m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00141)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
MALAT1
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Browse Drug
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | DKO-1 cell line | Homo sapiens |
|
Treatment: METTL3 knockdown DKO-1 cell
Control: DKO-1 cell
|
GSE182382 | |
| Regulation |
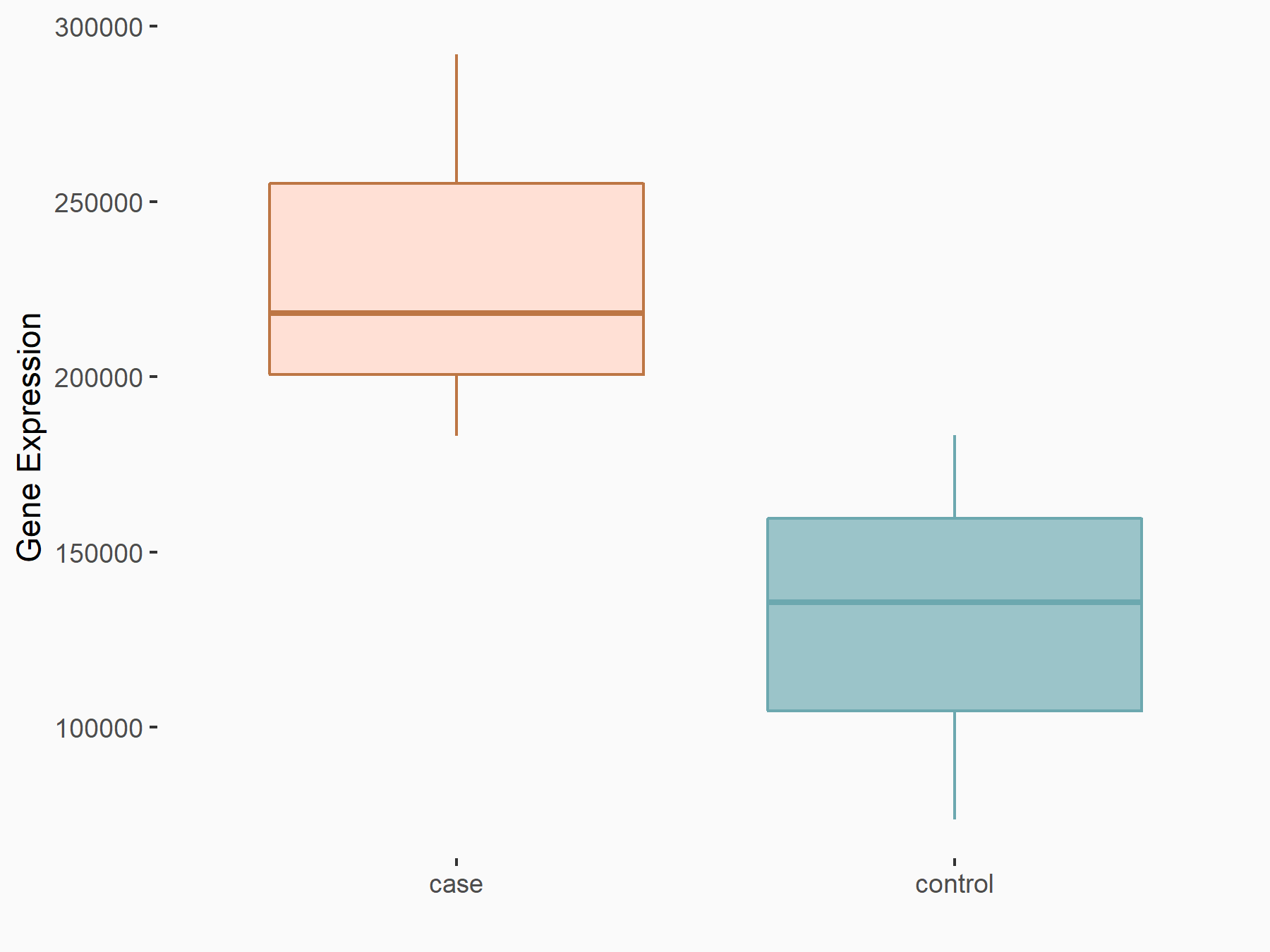  |
logFC: 8.20E-01 p-value: 5.93E-03 |
| More Results | Click to View More RNA-seq Results | |
| Representative RIP-seq result supporting the interaction between MALAT1 and the regulator | ||
| Cell Line | MDA-MB-231 | Homo sapiens |
| Regulation | logFC: 1.28E+00 | GSE60213 |
| In total 9 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | METTL3 promoted the malignant progression of IDH-wildtype gliomas and revealed important insight into the upstream regulatory mechanism of Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and NF-Kappa-B with a primary focus on m6A modification. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Glioma | ICD-11: 2A00.0 | ||
| Pathway Response | TNF signaling pathway | hsa04668 | ||
| Cell Process | Cell proliferation and metastasis | |||
| In-vitro Model | H4 | Astrocytoma | Homo sapiens | CVCL_1239 |
| LN-229 | Glioblastoma | Homo sapiens | CVCL_0393 | |
| U87 (A primary glioblastoma cell line) | ||||
| In-vivo Model | U87 cells (5 × 105) transfected with an empty vector, METTL3 shRNA, or METTL3 overexpression vector were inoculated into the right frontal node of nude mice. | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
| Experiment 3 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | This study highlighted METTL3 as a tumor promoter in Thymic tumors and c-MYC as a promising target to be exploited for the treatment of TET. High expression of c-MYC protein is enabled by lncRNA Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), which is methylated and delocalized by METTL3. Silencing of METTL3 combined with cisplatin or c-MYC inhibitor induces cell death in TET cells. Blocking of c-MYC by using JQ1 inhibitor cooperates with METTL3 depletion in the inhibition of proliferation and induction of cell death. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Thymic epithelial tumors | ICD-11: 2C27.Y | ||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Cellular senescence | hsa04218 | ||
| Cell Process | Cell viability and proliferation | |||
| In-vitro Model | T1889 | Thymic undifferentiated carcinoma | Homo sapiens | CVCL_D024 |
| Experiment 4 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | This study highlighted METTL3 as a tumor promoter in Thymic tumors and c-MYC as a promising target to be exploited for the treatment of TET. High expression of c-MYC protein is enabled by lncRNA Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), which is methylated and delocalized by METTL3. Silencing of METTL3 combined with cisplatin or c-MYC inhibitor induces cell death in TET cells. Blocking of c-MYC by using JQ1 inhibitor cooperates with METTL3 depletion in the inhibition of proliferation and induction of cell death. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Thymic epithelial tumors | ICD-11: 2C27.Y | ||
| Responsed Drug | JQ-1 | Phase 1 | ||
| Pathway Response | Cellular senescence | hsa04218 | ||
| Cell Process | Cell viability and proliferation | |||
| In-vitro Model | T1889 | Thymic undifferentiated carcinoma | Homo sapiens | CVCL_D024 |
| Experiment 5 Reporting the m6A Methylation Regulator of This Target Gene | [4] | |||
| Response Summary | METTL3 can regulate the expression of Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) through m6A, mediate the E2F1/AGR2 axis, and promote the adriamycin resistance of breast cancer. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Responsed Drug | Doxil | Approved | ||
| In-vitro Model | MCF7-DoxR (Adriamycin-resistant cell line MCF7-DoxR) | |||
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| In-vivo Model | Once the tumor volume increased to about 1 cm3, six groups of MCF7 bearing mice (n = 10 in each group) were injected with PBS (0.1 ml, caudal vein) and adriamycin (0.1 ml, 10 mg/kg), respectively. When the tumor reached 1.5 cm in any direction (defined as event-free survival analysis), 10 mice in each group were selected to measure the tumor size and weight on the 12th day after adriamycin injection. | |||
| Experiment 6 Reporting the m6A Methylation Regulator of This Target Gene | [5] | |||
| Response Summary | Silencing METTL3 down-regulate Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and HMGA2 by sponging miR-26b, and finally inhibit EMT, migration and invasion in breast cancer, providing a theoretical basis for clinical treatment of breast cancer. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Cell Process | Epithelial-mesenchymal transition | |||
| In-vitro Model | MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 |
| MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MDA-MB-468 | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | |
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | |
| Experiment 7 Reporting the m6A Methylation Regulator of This Target Gene | [5] | |||
| Response Summary | Silencing METTL3 down-regulate Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and HMGA2 by sponging miR-26b, and finally inhibit EMT, migration and invasion in BC, providing a theoretical basis for clinical treatment of BC. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| Cell Process | Epithelial-mesenchymal transition | |||
| In-vitro Model | MDA-MB-468 | Breast adenocarcinoma | Homo sapiens | CVCL_0419 |
| MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | |
| In-vivo Model | Eighteen BALB/C female nude mice aged 4-5 weeks and weighing 15-18 g were randomly assigned into three groups of six mice. The MCF-7 cell lines stably transfected with sh-NC + oe-NC, sh-METTL3 + oe-NC and sh-METTL3 + oe-HMGA2 were selected for subcutaneous establishment of the BC cell line MCF-7 as xenografts in the nude mice. For this purpose, MCF-7 cell lines in the logarithmic growth stage were prepared into a suspension with a concentration of about 1 × 107 cells/ml. The prepared cell suspension was injected into the left armpit of the mice, and the subsequent tumor growth was recorded. | |||
| Experiment 8 Reporting the m6A Methylation Regulator of This Target Gene | [6] | |||
| Response Summary | Renal fibrosis is a key factor in chronic kidney disease (CKD). Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)/miR-145/FAK pathway was involved in the effect of dihydroartemisinin (DHA) on TGF-beta1-induced renal fibrosis in vitro and in vivo. | |||
| Responsed Disease | Chronic kidney disease | ICD-11: GB61 | ||
| Responsed Drug | Artenimol | Approved | ||
| Cell Process | Epithelial-mesenchymal transition | |||
| In-vitro Model | HK-2 [Human kidney] | Normal | Homo sapiens | CVCL_0302 |
| HK2 | Normal | Acipenser baerii | CVCL_YE28 | |
| In-vivo Model | For the unilateral ureteral obstruction (UUO) model, male C57BL/6J mice at 8 weeks of age (20-22 g body weight) were first anaesthetized with pentobarbital sodium (50 mg/kg) via intraperitoneal injection. Then, the left ureter was ligated using 3-0 silk and a left lateral incision. | |||
| Experiment 9 Reporting the m6A Methylation Regulator of This Target Gene | [7] | |||
| Response Summary | m6A modification is co-regulated by METTL3 and FTO in cadmium-treated cells. Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), LncRNA-PVT1 and m6A modification could be key nodes for cadmium-induced oxidative damage, and highlight their importance as promising preventive and therapeutic targets in cadmium toxicity. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Kidney failure | ICD-11: GB6Z | ||
| In-vitro Model | NIT-1 | Insulin tumor | Mus musculus | CVCL_3561 |
Fat mass and obesity-associated protein (FTO) [ERASER]
| Representative RNA-seq result indicating the expression of this target gene regulated by FTO | ||
| Cell Line | NB4 cell line | Homo sapiens |
|
Treatment: shFTO NB4 cells
Control: shNS NB4 cells
|
GSE103494 | |
| Regulation |
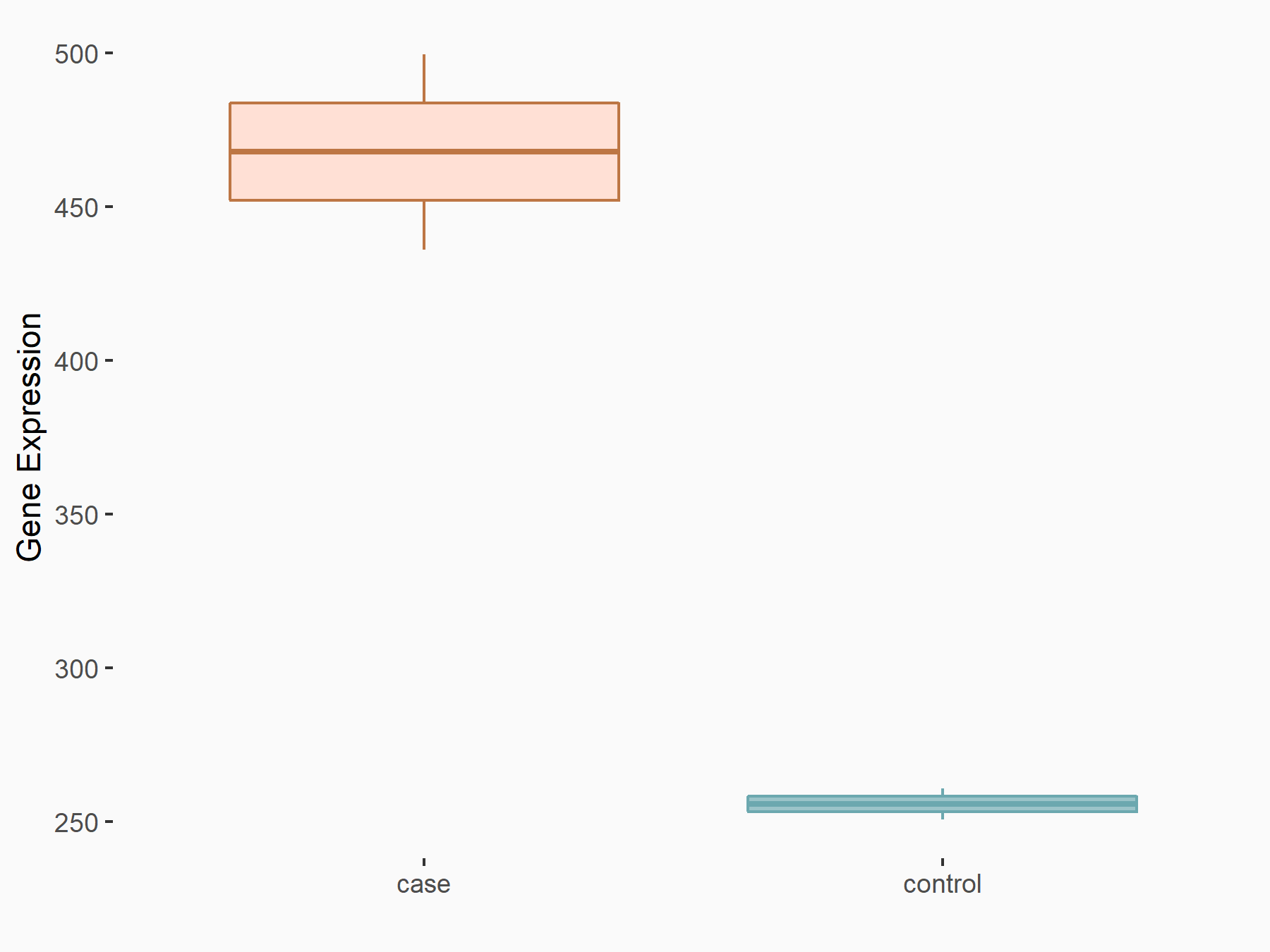  |
logFC: 8.67E-01 p-value: 5.83E-03 |
| More Results | Click to View More RNA-seq Results | |
| In total 3 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [8] | |||
| Response Summary | In bladder cancer, the changes in m6A methylation level mainly appeared at 5' untranslated region (5' UTR) of Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and NOTCH1 transcripts, and at 3' UTR of CSNK2A2 and ITGA6 transcripts, responding to the overexpression of FTO. SFPQ could influence the FTO-mediated m6A RNA demethylation, eventually affecting the gene expression. | |||
| Target Regulation | Down regulation | |||
| Responsed Disease | Bladder cancer | ICD-11: 2C94 | ||
| Pathway Response | Notch signaling pathway | hsa04330 | ||
| Cell Process | Cell proliferation | |||
| Cell invasion | ||||
| Cell apoptosis | ||||
| In-vitro Model | HT-1197 | Recurrent bladder carcinoma | Homo sapiens | CVCL_1291 |
| HT-1376 | Bladder carcinoma | Homo sapiens | CVCL_1292 | |
| In-vivo Model | BALB/cnu/nu mice (4-5 weeks old) were used for the xenograft experiment. The mice were randomly divided into 2 groups (n = 6 for each group) and injected with 5 × 106 HT-1197 cells in control group or FTO plasmid group, respectively. | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [9] | |||
| Response Summary | FTO facilitates the tumorigenesis of bladder cancer through regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)/miR-384/MAL2 axis in m6A RNA modification manner, which ensures the potential of FTO for serving as a diagnostic or prognostic biomarker in bladder cancer. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Bladder cancer | ICD-11: 2C94 | ||
