCTGACTTAAGCAAAAGAAAAAGAT | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152867 | ||
| mod ID: M6ASITE007800 | Click to Show/Hide the Full List | ||
| mod site | chr11:69651481-69651482:+ | [22] | |
| Sequence | AAGAAAAAGATTACCCAAAAACTGTCTTTAAAAGAGAGAGA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T; A549; U2OS; hNPCs; hESCs; fibroblasts; H1299; Huh7; iSLK; MSC; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152868 | ||
| mod ID: M6ASITE007801 | Click to Show/Hide the Full List | ||
| mod site | chr11:69651711-69651712:+ | [23] | |
| Sequence | GTTTAAAAAAAAGCATAAAAACATTTTAAAAACATAGAAAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | hESC-HEK293T; Huh7 | ||
| Seq Type List | MAZTER-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152869 | ||
| mod ID: M6ASITE007802 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652242-69652243:+ | [25] | |
| Sequence | CTCACGTCCAGGTTCAACCCACAGCTACTTGGTTTGTGTTC | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152870 | ||
| mod ID: M6ASITE007803 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652279-69652280:+ | [22] | |
| Sequence | GTTCTTCTTCATATTCTAAAACCATTCCATTTCCAAGCACT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152871 | ||
| mod ID: M6ASITE007804 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652391-69652392:+ | [24] | |
| Sequence | TGGTTTGGGAATATCCATGTACTTGTTTGCAAGCAGGACTT | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152872 | ||
| mod ID: M6ASITE007805 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652408-69652409:+ | [26] | |
| Sequence | TGTACTTGTTTGCAAGCAGGACTTTGAGGCAAGTGTGGGCC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152873 | ||
| mod ID: M6ASITE007806 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652591-69652592:+ | [26] | |
| Sequence | ACTGGTGTTTGAAAGTAGGGACCTCAGAGGTTTACCTAGAG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152874 | ||
| mod ID: M6ASITE007807 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652769-69652770:+ | [22] | |
| Sequence | GCAATCTCCCCTTGATTTAAACACACAGATACACACACACA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | rmsk_3579101; ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152875 | ||
| mod ID: M6ASITE007808 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652805-69652806:+ | [22] | |
| Sequence | ACACACACACACACACACAAACCTTCTGCCTTTGATGTTAC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152876 | ||
| mod ID: M6ASITE007809 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652879-69652880:+ | [22] | |
| Sequence | CTTTTATAGGTGAGAAAAAAACAATCTGGAAGAAAAAAACC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152877 | ||
| mod ID: M6ASITE007810 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652897-69652898:+ | [26] | |
| Sequence | AAACAATCTGGAAGAAAAAAACCACACAAAGACATTGATTC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152878 | ||
| mod ID: M6ASITE007811 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652908-69652909:+ | [26] | |
| Sequence | AAGAAAAAAACCACACAAAGACATTGATTCAGCCTGTTTGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152879 | ||
| mod ID: M6ASITE007812 | Click to Show/Hide the Full List | ||
| mod site | chr11:69652953-69652954:+ | [26] | |
| Sequence | TCCCAGAGTCATCTGATTGGACAGGCATGGGTGCAAGGAAA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152880 | ||
| mod ID: M6ASITE007813 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653038-69653039:+ | [26] | |
| Sequence | CCTGCCCCTTCCTTTAAAAAACTTAGTGACAAAATAGACAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152881 | ||
| mod ID: M6ASITE007814 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653055-69653056:+ | [26] | |
| Sequence | AAAACTTAGTGACAAAATAGACAATTTGCACATCTTGGCTA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152882 | ||
| mod ID: M6ASITE007815 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653152-69653153:+ | [22] | |
| Sequence | AGGCTGACGTGTGAGGGAGGACAGGCGGGAGGAGGTGTGAG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2; rmsk_3579102 | ||
| External Link | RMBase: m6A_site_152883 | ||
| mod ID: M6ASITE007816 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653214-69653215:+ | [22] | |
| Sequence | GGGCGGTGCCCACACCGGGGACAGGCCGCAGCTCCATTTTC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152884 | ||
| mod ID: M6ASITE007817 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653345-69653346:+ | [24] | |
| Sequence | GAGGATCAGTTTTTTGTTTTACAATGTCATATACTGCCATG | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152887 | ||
| mod ID: M6ASITE007818 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653357-69653358:+ | [24] | |