| Cell Process | RNA stability | |||
| In-vitro Model | T24 | Bladder carcinoma | Homo sapiens | CVCL_0554 |
| SV-HUC-1 | Normal | Homo sapiens | CVCL_3798 | |
| SCaBER | Bladder squamous cell carcinoma | Homo sapiens | CVCL_3599 | |
| J82 | Bladder carcinoma | Homo sapiens | CVCL_0359 | |
| 253J | Bladder carcinoma | Homo sapiens | CVCL_7935 | |
| 5637 | Bladder carcinoma | Homo sapiens | CVCL_0126 | |
| In-vivo Model | Approximately 5 × 106 253J and 5637 cells infected with indicated vectors were injected subcutaneously into the flank of the mice. | |||
| Experiment 3 Reporting the m6A Methylation Regulator of This Target Gene | [7] | |||
| Response Summary | m6A modification is co-regulated by METTL3 and FTO in cadmium-treated cells. Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), LncRNA-PVT1 and m6A modification could be key nodes for cadmium-induced oxidative damage, and highlight their importance as promising preventive and therapeutic targets in cadmium toxicity. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Kidney failure | ICD-11: GB6Z | ||
| In-vitro Model | NIT-1 | Insulin tumor | Mus musculus | CVCL_3561 |
RNA binding protein X (RBMX) [READER]
| Representative RNA-seq result indicating the expression of this target gene regulated by RBMX | ||
| Cell Line | HEK293 cell line | Homo sapiens |
|
Treatment: RBMX overexpressed HEK293 cells
Control: Wild type HEK293 cells
|
GSE68990 | |
| Regulation |
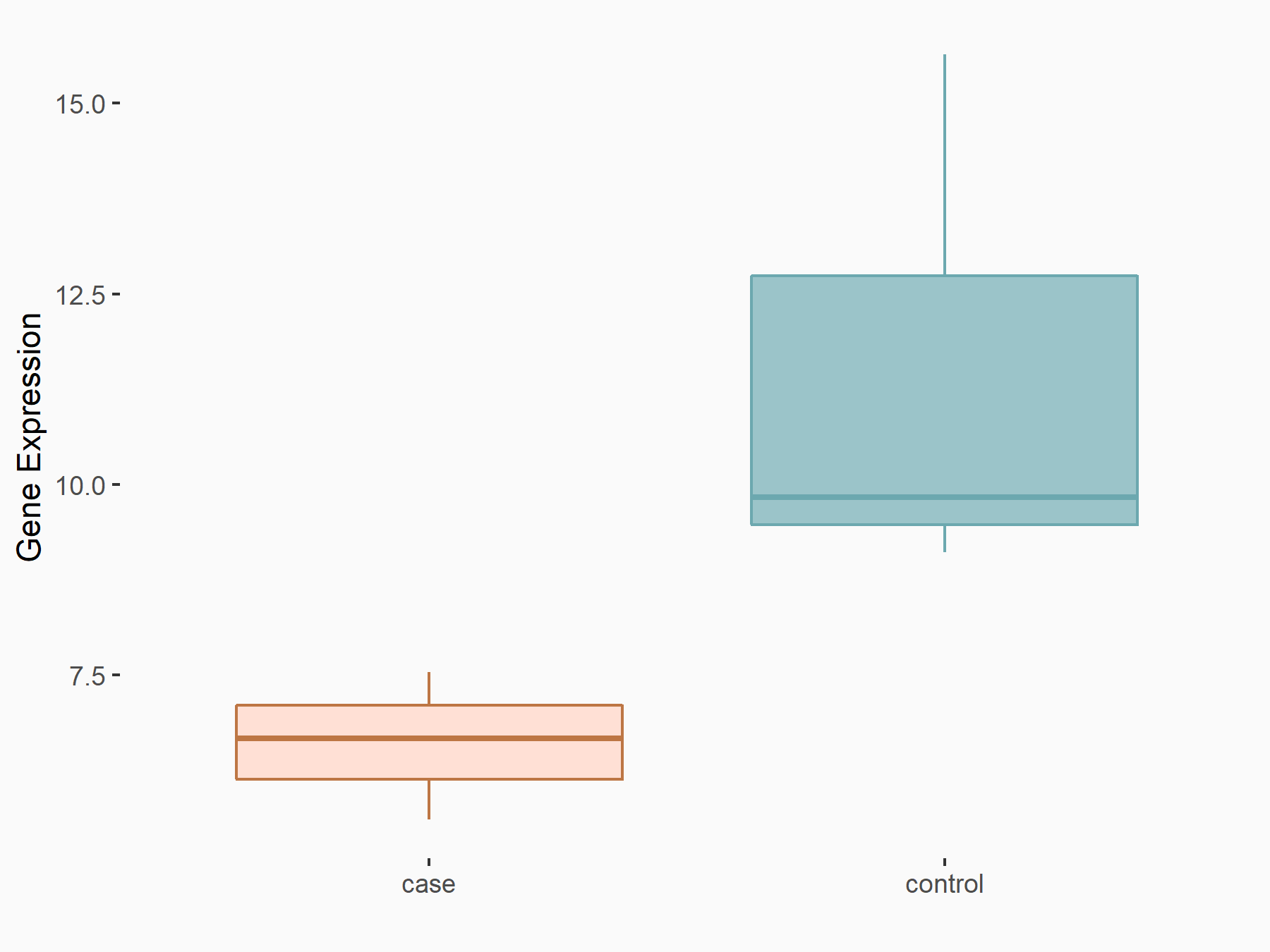  |
logFC: -6.91E-01 p-value: 3.81E-02 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [10] | |||
| Response Summary | HNRNPG can bind the m6A-modified hairpin of Metastasis associated lung adenocarcinoma transcript 1 (MALAT1). | |||
RNA demethylase ALKBH5 (ALKBH5) [ERASER]
| Representative RNA-seq result indicating the expression of this target gene regulated by ALKBH5 | ||
| Cell Line | NOMO-1 cell line | Homo sapiens |
|
Treatment: shALKBH5 NOMO-1 cells
Control: shNS NOMO-1 cells
|
GSE144968 | |
| Regulation |
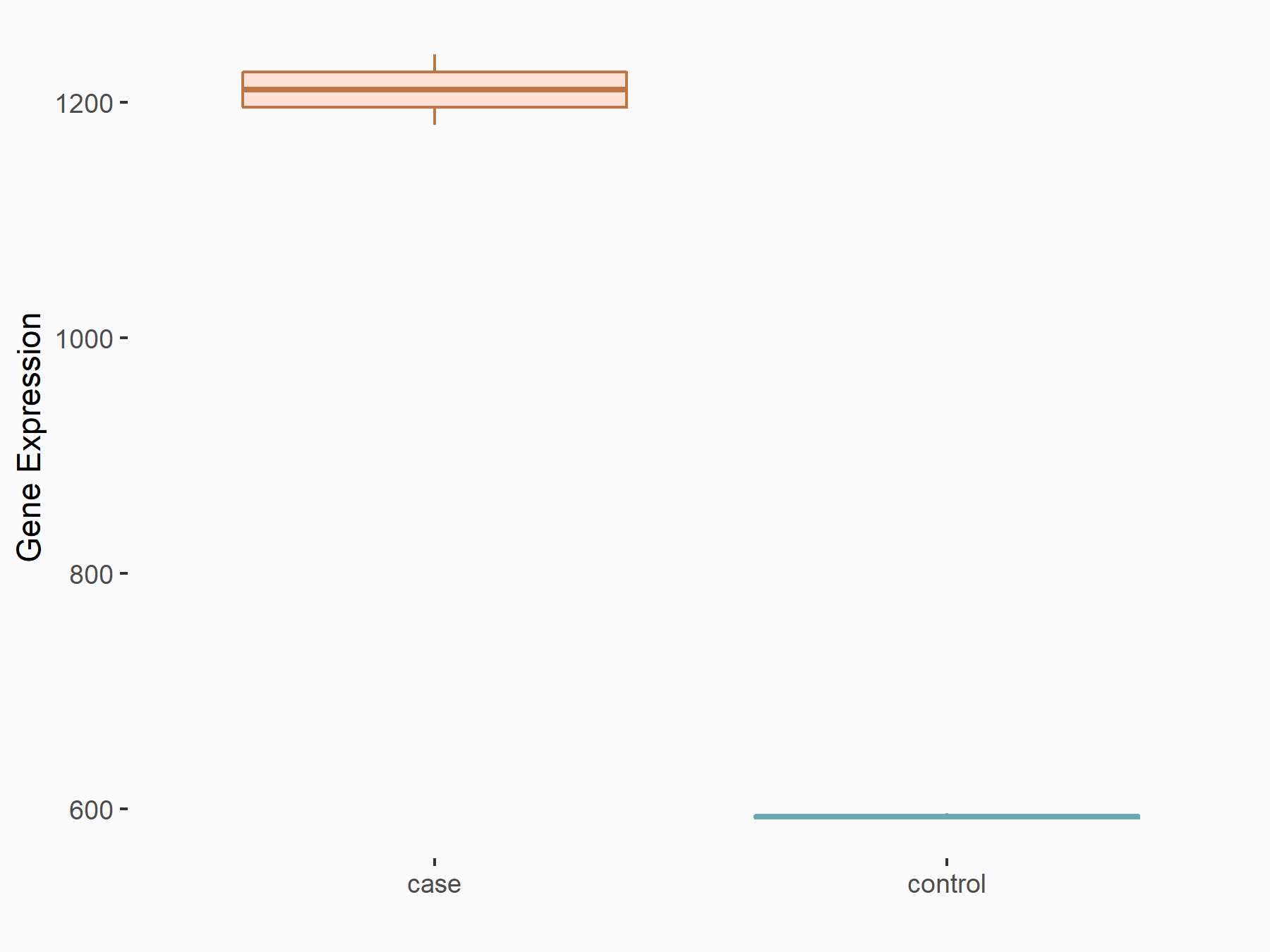  |
logFC: 1.03E+00 p-value: 3.88E-04 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [11] | |||
| Response Summary | ALKBH5 could up-regulate Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) expression by demethylation. Furthermore, dexmedetomidine inhibited the expression of ALKBH5 in LPS-treated HK-2 cells. Dexmedetomidine suppressed the biological behavior of HK-2 cells treated with LPS by inhibiting the expression of ALKBH5 in vitro, which provides potential targets for the prevention and treatment of sepsis-induced kidney injury. Dexmedetomidine suppressed the biological behavior of HK-2 cells treated with LPS by inhibiting the expression of ALKBH5 in vitro, which provides potential targets for the prevention and treatment of sepsis-induced kidney injury. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Injury of kidney | ICD-11: NB92.0 | ||
| Cell Process | Cell cycle | |||
| Cell proliferation | ||||
| Cell apoptosis | ||||
| In-vitro Model | HK2 | Normal | Acipenser baerii | CVCL_YE28 |
YTH domain-containing protein 1 (YTHDC1) [READER]
| Representative RNA-seq result indicating the expression of this target gene regulated by YTHDC1 | ||
| Cell Line | MOLM-13 cell line | Homo sapiens |
|
Treatment: shYTHDC1 MOLM13 cells
Control: shControl MOLM13 cells
|
GSE168565 | |
| Regulation |
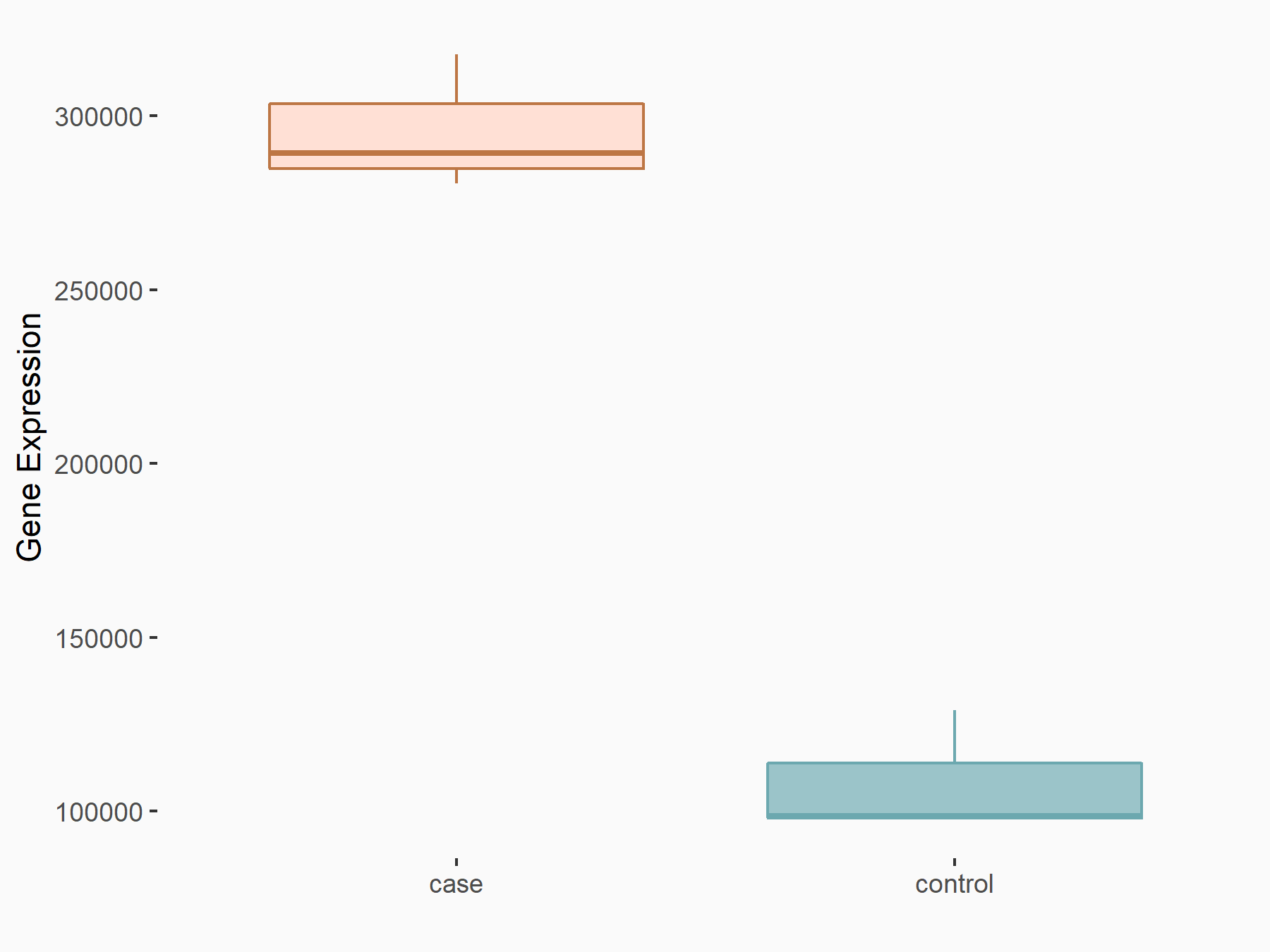 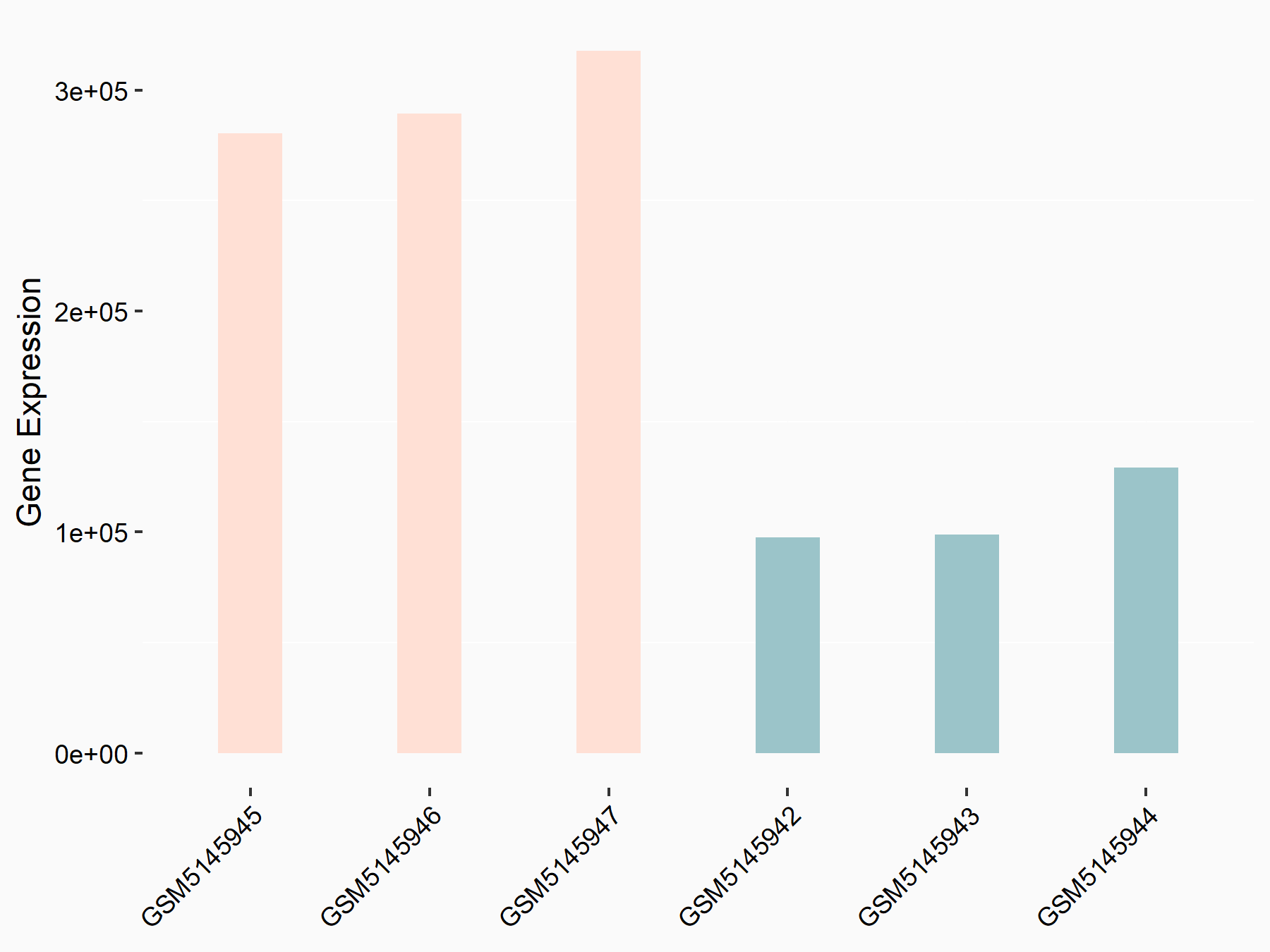 |
logFC: 1.45E+00 p-value: 5.75E-21 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [12] | |||
| Response Summary | MALAT1 hijacks both chimeric mRNAs and fusion protein in nuclear speckles during chromosomal translocation and mediates colocalization with METTL14 in an oncogenic fusion protein such as PML-RARalpha. Reducing MALAT1 or m6A methyltransferases and the 'reader' YTHDC1 result in the universal retention of distinct oncogenic gene (PML-RARalpha) mRNAs in nucleus. Targeting the lncRNA-triggered autoregulatory loop to disrupt chimeric mRNA transport represents a new common paradigm for treating blood malignancies. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Blood malignancies | ICD-11: 2B33.Y | ||
| Cell Process | Oncogenic fusion protein expression | |||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HL-60 | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| K-562 | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | |
| Kasumi-1 | Myeloid leukemia with maturation | Homo sapiens | CVCL_0589 | |
| MOLM-13 | Adult acute myeloid leukemia | Homo sapiens | CVCL_2119 | |
| NB4 | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | |
| In-vivo Model | The NOD-SCID mice were intravenously (tail vein) implanted with sh-RNA-established NB4 cells. Direct injection of 5 × 106 shRNA-transformed NB4 cells into 150 uL of PBS was performed to establish intravenous (tail vein) leukemia. | |||
Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) [READER]
| Representative RIP-seq result supporting the interaction between MALAT1 and the regulator | ||
| Cell Line | HEK293T | Homo sapiens |
| Regulation | logFC: 1.31E+00 | GSE90639 |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [13] | |||
| Response Summary | IGF2BP2 promotes Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) stability in an m6A-dependent mechanism, thus promoting its downstream target autophagy-related (ATG)12 expression and NSCLC proliferation. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Pathway Response | Lysosome | hsa04142 | ||
| Cell Process | Cell autophagy | |||
| In-vitro Model | NCI-H157 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0463 |
| NCI-H460 | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | |
| NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| NCI-H1703 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1490 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| BEAS-2B | Normal | Homo sapiens | CVCL_0168 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In-vivo Model | Mice (male and 6 weeks old) were subcutaneously injected with NSCLC cells (1.0*106 cells/200 uL). The mice were terminated after 4 weeks of induction, and the tumor volume and tumor weight were measured. | |||
Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) [READER]
| Representative RIP-seq result supporting the interaction between MALAT1 and the regulator | ||
| Cell Line | HEK293T | Homo sapiens |
| Regulation | logFC: 2.96E+00 | GSE90639 |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [14] | |||
| Response Summary | CircRNA hsa_circ_0004287 was upregulated in peripheral blood mononuclear cells of both AD and psoriasis patients. hsa_circ_0004287 reduced the stability of its host gene Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) by competitively binding to IGF2BP3 with MALAT1 in an N6-methyladenosine (m6A)-dependent manner. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Atopic eczema | ICD-11: EA80 | ||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Inflammation | |||
| Proteasome pathway degradation | ||||
| In-vitro Model | RAW 264.7 | Mouse leukemia | Mus musculus | CVCL_0493 |
| In-vivo Model | IMQ-induced psoriatic model was constructed by applying 10 mg per ear 5% IMQ for 8 consecutive days, and 6 ug macrophage-specific control or hsa_circ_0004287 plasmid was topically applied every 2 days (5 mice per group per experiment). | |||
YTH domain-containing family protein 1 (YTHDF1) [READER]
| Representative RIP-seq result supporting the interaction between MALAT1 and the regulator | ||
| Cell Line | Hela | Homo sapiens |
| Regulation | logFC: 2.44E+00 | GSE63591 |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
YTH domain-containing family protein 3 (YTHDF3) [READER]
| Representative RIP-seq result supporting the interaction between MALAT1 and the regulator | ||
| Cell Line | Hela | Homo sapiens |
| Regulation | logFC: 1.66E+00 | GSE86214 |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
Methyltransferase-like 14 (METTL14) [WRITER]
| In total 2 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [12] | |||
| Response Summary | MALAT1 hijacks both chimeric mRNAs and fusion protein in nuclear speckles during chromosomal translocation and mediates colocalization with METTL14 in an oncogenic fusion protein such as PML-RARalpha. Reducing MALAT1 or m6A methyltransferases and the 'reader' YTHDC1 result in the universal retention of distinct oncogenic gene (PML-RARalpha) mRNAs in nucleus. Targeting the lncRNA-triggered autoregulatory loop to disrupt chimeric mRNA transport represents a new common paradigm for treating blood malignancies. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Blood malignancies | ICD-11: 2B33.Y | ||
| Cell Process | Oncogenic fusion protein expression | |||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HL-60 | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| K-562 | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | |
| Kasumi-1 | Myeloid leukemia with maturation | Homo sapiens | CVCL_0589 | |
| MOLM-13 | Adult acute myeloid leukemia | Homo sapiens | CVCL_2119 | |
| NB4 | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | |
| In-vivo Model | The NOD-SCID mice were intravenously (tail vein) implanted with sh-RNA-established NB4 cells. Direct injection of 5 × 106 shRNA-transformed NB4 cells into 150 uL of PBS was performed to establish intravenous (tail vein) leukemia. | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [16] | |||
| Response Summary | METTL14 and lncRNA Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) were upregulated, and miR-224-5p was downregulated in OSCC tissues and cells. METTL14 induced m6A modification of MALAT1 to upregulate MALAT1. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Oral squamous cell carcinoma | ICD-11: 2B6E.0 | ||
| In-vitro Model | SCC-25 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1682 |
| SCC-15 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1681 | |
| Hs 680.Tg | Normal | Homo sapiens | CVCL_0842 | |
| FaDu | Hypopharyngeal squamous cell carcinoma | Homo sapiens | CVCL_1218 | |
| CAL-27 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1107 | |
| In-vivo Model | Lentiviruses containing sh-METTL-14 and its negative control (RiboBio Co., Ltd., Guangzhou, China) were transduced into CAL27 cells and stably transduced cells were screened using puromycin. CAL27 cells (3 × 106 cells/mouse) were subcutaneously inoculated into the posterior flank of each mouse (N = 12/group). | |||
Methyltransferase-like 16 (METTL16) [WRITER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [17] | |||
| Response Summary | LncRNAs are involved in a plethora of cellular signaling pathways and actively regulate gene expression via a broad selection of molecular mechanisms. Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) could serve a role as a regulator of RNA processing or modification events through guiding METTL16 onto its RNA targets. | |||
Brain cancer [ICD-11: 2A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | METTL3 promoted the malignant progression of IDH-wildtype gliomas and revealed important insight into the upstream regulatory mechanism of Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and NF-Kappa-B with a primary focus on m6A modification. | |||
| Responsed Disease | Glioma [ICD-11: 2A00.0] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | TNF signaling pathway | hsa04668 | ||
| Cell Process | Cell proliferation and metastasis | |||
| In-vitro Model | H4 | Astrocytoma | Homo sapiens | CVCL_1239 |
| LN-229 | Glioblastoma | Homo sapiens | CVCL_0393 | |
| U87 (A primary glioblastoma cell line) | ||||
| In-vivo Model | U87 cells (5 × 105) transfected with an empty vector, METTL3 shRNA, or METTL3 overexpression vector were inoculated into the right frontal node of nude mice. | |||
Malignant haematopoietic neoplasm [ICD-11: 2B33]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [12] | |||
| Response Summary | MALAT1 hijacks both chimeric mRNAs and fusion protein in nuclear speckles during chromosomal translocation and mediates colocalization with METTL14 in an oncogenic fusion protein such as PML-RARalpha. Reducing MALAT1 or m6A methyltransferases and the 'reader' YTHDC1 result in the universal retention of distinct oncogenic gene (PML-RARalpha) mRNAs in nucleus. Targeting the lncRNA-triggered autoregulatory loop to disrupt chimeric mRNA transport represents a new common paradigm for treating blood malignancies. | |||
| Responsed Disease | Blood malignancies [ICD-11: 2B33.Y] | |||
| Target Regulator | Methyltransferase-like 14 (METTL14) | WRITER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Oncogenic fusion protein expression | |||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HL-60 | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| K-562 | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | |
| Kasumi-1 | Myeloid leukemia with maturation | Homo sapiens | CVCL_0589 | |
| MOLM-13 | Adult acute myeloid leukemia | Homo sapiens | CVCL_2119 | |
| NB4 | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | |
| In-vivo Model | The NOD-SCID mice were intravenously (tail vein) implanted with sh-RNA-established NB4 cells. Direct injection of 5 × 106 shRNA-transformed NB4 cells into 150 uL of PBS was performed to establish intravenous (tail vein) leukemia. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [12] | |||
| Response Summary | MALAT1 hijacks both chimeric mRNAs and fusion protein in nuclear speckles during chromosomal translocation and mediates colocalization with METTL14 in an oncogenic fusion protein such as PML-RARalpha. Reducing MALAT1 or m6A methyltransferases and the 'reader' YTHDC1 result in the universal retention of distinct oncogenic gene (PML-RARalpha) mRNAs in nucleus. Targeting the lncRNA-triggered autoregulatory loop to disrupt chimeric mRNA transport represents a new common paradigm for treating blood malignancies. | |||
| Responsed Disease | Blood malignancies [ICD-11: 2B33.Y] | |||
| Target Regulator | YTH domain-containing protein 1 (YTHDC1) | READER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Oncogenic fusion protein expression | |||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HL-60 | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| K-562 | Chronic myelogenous leukemia | Homo sapiens | CVCL_0004 | |
| Kasumi-1 | Myeloid leukemia with maturation | Homo sapiens | CVCL_0589 | |
| MOLM-13 | Adult acute myeloid leukemia | Homo sapiens | CVCL_2119 | |
| NB4 | Acute promyelocytic leukemia | Homo sapiens | CVCL_0005 | |
| In-vivo Model | The NOD-SCID mice were intravenously (tail vein) implanted with sh-RNA-established NB4 cells. Direct injection of 5 × 106 shRNA-transformed NB4 cells into 150 uL of PBS was performed to establish intravenous (tail vein) leukemia. | |||
Head and neck squamous carcinoma [ICD-11: 2B6E]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [16] | |||
| Response Summary | METTL14 and lncRNA Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) were upregulated, and miR-224-5p was downregulated in OSCC tissues and cells. METTL14 induced m6A modification of MALAT1 to upregulate MALAT1. | |||
| Responsed Disease | Oral squamous cell carcinoma [ICD-11: 2B6E.0] | |||
| Target Regulator | Methyltransferase-like 14 (METTL14) | WRITER | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | SCC-25 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1682 |
| SCC-15 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1681 | |
| Hs 680.Tg | Normal | Homo sapiens | CVCL_0842 | |
| FaDu | Hypopharyngeal squamous cell carcinoma | Homo sapiens | CVCL_1218 | |
| CAL-27 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1107 | |
| In-vivo Model | Lentiviruses containing sh-METTL-14 and its negative control (RiboBio Co., Ltd., Guangzhou, China) were transduced into CAL27 cells and stably transduced cells were screened using puromycin. CAL27 cells (3 × 106 cells/mouse) were subcutaneously inoculated into the posterior flank of each mouse (N = 12/group). | |||
Lung cancer [ICD-11: 2C25]
| In total 4 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [13] | |||
| Response Summary | IGF2BP2 promotes Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) stability in an m6A-dependent mechanism, thus promoting its downstream target autophagy-related (ATG)12 expression and NSCLC proliferation. | |||
| Responsed Disease | Non-small-cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Target Regulator | Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Lysosome | hsa04142 | ||
| Cell Process | Cell autophagy | |||
| In-vitro Model | NCI-H157 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0463 |
| NCI-H460 | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | |
| NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| NCI-H1703 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1490 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| BEAS-2B | Normal | Homo sapiens | CVCL_0168 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | |
| In-vivo Model | Mice (male and 6 weeks old) were subcutaneously injected with NSCLC cells (1.0*106 cells/200 uL). The mice were terminated after 4 weeks of induction, and the tumor volume and tumor weight were measured. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Responsed Disease | Non-small-cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
| Experiment 3 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Responsed Disease | Non-small-cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Target Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
| Experiment 4 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Responsed Disease | Non-small-cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Target Regulator | YTH domain-containing family protein 3 (YTHDF3) | READER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
Thymoma [ICD-11: 2C27]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [3] | |||
| Response Summary | This study highlighted METTL3 as a tumor promoter in Thymic tumors and c-MYC as a promising target to be exploited for the treatment of TET. High expression of c-MYC protein is enabled by lncRNA Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), which is methylated and delocalized by METTL3. Silencing of METTL3 combined with cisplatin or c-MYC inhibitor induces cell death in TET cells. Blocking of c-MYC by using JQ1 inhibitor cooperates with METTL3 depletion in the inhibition of proliferation and induction of cell death. | |||
| Responsed Disease | Thymic epithelial tumors [ICD-11: 2C27.Y] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Cellular senescence | hsa04218 | ||
| Cell Process | Cell viability and proliferation | |||
| In-vitro Model | T1889 | Thymic undifferentiated carcinoma | Homo sapiens | CVCL_D024 |
| Experiment 2 Reporting the m6A-centered Disease Response | [3] | |||
| Response Summary | This study highlighted METTL3 as a tumor promoter in Thymic tumors and c-MYC as a promising target to be exploited for the treatment of TET. High expression of c-MYC protein is enabled by lncRNA Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), which is methylated and delocalized by METTL3. Silencing of METTL3 combined with cisplatin or c-MYC inhibitor induces cell death in TET cells. Blocking of c-MYC by using JQ1 inhibitor cooperates with METTL3 depletion in the inhibition of proliferation and induction of cell death. | |||