| Sequence | TTTGTTTTACAATGTCATATACTGCCATGTACTAGTTTTAG | ||
| Motif Score | 2.53247619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152888 | ||
| mod ID: M6ASITE007819 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653367-69653368:+ | [24] | |
| Sequence | AATGTCATATACTGCCATGTACTAGTTTTAGTTTTCTCTTA | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152889 | ||
| mod ID: M6ASITE007820 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653390-69653391:+ | [22] | |
| Sequence | AGTTTTAGTTTTCTCTTAGAACATTGTATTACAGATGCCTT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152890 | ||
| mod ID: M6ASITE007821 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653559-69653560:+ | [26] | |
| Sequence | CTGCCTGCTTTGGCGGGCAGACACGCGGGCGCGATCCCACA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152891 | ||
| mod ID: M6ASITE007822 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653620-69653621:+ | [26] | |
| Sequence | CCGAGGCCGCGTGCGTGAGAACCGCGCCGGTGTCCCCAGAG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152892 | ||
| mod ID: M6ASITE007823 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653641-69653642:+ | [26] | |
| Sequence | CCGCGCCGGTGTCCCCAGAGACCAGGCTGTGTCCCTCTTCT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152893 | ||
| mod ID: M6ASITE007824 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653789-69653790:+ | [23] | |
| Sequence | TTATTATTATTATTATTATAACAAGTGTGTCTTACGTGCCA | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152894 | ||
| mod ID: M6ASITE007825 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653812-69653813:+ | [24] | |
| Sequence | AGTGTGTCTTACGTGCCACCACGGCGTTGTACCTGTAGGAC | ||
| Motif Score | 2.027047619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152895 | ||
| mod ID: M6ASITE007826 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653822-69653823:+ | [24] | |
| Sequence | ACGTGCCACCACGGCGTTGTACCTGTAGGACTCTCATTCGG | ||
| Motif Score | 2.8355 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152896 | ||
| mod ID: M6ASITE007827 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653935-69653936:+ | [23] | |
| Sequence | TTGTTATTGTTTTGTTAATTACACCATAATGCTAATTTAAA | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152897 | ||
| mod ID: M6ASITE007828 | Click to Show/Hide the Full List | ||
| mod site | chr11:69653959-69653960:+ | [22] | |
| Sequence | CATAATGCTAATTTAAAGAGACTCCAAATCTCAATGAAGCC | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152898 | ||
| mod ID: M6ASITE007829 | Click to Show/Hide the Full List | ||
| mod site | chr11:69654023-69654024:+ | [26] | |
| Sequence | GTCACCTAGCAAGCTGCCGAACCAAAAGAATTTGCACCCCG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152899 | ||
| mod ID: M6ASITE007830 | Click to Show/Hide the Full List | ||
| mod site | chr11:69654140-69654141:+ | [22] | |
| Sequence | CCCCGCCCCACCCCTCCAGAACACGGCTCACGCTTACCTCA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; A549; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152900 | ||
| mod ID: M6ASITE007831 | Click to Show/Hide the Full List | ||
| mod site | chr11:69654188-69654189:+ | [22] | |
| Sequence | TGGCTGCGGCGTCTGTCTGAACCACGCGGGGGCCTTGAGGG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; H1B; A549; Huh7; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152902 | ||
| mod ID: M6ASITE007832 | Click to Show/Hide the Full List | ||
| mod site | chr11:69654209-69654210:+ | [27] | |
| Sequence | CCACGCGGGGGCCTTGAGGGACGCTTTGTCTGTCGTGATGG | ||
| Motif Score | 3.616982143 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152903 | ||
| mod ID: M6ASITE007833 | Click to Show/Hide the Full List | ||
| mod site | chr11:69654426-69654427:+ | [22] | |
| Sequence | CGGCATGTTTCCAGCAGAAGACAAAAAGACAAACATGAAAG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152910 | ||
| mod ID: M6ASITE007834 | Click to Show/Hide the Full List | ||
| mod site | chr11:69654434-69654435:+ | [22] | |
| Sequence | TTCCAGCAGAAGACAAAAAGACAAACATGAAAGTCTAGAAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152911 | ||
| mod ID: M6ASITE007835 | Click to Show/Hide the Full List | ||
| mod site | chr11:69654459-69654460:+ | [22] | |
| Sequence | CATGAAAGTCTAGAAATAAAACTGGTAAAACCCCAGCGTGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152912 | ||
| mod ID: M6ASITE007836 | Click to Show/Hide the Full List | ||
| mod site | chr11:69654468-69654469:+ | [22] | |
| Sequence | CTAGAAATAAAACTGGTAAAACCCCAGCGTGGTGCCTGCCT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000227507.2 | ||
| External Link | RMBase: m6A_site_152913 | ||
References