| Responsed Disease | Thymic epithelial tumors [ICD-11: 2C27.Y] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | JQ-1 | Phase 1 | ||
| Pathway Response | Cellular senescence | hsa04218 | ||
| Cell Process | Cell viability and proliferation | |||
| In-vitro Model | T1889 | Thymic undifferentiated carcinoma | Homo sapiens | CVCL_D024 |
Breast cancer [ICD-11: 2C60]
| In total 3 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [4] | |||
| Response Summary | METTL3 can regulate the expression of Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) through m6A, mediate the E2F1/AGR2 axis, and promote the adriamycin resistance of breast cancer. | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Doxil | Approved | ||
| In-vitro Model | MCF7-DoxR (Adriamycin-resistant cell line MCF7-DoxR) | |||
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| In-vivo Model | Once the tumor volume increased to about 1 cm3, six groups of MCF7 bearing mice (n = 10 in each group) were injected with PBS (0.1 ml, caudal vein) and adriamycin (0.1 ml, 10 mg/kg), respectively. When the tumor reached 1.5 cm in any direction (defined as event-free survival analysis), 10 mice in each group were selected to measure the tumor size and weight on the 12th day after adriamycin injection. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [5] | |||
| Response Summary | Silencing METTL3 down-regulate Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and HMGA2 by sponging miR-26b, and finally inhibit EMT, migration and invasion in breast cancer, providing a theoretical basis for clinical treatment of breast cancer. | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Epithelial-mesenchymal transition | |||
| In-vitro Model | MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 |
| MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MDA-MB-468 | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | |
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | |
| Experiment 3 Reporting the m6A-centered Disease Response | [5] | |||
| Response Summary | Silencing METTL3 down-regulate Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and HMGA2 by sponging miR-26b, and finally inhibit EMT, migration and invasion in BC, providing a theoretical basis for clinical treatment of BC. | |||
| Responsed Disease | Breast cancer [ICD-11: 2C60] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Epithelial-mesenchymal transition | |||
| In-vitro Model | MDA-MB-468 | Breast adenocarcinoma | Homo sapiens | CVCL_0419 |
| MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | |
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| MCF-10A | Normal | Homo sapiens | CVCL_0598 | |
| In-vivo Model | Eighteen BALB/C female nude mice aged 4-5 weeks and weighing 15-18 g were randomly assigned into three groups of six mice. The MCF-7 cell lines stably transfected with sh-NC + oe-NC, sh-METTL3 + oe-NC and sh-METTL3 + oe-HMGA2 were selected for subcutaneous establishment of the BC cell line MCF-7 as xenografts in the nude mice. For this purpose, MCF-7 cell lines in the logarithmic growth stage were prepared into a suspension with a concentration of about 1 × 107 cells/ml. The prepared cell suspension was injected into the left armpit of the mice, and the subsequent tumor growth was recorded. | |||
Bladder cancer [ICD-11: 2C94]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [8] | |||
| Response Summary | In bladder cancer, the changes in m6A methylation level mainly appeared at 5' untranslated region (5' UTR) of Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and NOTCH1 transcripts, and at 3' UTR of CSNK2A2 and ITGA6 transcripts, responding to the overexpression of FTO. SFPQ could influence the FTO-mediated m6A RNA demethylation, eventually affecting the gene expression. | |||
| Responsed Disease | Bladder cancer [ICD-11: 2C94] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Down regulation | |||
| Pathway Response | Notch signaling pathway | hsa04330 | ||
| Cell Process | Cell proliferation | |||
| Cell invasion | ||||
| Cell apoptosis | ||||
| In-vitro Model | HT-1197 | Recurrent bladder carcinoma | Homo sapiens | CVCL_1291 |
| HT-1376 | Bladder carcinoma | Homo sapiens | CVCL_1292 | |
| In-vivo Model | BALB/cnu/nu mice (4-5 weeks old) were used for the xenograft experiment. The mice were randomly divided into 2 groups (n = 6 for each group) and injected with 5 × 106 HT-1197 cells in control group or FTO plasmid group, respectively. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [9] | |||
| Response Summary | FTO facilitates the tumorigenesis of bladder cancer through regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)/miR-384/MAL2 axis in m6A RNA modification manner, which ensures the potential of FTO for serving as a diagnostic or prognostic biomarker in bladder cancer. | |||
| Responsed Disease | Bladder cancer [ICD-11: 2C94] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Cell Process | RNA stability | |||
| In-vitro Model | T24 | Bladder carcinoma | Homo sapiens | CVCL_0554 |
| SV-HUC-1 | Normal | Homo sapiens | CVCL_3798 | |
| SCaBER | Bladder squamous cell carcinoma | Homo sapiens | CVCL_3599 | |
| J82 | Bladder carcinoma | Homo sapiens | CVCL_0359 | |
| 253J | Bladder carcinoma | Homo sapiens | CVCL_7935 | |
| 5637 | Bladder carcinoma | Homo sapiens | CVCL_0126 | |
| In-vivo Model | Approximately 5 × 106 253J and 5637 cells infected with indicated vectors were injected subcutaneously into the flank of the mice. | |||
Atopic eczema [ICD-11: EA80]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [14] | |||
| Response Summary | CircRNA hsa_circ_0004287 was upregulated in peripheral blood mononuclear cells of both AD and psoriasis patients. hsa_circ_0004287 reduced the stability of its host gene Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) by competitively binding to IGF2BP3 with MALAT1 in an N6-methyladenosine (m6A)-dependent manner. | |||
| Responsed Disease | Atopic eczema [ICD-11: EA80] | |||
| Target Regulator | Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| Ubiquitin mediated proteolysis | hsa04120 | |||
| Cell Process | Inflammation | |||
| Proteasome pathway degradation | ||||
| In-vitro Model | RAW 264.7 | Mouse leukemia | Mus musculus | CVCL_0493 |
| In-vivo Model | IMQ-induced psoriatic model was constructed by applying 10 mg per ear 5% IMQ for 8 consecutive days, and 6 ug macrophage-specific control or hsa_circ_0004287 plasmid was topically applied every 2 days (5 mice per group per experiment). | |||
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [6] | |||
| Response Summary | Renal fibrosis is a key factor in chronic kidney disease (CKD). Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)/miR-145/FAK pathway was involved in the effect of dihydroartemisinin (DHA) on TGF-beta1-induced renal fibrosis in vitro and in vivo. | |||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Responsed Drug | Artenimol | Approved | ||
| Cell Process | Epithelial-mesenchymal transition | |||
| In-vitro Model | HK-2 [Human kidney] | Normal | Homo sapiens | CVCL_0302 |
| HK2 | Normal | Acipenser baerii | CVCL_YE28 | |
| In-vivo Model | For the unilateral ureteral obstruction (UUO) model, male C57BL/6J mice at 8 weeks of age (20-22 g body weight) were first anaesthetized with pentobarbital sodium (50 mg/kg) via intraperitoneal injection. Then, the left ureter was ligated using 3-0 silk and a left lateral incision. | |||
Kidney failure [ICD-11: GB6Z]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [7] | |||
| Response Summary | m6A modification is co-regulated by METTL3 and FTO in cadmium-treated cells. Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), LncRNA-PVT1 and m6A modification could be key nodes for cadmium-induced oxidative damage, and highlight their importance as promising preventive and therapeutic targets in cadmium toxicity. | |||
| Responsed Disease | Kidney failure [ICD-11: GB6Z] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | NIT-1 | Insulin tumor | Mus musculus | CVCL_3561 |
| Experiment 2 Reporting the m6A-centered Disease Response | [7] | |||
| Response Summary | m6A modification is co-regulated by METTL3 and FTO in cadmium-treated cells. Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), LncRNA-PVT1 and m6A modification could be key nodes for cadmium-induced oxidative damage, and highlight their importance as promising preventive and therapeutic targets in cadmium toxicity. | |||
| Responsed Disease | Kidney failure [ICD-11: GB6Z] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| In-vitro Model | NIT-1 | Insulin tumor | Mus musculus | CVCL_3561 |
Urinary/pelvic organs injury [ICD-11: NB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [11] | |||
| Response Summary | ALKBH5 could up-regulate Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) expression by demethylation. Furthermore, dexmedetomidine inhibited the expression of ALKBH5 in LPS-treated HK-2 cells. Dexmedetomidine suppressed the biological behavior of HK-2 cells treated with LPS by inhibiting the expression of ALKBH5 in vitro, which provides potential targets for the prevention and treatment of sepsis-induced kidney injury. Dexmedetomidine suppressed the biological behavior of HK-2 cells treated with LPS by inhibiting the expression of ALKBH5 in vitro, which provides potential targets for the prevention and treatment of sepsis-induced kidney injury. | |||
| Responsed Disease | Injury of kidney [ICD-11: NB92.0] | |||
| Target Regulator | RNA demethylase ALKBH5 (ALKBH5) | ERASER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Cell cycle | |||
| Cell proliferation | ||||
| Cell apoptosis | ||||
| In-vitro Model | HK2 | Normal | Acipenser baerii | CVCL_YE28 |
Artenimol
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [6] | |||
| Response Summary | Renal fibrosis is a key factor in chronic kidney disease (CKD). Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)/miR-145/FAK pathway was involved in the effect of dihydroartemisinin (DHA) on TGF-beta1-induced renal fibrosis in vitro and in vivo. | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Responsed Disease | Chronic kidney disease | ICD-11: GB61 | ||
| Cell Process | Epithelial-mesenchymal transition | |||
| In-vitro Model | HK-2 [Human kidney] | Normal | Homo sapiens | CVCL_0302 |
| HK2 | Normal | Acipenser baerii | CVCL_YE28 | |
| In-vivo Model | For the unilateral ureteral obstruction (UUO) model, male C57BL/6J mice at 8 weeks of age (20-22 g body weight) were first anaesthetized with pentobarbital sodium (50 mg/kg) via intraperitoneal injection. Then, the left ureter was ligated using 3-0 silk and a left lateral incision. | |||
Cisplatin
[Approved]
| In total 4 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
| Experiment 2 Reporting the m6A-centered Drug Response | [3] | |||
| Response Summary | This study highlighted METTL3 as a tumor promoter in Thymic tumors and c-MYC as a promising target to be exploited for the treatment of TET. High expression of c-MYC protein is enabled by lncRNA Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), which is methylated and delocalized by METTL3. Silencing of METTL3 combined with cisplatin or c-MYC inhibitor induces cell death in TET cells. Blocking of c-MYC by using JQ1 inhibitor cooperates with METTL3 depletion in the inhibition of proliferation and induction of cell death. | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Thymic epithelial tumors | ICD-11: 2C27.Y | ||
| Pathway Response | Cellular senescence | hsa04218 | ||
| Cell Process | Cell viability and proliferation | |||
| In-vitro Model | T1889 | Thymic undifferentiated carcinoma | Homo sapiens | CVCL_D024 |
| Experiment 3 Reporting the m6A-centered Drug Response | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Target Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
| Experiment 4 Reporting the m6A-centered Drug Response | [2] | |||
| Response Summary | METTL3, YTHDF3, YTHDF1, and eIF3b directly promoted YAP translation through an interaction with the translation initiation machinery. METTL3 knockdown inhibits tumor growth and enhances sensitivity to DDP in vivo.m6A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the Metastasis associated lung adenocarcinoma transcript 1 (MALAT1)-miR-1914-3p-YAP axis to induce Non-small cell lung cancer drug resistance and metastasis. | |||
| Target Regulator | YTH domain-containing family protein 3 (YTHDF3) | READER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Pathway Response | Hippo signaling pathway | hsa04390 | ||
| Cell Process | Metabolic | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| Calu-6 | Lung adenocarcinoma | Homo sapiens | CVCL_0236 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H520 | Lung squamous cell carcinoma | Homo sapiens | CVCL_1566 | |
| In-vivo Model | Mice were injected with 5 × 106 lung cancer cells with stably expression of relevant plasmids and randomly divided into two groups (five mice per group) after the diameter of the xenografted tumors had reached approximately 5 mm in diameter. Xenografted mice were then administrated with PBS or DDP (3 mg/kg per day) for three times a week, and tumor volume were measured every second day. | |||
Doxil
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [4] | |||
| Response Summary | METTL3 can regulate the expression of Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) through m6A, mediate the E2F1/AGR2 axis, and promote the adriamycin resistance of breast cancer. | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | ||
| In-vitro Model | MCF7-DoxR (Adriamycin-resistant cell line MCF7-DoxR) | |||
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| In-vivo Model | Once the tumor volume increased to about 1 cm3, six groups of MCF7 bearing mice (n = 10 in each group) were injected with PBS (0.1 ml, caudal vein) and adriamycin (0.1 ml, 10 mg/kg), respectively. When the tumor reached 1.5 cm in any direction (defined as event-free survival analysis), 10 mice in each group were selected to measure the tumor size and weight on the 12th day after adriamycin injection. | |||
JQ-1
[Phase 1]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [3] | |||
| Response Summary | This study highlighted METTL3 as a tumor promoter in Thymic tumors and c-MYC as a promising target to be exploited for the treatment of TET. High expression of c-MYC protein is enabled by lncRNA Metastasis associated lung adenocarcinoma transcript 1 (MALAT1), which is methylated and delocalized by METTL3. Silencing of METTL3 combined with cisplatin or c-MYC inhibitor induces cell death in TET cells. Blocking of c-MYC by using JQ1 inhibitor cooperates with METTL3 depletion in the inhibition of proliferation and induction of cell death. | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Thymic epithelial tumors | ICD-11: 2C27.Y | ||
| Pathway Response | Cellular senescence | hsa04218 | ||
| Cell Process | Cell viability and proliferation | |||
| In-vitro Model | T1889 | Thymic undifferentiated carcinoma | Homo sapiens | CVCL_D024 |
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
RNA modification
m6A Regulator: Methyltransferase-like 3 (METTL3)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT00533 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 26a-1 (MIR26A1) | |
| Crosstalk relationship | m6A → A-to-I | |
m6A Regulator: Methyltransferase-like 16 (METTL16)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT00534 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 26a-1 (MIR26A1) | |
| Crosstalk relationship | m6A → A-to-I | |
m6A Regulator: RNA demethylase ALKBH5 (ALKBH5)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT00535 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 26a-1 (MIR26A1) | |
| Crosstalk relationship | m6A → A-to-I | |
m6A Regulator: Fat mass and obesity-associated protein (FTO)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT00536 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 26a-1 (MIR26A1) | |
| Crosstalk relationship | m6A → A-to-I | |
DNA modification
m6A Regulator: Methyltransferase-like 3 (METTL3)
| In total 3 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT02047 | ||
| Epigenetic Regulator | Cysteine methyltransferase DNMT3A (DNMT3A) | |
| Regulated Target | Secreted frizzled-related protein 2 (SFRP2) | |
| Crosstalk relationship | m6A → DNA modification | |
| Drug | Simvastatin | |
| Crosstalk ID: M6ACROT02048 | ||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | |
| Regulated Target | Secreted frizzled-related protein 2 (SFRP2) | |
| Crosstalk relationship | m6A → DNA modification | |
| Drug | Simvastatin | |
| Crosstalk ID: M6ACROT02049 | ||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 1 (DNMT1) | |
| Regulated Target | Secreted frizzled-related protein 2 (SFRP2) | |
| Crosstalk relationship | m6A → DNA modification | |
| Drug | Simvastatin | |
Histone modification
m6A Regulator: Methyltransferase-like 14 (METTL14)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT03116 | ||
| Epigenetic Regulator | Lysine-specific demethylase 2A (KDM2A) | |
| Crosstalk relationship | m6A → Histone modification | |
| Disease | Oral squamous cell carcinoma | |
m6A Regulator: Methyltransferase-like 3 (METTL3)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT03316 | ||
| Epigenetic Regulator | Probable JmjC domain-containing histone demethylation protein 2C (JMJD1C) | |
| Regulated Target | Histone H3 lysine 9 monomethylation (H3K9me1) | |
| Crosstalk relationship | Histone modification → m6A | |
| Disease | Brain cancer | |
Non-coding RNA
m6A Regulator: Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3)
| In total 3 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05275 | ||
| Epigenetic Regulator | hsa_circ_0004287 (Circ_MALAT1) | |
| Regulated Target | Insulin like growth factor 2 mRNA binding protein 3 (IGF2BP3) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Atopic eczema | |
| Crosstalk ID: M6ACROT05638 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Protein S100-A8 (S100A8) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Atopic eczema | |
| Crosstalk ID: M6ACROT06028 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Protein S100-A9 (S100A9) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Atopic eczema | |
m6A Regulator: Methyltransferase-like 16 (METTL16)
| In total 2 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05374 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Crosstalk ID: M6ACROT05794 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
m6A Regulator: YTH domain-containing family protein 3 (YTHDF3)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05403 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Transcriptional coactivator YAP1 (YAP1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Breast cancer | |
| Drug | Tamoxifen | |
m6A Regulator: Methyltransferase-like 3 (METTL3)
| In total 14 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05404 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Transcriptional coactivator YAP1 (YAP1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Non-small cell lung cancer | |
| Drug | Cisplatin | |
| Crosstalk ID: M6ACROT05424 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | MicroRNA 145 (MIR145) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Chronic kidney disease | |
| Drug | Dihydroartemisinin | |
| Crosstalk ID: M6ACROT05479 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Kidney failure | |
| Crosstalk ID: M6ACROT05492 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Transcription factor p65 (RELA) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Brain cancer | |
| Crosstalk ID: M6ACROT05510 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | hsa-miR-26b | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Breast cancer | |
| Crosstalk ID: M6ACROT05515 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Myc proto-oncogene protein (MYC) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Thymic epithelial tumors | |
| Drug | JQ1 | |
| Crosstalk ID: M6ACROT05516 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Myc proto-oncogene protein (MYC) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Thymic epithelial tumors | |
| Drug | Cisplatin | |
| Crosstalk ID: M6ACROT05608 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | hsa-miR-26b | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Breast cancer | |
| Crosstalk ID: M6ACROT05613 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Transcription factor E2F1 (E2F1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Breast cancer | |
| Drug | Adriamycin | |
| Crosstalk ID: M6ACROT05644 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Osteosarcoma | |
| Crosstalk ID: M6ACROT05694 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | hsa-miR-124-3p | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Ewing's sarcoma | |
| Crosstalk ID: M6ACROT05728 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Non-small cell lung cancer | |
| Crosstalk ID: M6ACROT05822 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Osteosarcoma | |
| Crosstalk ID: M6ACROT05930 | ||
| Epigenetic Regulator | MicroRNA 145 (MIR145) | |
| Regulated Target | Focal adhesion kinase 1 (FAK) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Chronic kidney disease | |
| Drug | Dihydroartemisinin | |
m6A Regulator: YTH domain-containing family protein 1 (YTHDF1)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05405 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Transcriptional coactivator YAP1 (YAP1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Non-small cell lung cancer | |
| Drug | Cisplatin | |
m6A Regulator: Fat mass and obesity-associated protein (FTO)
| In total 3 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05477 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Kidney failure | |
| Crosstalk ID: M6ACROT05514 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Bladder cancer | |
| Crosstalk ID: M6ACROT05567 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | MicroRNA 384 (MIR384) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Bladder cancer | |
m6A Regulator: RNA demethylase ALKBH5 (ALKBH5)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05552 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Injury of kidney | |
| Drug | Dexmedetomidine* | |
m6A Regulator: Methyltransferase-like 14 (METTL14)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05624 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | hsa-miR-224-5p | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Oral squamous cell carcinoma | |
m6A Regulator: Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05631 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Regulated Target | Ubiquitin-like protein ATG12 (ATG12) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Non-small cell lung cancer | |
m6A Regulator: Dihydropyrimidinase-related protein 2 (DPYSL2)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05652 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
m6A Regulator: Cytoplasmic FMR1-interacting protein 2 (CYFIP2)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05653 | ||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | |
| Crosstalk relationship | m6A → ncRNA | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00141)
| In total 32 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE004917 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500886-65500887:+ | [23] | |
| Sequence | AAAGGGATTTATATGGGGACGTAGGCCGATTTCCGGGTGTT | ||
| Transcript ID List | ENST00000619449.2; ENST00000544868.2; ENST00000534336.1 | ||
| External Link | RMBase: RNA-editing_site_22723 | ||
| mod ID: A2ISITE004918 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501190-65501191:+ | [23] | |
| Sequence | TTTTGTAAATGTAGAGTTTGGATGTGTAACTGAGGCGGGGG | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: RNA-editing_site_22724 | ||
| mod ID: A2ISITE004919 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501357-65501358:+ | [23] | |
| Sequence | TATCAGGATAATCAGACCACCACAGGTTTACAGTTTATAGA | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: RNA-editing_site_22725 | ||
| mod ID: A2ISITE004920 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501391-65501392:+ | [23] | |
| Sequence | TTATAGAAACTAGAGCAGTTCTCACGTTGAGGTCTGTGGAA | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: RNA-editing_site_22726 | ||
| mod ID: A2ISITE004921 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501628-65501629:+ | [23] | |
| Sequence | GACAAACTGGGTTAGAGAAGGAGTGTACCGCTGTGCTGTTG | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: RNA-editing_site_22727 | ||
| mod ID: A2ISITE004922 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501630-65501631:+ | [23] | |
| Sequence | CAAACTGGGTTAGAGAAGGAGTGTACCGCTGTGCTGTTGGC | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: RNA-editing_site_22728 | ||
| mod ID: A2ISITE004923 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501844-65501845:+ | [23] | |
| Sequence | TAAAAGTTTTATTAAAGGGGAGGGGCAAATATTGGCAATTA | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: RNA-editing_site_22729 | ||
| mod ID: A2ISITE004924 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501849-65501850:+ | [23] | |
| Sequence | GTTTTATTAAAGGGGAGGGGCAAATATTGGCAATTAGTTGG | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000508832.2 | ||
| External Link | RMBase: RNA-editing_site_22730 | ||
| mod ID: A2ISITE004925 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502195-65502196:+ | [23] | |
| Sequence | GTATTGAACTGGGGGTTGGTCTGGCCTACTGGGCTGACATT | ||
| Transcript ID List | ENST00000619449.2; ENST00000616527.4; ENST00000534336.1; ENST00000508832.2 | ||
| External Link | RMBase: RNA-editing_site_22731 | ||
| mod ID: A2ISITE004926 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502630-65502631:+ | [23] | |
| Sequence | AGGGGAAACTTTTTTTTTTTCTATAGACTTTTTTCAGATAA | ||
| Transcript ID List | ENST00000619449.2; ENST00000508832.2; ENST00000616527.4; ENST00000534336.1 | ||
| External Link | RMBase: RNA-editing_site_22732 | ||
| mod ID: A2ISITE004927 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502675-65502676:+ | [23] | |
| Sequence | TTCTGAGTCATAACCAGCCTGGCAGTATGATGGCCTAGATG | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000616527.4; ENST00000508832.2 | ||
| External Link | RMBase: RNA-editing_site_22733 | ||
| mod ID: A2ISITE004928 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503000-65503001:+ | [23] | |
| Sequence | GAATAAAAGCGAAAAGAAATGAAAATGTTACACTACATTAA | ||
| Transcript ID List | ENST00000618249.1; ENST00000508832.2; ENST00000616527.4; ENST00000534336.1; ENST00000619449.2; ENST00000618925.1 | ||
| External Link | RMBase: RNA-editing_site_22734 | ||
| mod ID: A2ISITE004929 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503764-65503765:+ | [23] | |
| Sequence | CACTCTTTAATGGACCAGATCAGGATTTGAGCGGAAGAACG | ||
| Transcript ID List | ENST00000620465.4; ENST00000534336.1; ENST00000612781.1; ENST00000619449.2; ENST00000616527.4 | ||
| External Link | RMBase: RNA-editing_site_22735 | ||
| mod ID: A2ISITE004930 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503784-65503785:+ | [23] | |
| Sequence | CAGGATTTGAGCGGAAGAACGAATGTAACTTTAAGGCAGGA | ||
| Transcript ID List | ENST00000620465.4; ENST00000616527.4; ENST00000534336.1; ENST00000619449.2; ENST00000612781.1 | ||
| External Link | RMBase: RNA-editing_site_22736 | ||
| mod ID: A2ISITE004931 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503909-65503910:+ | [23] | |
| Sequence | ATAACCTCTTAGACAGGTGGGAGATTATGATCAGAGTAAAA | ||
| Transcript ID List | ENST00000620465.4; ENST00000616527.4; ENST00000534336.1; ENST00000619449.2; ENST00000612781.1 | ||
| External Link | RMBase: RNA-editing_site_22737 | ||
| mod ID: A2ISITE004932 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504091-65504092:+ | [23] | |
| Sequence | TTGACCTTATATAGGGAAGGGAGGGGGTGCCTGTGGGGTTT | ||
| Transcript ID List | ENST00000620465.4; ENST00000619449.2; ENST00000616527.4; ENST00000534336.1; ENST00000618132.1; ENST00000612781.1 | ||
| External Link | RMBase: RNA-editing_site_22738 | ||
| mod ID: A2ISITE004933 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504094-65504095:+ | [23] | |
| Sequence | ACCTTATATAGGGAAGGGAGGGGGTGCCTGTGGGGTTTTAA | ||
| Transcript ID List | ENST00000618132.1; ENST00000616527.4; ENST00000620465.4; ENST00000534336.1; ENST00000612781.1; ENST00000619449.2 | ||
| External Link | RMBase: RNA-editing_site_22739 | ||
| mod ID: A2ISITE004934 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504798-65504799:+ | [23] | |
| Sequence | GTTTCTCTCTCCCCTCCCTTGGTCTTAATTCTTACATGCAG | ||
| Transcript ID List | ENST00000534336.1; ENST00000618132.1; ENST00000619449.2; ENST00000613376.1; ENST00000610851.1 | ||
| External Link | RMBase: RNA-editing_site_22740 | ||
| mod ID: A2ISITE004935 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504822-65504823:+ | [23] | |
| Sequence | TTAATTCTTACATGCAGGAACACTCAGCAGACACACGTATG | ||
| Transcript ID List | ENST00000534336.1; ENST00000610851.1; ENST00000618132.1; ENST00000619449.2; ENST00000613376.1 | ||
| External Link | RMBase: RNA-editing_site_22741 | ||
| mod ID: A2ISITE004936 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504825-65504826:+ | [23] | |
| Sequence | ATTCTTACATGCAGGAACACTCAGCAGACACACGTATGCGA | ||
| Transcript ID List | ENST00000610851.1; ENST00000618132.1; ENST00000613376.1; ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: RNA-editing_site_22742 | ||
| mod ID: A2ISITE004937 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504853-65504854:+ | [23] | |
| Sequence | CACACGTATGCGAAGGGCCAGAGAAGCCAGACCCAGTAAGA | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000618227.1; ENST00000610851.1 | ||
| External Link | RMBase: RNA-editing_site_22743 | ||
| mod ID: A2ISITE004938 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504868-65504869:+ | [23] | |
| Sequence | GGCCAGAGAAGCCAGACCCAGTAAGAAAAAATAGCCTATTT | ||
| Transcript ID List | ENST00000610851.1; ENST00000534336.1; ENST00000619449.2; ENST00000618227.1 | ||
| External Link | RMBase: RNA-editing_site_22744 | ||
| mod ID: A2ISITE004939 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505117-65505118:+ | [23] | |
| Sequence | TGCTGGAGTAACTGGCATGTGAGCAAACTGTGTTGGCGTGG | ||
| Transcript ID List | ENST00000610851.1; ENST00000534336.1; ENST00000618227.1; ENST00000619449.2 | ||
| External Link | RMBase: RNA-editing_site_22745 | ||
| mod ID: A2ISITE004940 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505387-65505388:+ | [23] | |
| Sequence | TGCTTGAAGTACCCCTGGGCTTCTCTTAACATTTAAGCAAG | ||
| Transcript ID List | ENST00000616691.1; ENST00000610851.1; ENST00000619449.2; ENST00000617489.1; ENST00000618227.1; ENST00000534336.1 | ||
| External Link | RMBase: RNA-editing_site_22746 | ||
| mod ID: A2ISITE004941 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505561-65505562:+ | [23] | |
| Sequence | AGCAACTTCTCTGCCACATCGCCACCCCGTGCCTTTTGATC | ||
| Transcript ID List | ENST00000616691.1; ENST00000619449.2; ENST00000610851.1; ENST00000618227.1; ENST00000534336.1; ENST00000617489.1 | ||
| External Link | RMBase: RNA-editing_site_22747 | ||
| mod ID: A2ISITE004942 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505565-65505566:+ | [23] | |
| Sequence | ACTTCTCTGCCACATCGCCACCCCGTGCCTTTTGATCTAGC | ||
| Transcript ID List | ENST00000616691.1; ENST00000619449.2; ENST00000610851.1; ENST00000618227.1; ENST00000534336.1; ENST00000617489.1 | ||
| External Link | RMBase: RNA-editing_site_22748 | ||
| mod ID: A2ISITE004943 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505566-65505567:+ | [23] | |
| Sequence | CTTCTCTGCCACATCGCCACCCCGTGCCTTTTGATCTAGCA | ||
| Transcript ID List | ENST00000610851.1; ENST00000617489.1; ENST00000534336.1; ENST00000616691.1; ENST00000619449.2; ENST00000618227.1 | ||
| External Link | RMBase: RNA-editing_site_22749 | ||
| mod ID: A2ISITE004944 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505567-65505568:+ | [23] | |
| Sequence | TTCTCTGCCACATCGCCACCCCGTGCCTTTTGATCTAGCAC | ||
| Transcript ID List | ENST00000616691.1; ENST00000619449.2; ENST00000618227.1; ENST00000610851.1; ENST00000534336.1; ENST00000617489.1 | ||
| External Link | RMBase: RNA-editing_site_22750 | ||
| mod ID: A2ISITE004945 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505569-65505570:+ | [23] | |
| Sequence | CTCTGCCACATCGCCACCCCGTGCCTTTTGATCTAGCACAG | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000616691.1; ENST00000618227.1; ENST00000610851.1; ENST00000617489.1 | ||
| External Link | RMBase: RNA-editing_site_22751 | ||
| mod ID: A2ISITE004946 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505596-65505597:+ | [23] | |
| Sequence | TTGATCTAGCACAGACCCTTCACCCCTCACCTCGATGCAGC | ||
| Transcript ID List | ENST00000610851.1; ENST00000617489.1; ENST00000618227.1; ENST00000619449.2; ENST00000616691.1; ENST00000534336.1 | ||
| External Link | RMBase: RNA-editing_site_22752 | ||
| mod ID: A2ISITE004947 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505856-65505857:+ | [23] | |
| Sequence | GAACTGTAATGCTGGGTGGGAACATGTAACTTGTAGACTGG | ||
| Transcript ID List | ENST00000534336.1; ENST00000610851.1; ENST00000619449.2; ENST00000617489.1; ENST00000616691.1; ENST00000618227.1 | ||
| External Link | RMBase: RNA-editing_site_22753 | ||
| mod ID: A2ISITE004948 | Click to Show/Hide the Full List | ||
| mod site | chr11:65506158-65506159:+ | [24] | |
| Sequence | GGTTTCCAGGACGGGGTTCAAATCCCTGCGGCGTCTTTGCT | ||
| Transcript ID List | ENST00000619449.2; ENST00000611300.1; ENST00000618227.1; ENST00000617489.1; ENST00000610851.1; ENST00000534336.1; ENST00000616691.1 | ||
| External Link | RMBase: RNA-editing_site_22754 | ||
2'-O-Methylation (2'-O-Me)
| In total 18 m6A sequence/site(s) in this target gene | |||
| mod ID: 2OMSITE000005 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499674-65499675:+ | [25] | |
| Sequence | GAAGTGGAAAACTGGAAGACAGAAGTACGGGAAGGCGAAGA | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | rmsk_3569609; ENST00000620902.1; ENST00000544868.2; ENST00000617791.1; ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: Nm_site_1004 | ||
| mod ID: 2OMSITE000007 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500431-65500432:+ | [25] | |
| Sequence | GTGGATTCAGTGAATCTAGGAAGACAGCAGCAGACAGGATT | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000534336.1; ENST00000619449.2; ENST00000610481.1 | ||
| External Link | RMBase: Nm_site_1006 | ||
| mod ID: 2OMSITE000008 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501295-65501296:+ | [25] | |
| Sequence | AGGTCTGTCTAGAATCCTAAAGGCAAATGACTCAAGGTGTA | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: Nm_site_1007 | ||
| mod ID: 2OMSITE000014 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503531-65503532:+ | [25] | |
| Sequence | ATAGCATGATGTGCTGTTAGAATCAGATGTTACTGCTAAAA | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000508832.2; ENST00000616527.4; ENST00000619449.2; ENST00000534336.1; ENST00000618925.1 | ||
| External Link | RMBase: Nm_site_1013 | ||
| mod ID: 2OMSITE000004 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499673-65499674:+ | [25] | |
| Sequence | TGAAGTGGAAAACTGGAAGACAGAAGTACGGGAAGGCGAAG | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000619449.2; ENST00000617791.1; ENST00000534336.1; rmsk_3569609; ENST00000620902.1 | ||
| External Link | RMBase: Nm_site_1003 | ||
| mod ID: 2OMSITE000021 | Click to Show/Hide the Full List | ||
| mod site | chr11:65506000-65506001:+ | [25] | |
| Sequence | ATCTTAGCGGAAGCTGATCTCCAATGCTCTTCAGTAGGGTC | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000618227.1; ENST00000534336.1; ENST00000610851.1; ENST00000619449.2; ENST00000617489.1; ENST00000616691.1 | ||
| External Link | RMBase: Nm_site_1020 | ||
| mod ID: 2OMSITE000006 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499679-65499680:+ | [25] | |
| Sequence | GGAAAACTGGAAGACAGAAGTACGGGAAGGCGAAGAAAAGA | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | rmsk_3569609; ENST00000534336.1; ENST00000617791.1; ENST00000619449.2; ENST00000544868.2; ENST00000620902.1 | ||
| External Link | RMBase: Nm_site_1005 | ||
| mod ID: 2OMSITE000009 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501306-65501307:+ | [25] | |
| Sequence | GAATCCTAAAGGCAAATGACTCAAGGTGTAACAGAAAACAA | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: Nm_site_1008 | ||
| mod ID: 2OMSITE000010 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502273-65502274:+ | [25] | |
| Sequence | TGGTAGTGTGTGGTTCTCTTTTGGAATTTTTTTCAGGTGAT | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000616527.4; ENST00000508832.2; ENST00000619449.2 | ||
| External Link | RMBase: Nm_site_1009 | ||
| mod ID: 2OMSITE000011 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503262-65503263:+ | [25] | |
| Sequence | TTTAAGAGCTGTGGAGTTCTTAAATATCAACCATGGCACTT | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000616527.4; ENST00000618925.1; ENST00000619449.2; ENST00000508832.2 | ||
| External Link | RMBase: Nm_site_1010 | ||
| mod ID: 2OMSITE000012 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503324-65503325:+ | [25] | |
| Sequence | GGATTTCAGGATTGAGAAATTTTTCCATCGAGCCTTTTTAA | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000508832.2; ENST00000616527.4; ENST00000619449.2; ENST00000618925.1; ENST00000534336.1 | ||
| External Link | RMBase: Nm_site_1011 | ||
| mod ID: 2OMSITE000013 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503326-65503327:+ | [25] | |
| Sequence | ATTTCAGGATTGAGAAATTTTTCCATCGAGCCTTTTTAAAA | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000508832.2; ENST00000618925.1; ENST00000616527.4; ENST00000534336.1 | ||
| External Link | RMBase: Nm_site_1012 | ||
| mod ID: 2OMSITE000015 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504172-65504173:+ | [25] | |
| Sequence | AGCCATTCAGGATTTTGAATTGCATATGAGTGCTTGGCTCT | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000616527.4; ENST00000620465.4; ENST00000618132.1; ENST00000534336.1; ENST00000612781.1; ENST00000619449.2 | ||
| External Link | RMBase: Nm_site_1014 | ||
| mod ID: 2OMSITE000016 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504201-65504202:+ | [25] | |
| Sequence | GTGCTTGGCTCTTCCTTCTGTTCTAGTGAGTGTATGAGACC | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000620465.4; ENST00000616527.4; ENST00000534336.1; ENST00000619449.2; ENST00000612781.1; ENST00000618132.1 | ||
| External Link | RMBase: Nm_site_1015 | ||
| mod ID: 2OMSITE000017 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504202-65504203:+ | [25] | |
| Sequence | TGCTTGGCTCTTCCTTCTGTTCTAGTGAGTGTATGAGACCT | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000618132.1; ENST00000534336.1; ENST00000616527.4; ENST00000620465.4; ENST00000612781.1 | ||
| External Link | RMBase: Nm_site_1016 | ||
| mod ID: 2OMSITE000018 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504520-65504521:+ | [25] | |
| Sequence | TGAGTGATAAAGGCTGAGTGTTGAGGAAATTTCTGCAGTTT | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000613376.1; ENST00000610851.1; ENST00000618132.1; ENST00000534336.1; ENST00000612781.1 | ||
| External Link | RMBase: Nm_site_1017 | ||
| mod ID: 2OMSITE000019 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504644-65504645:+ | [25] | |
| Sequence | TGGGAGGGGACTGAAGCCTTTAGTCTTTTCCAGATGCAACC | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000612781.1; ENST00000610851.1; ENST00000618132.1; ENST00000619449.2; ENST00000613376.1 | ||
| External Link | RMBase: Nm_site_1018 | ||
| mod ID: 2OMSITE000020 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505999-65506000:+ | [25] | |
| Sequence | CATCTTAGCGGAAGCTGATCTCCAATGCTCTTCAGTAGGGT | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000618227.1; ENST00000610851.1; ENST00000616691.1; ENST00000617489.1; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: Nm_site_1019 | ||
N1-methyladenosine (m1A)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: M1ASITE000020 | Click to Show/Hide the Full List | ||
| mod site | chr11:65506158-65506159:+ | [26] | |
| Sequence | GGTTTCCAGGACGGGGTTCAAATCCCTGCGGCGTCTTTGCT | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | m1A-seq | ||
| Transcript ID List | ENST00000610851.1; ENST00000617489.1; ENST00000611300.1; ENST00000619449.2; ENST00000616691.1; ENST00000618227.1; ENST00000534336.1 | ||
| External Link | RMBase: m1A_site_202 | ||
N6-methyladenosine (m6A)
| In total 134 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE006913 | Click to Show/Hide the Full List | ||
| mod site | chr11:65497755-65497756:+ | [27] | |
| Sequence | TCCGCAGCCTGCAGCCCGAGACTTCTGTAAAGGACTGGGGC | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147942 | ||
| mod ID: M6ASITE006914 | Click to Show/Hide the Full List | ||
| mod site | chr11:65497768-65497769:+ | [27] | |
| Sequence | GCCCGAGACTTCTGTAAAGGACTGGGGCCCCGCAACTGGCC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147943 | ||
| mod ID: M6ASITE006915 | Click to Show/Hide the Full List | ||
| mod site | chr11:65498483-65498484:+ | [28] | |
| Sequence | TTCTAAGATTTCCCAAGCAGACAGCCCGTGCTGCTCCGATT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147944 | ||
| mod ID: M6ASITE006916 | Click to Show/Hide the Full List | ||
| mod site | chr11:65498510-65498511:+ | [28] | |
| Sequence | GTGCTGCTCCGATTTCTCGAACAAAAAAGCAAAACGTGTGG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147945 | ||
| mod ID: M6ASITE006917 | Click to Show/Hide the Full List | ||
| mod site | chr11:65498918-65498919:+ | [27] | |
| Sequence | GAAAAATCTAGAAAAGTAAAACTAGAACCTATTTTTAACCG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147946 | ||
| mod ID: M6ASITE006918 | Click to Show/Hide the Full List | ||
| mod site | chr11:65498924-65498925:+ | [27] | |
| Sequence | TCTAGAAAAGTAAAACTAGAACCTATTTTTAACCGAAGAAC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147947 | ||
| mod ID: M6ASITE006919 | Click to Show/Hide the Full List | ||
| mod site | chr11:65498943-65498944:+ | [29] | |
| Sequence | AACCTATTTTTAACCGAAGAACTACTTTTTGCCTCCCTCAC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147948 | ||
| mod ID: M6ASITE006920 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499103-65499104:+ | [27] | |
| Sequence | GGCAGGCGGAGCTTGAGGAAACCGCAGATAAGTTTTTTTCT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; hESCs; fibroblasts; LCLs; H1299; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000620902.1; ENST00000619449.2; ENST00000617791.1; ENST00000544868.2 | ||
| External Link | RMBase: m6A_site_147949 | ||
| mod ID: M6ASITE006921 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499151-65499152:+ | [30] | |
| Sequence | AGATAGAGATTAATACAACTACTTAAAAAATATAGTCAATA | ||
| Motif Score | 2.500660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000620902.1; ENST00000617791.1; ENST00000544868.2 | ||
| External Link | RMBase: m6A_site_147950 | ||
| mod ID: M6ASITE006922 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499248-65499249:+ | [27] | |
| Sequence | TTTTAAGAGAAAATATGAAGACTTAGAAGAGTAGCATGAGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; A549; HepG2; hNPCs; hESCs; fibroblasts; LCLs; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000544868.2; ENST00000617791.1; ENST00000619449.2; ENST00000620902.1 | ||
| External Link | RMBase: m6A_site_147951 | ||
| mod ID: M6ASITE006923 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499294-65499295:+ | [27] | |
| Sequence | AAAGATAAAAGGTTTCTAAAACATGACGGAGGTTGAGATGA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; A549; HepG2; hNPCs; hESCs; fibroblasts; LCLs; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617791.1; ENST00000619449.2; ENST00000544868.2; ENST00000534336.1; ENST00000620902.1 | ||
| External Link | RMBase: m6A_site_147952 | ||
| mod ID: M6ASITE006924 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499299-65499300:+ | [30] | |
| Sequence | TAAAAGGTTTCTAAAACATGACGGAGGTTGAGATGAAGCTT | ||
| Motif Score | 2.833690476 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000620902.1; ENST00000544868.2; ENST00000619449.2; ENST00000617791.1 | ||
| External Link | RMBase: m6A_site_147953 | ||
| mod ID: M6ASITE006925 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499365-65499366:+ | [27] | |
| Sequence | AAAGAAAATTGAGAGAAAGGACTACAGAGCCCCGAATTAAT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; CD8T; Huh7; iSLK; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000620902.1; ENST00000617791.1; ENST00000534336.1; ENST00000544868.2 | ||
| External Link | RMBase: m6A_site_147954 | ||
| mod ID: M6ASITE006926 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499432-65499433:+ | [27] | |
| Sequence | TTAAAATGAAGGTGACTTAAACAGCTTAAAGTTTAGTTTAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; Huh7; iSLK; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000544868.2; ENST00000617791.1; ENST00000620902.1 | ||
| External Link | RMBase: m6A_site_147955 | ||
| mod ID: M6ASITE006927 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499505-65499506:+ | [27] | |
| Sequence | ATCTTTTAAAAAGAGATTAAACCGAAGGTGATTAAAAGACC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; Huh7; iSLK; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000620902.1; ENST00000544868.2; ENST00000617791.1; ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147956 | ||
| mod ID: M6ASITE006928 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499523-65499524:+ | [27] | |
| Sequence | AAACCGAAGGTGATTAAAAGACCTTGAAATCCATGACGCAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; Huh7; iSLK; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000617791.1; ENST00000619449.2; ENST00000544868.2; ENST00000620902.1; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147957 | ||
| mod ID: M6ASITE006929 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499586-65499587:+ | [30] | |
| Sequence | CTAGTTAACGCATTTACTAAACGCAGACGAAAATGGAAAGA | ||
| Motif Score | 2.179660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000620902.1; ENST00000534336.1; ENST00000544868.2; ENST00000619449.2; ENST00000617791.1 | ||
| External Link | RMBase: m6A_site_147958 | ||
| mod ID: M6ASITE006930 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499630-65499631:+ | [27] | |
| Sequence | ATTGGGAGTGGTAGGATGAAACAATTTGGAGAAGATAGAAG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; H1299; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617791.1; ENST00000620902.1; ENST00000619449.2; ENST00000544868.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147959 | ||
| mod ID: M6ASITE006931 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499664-65499665:+ | [27] | |
| Sequence | ATAGAAGTTTGAAGTGGAAAACTGGAAGACAGAAGTACGGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; CD8T; H1299; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | rmsk_3569609; ENST00000617791.1; ENST00000534336.1; ENST00000620902.1; ENST00000544868.2; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147960 | ||
| mod ID: M6ASITE006932 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499672-65499673:+ | [27] | |
| Sequence | TTGAAGTGGAAAACTGGAAGACAGAAGTACGGGAAGGCGAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; CD8T; H1299; Huh7; iSLK; TIME; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | rmsk_3569609; ENST00000617791.1; ENST00000534336.1; ENST00000619449.2; ENST00000544868.2; ENST00000620902.1 | ||
| External Link | RMBase: m6A_site_147961 | ||
| mod ID: M6ASITE006933 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499731-65499732:+ | [27] | |
| Sequence | AGGGAAATTAGAAGATAAAAACATACTTTTAGAAGAAAAAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; H1299; Huh7; iSLK; TIME; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | rmsk_3569609; ENST00000534336.1; ENST00000619449.2; ENST00000617791.1; ENST00000544868.2 | ||
| External Link | RMBase: m6A_site_147962 | ||
| mod ID: M6ASITE006934 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499763-65499764:+ | [27] | |
| Sequence | AAGAAAAAAGATAAATTTAAACCTGAAAAGTAGGAAGCAGA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; H1299; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000534336.1; rmsk_3569609; ENST00000619449.2; ENST00000617791.1 | ||
| External Link | RMBase: m6A_site_147963 | ||
| mod ID: M6ASITE006935 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499793-65499794:+ | [27] | |
| Sequence | TAGGAAGCAGAAGAAAAAAGACAAGCTAGGAAACAAAAAGC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; H1299; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544868.2; rmsk_3569609; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147964 | ||
| mod ID: M6ASITE006936 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499805-65499806:+ | [27] | |
| Sequence | GAAAAAAGACAAGCTAGGAAACAAAAAGCTAAGGGCAAAAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; H1299; Huh7; iSLK; TIME; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000544868.2; ENST00000534336.1; rmsk_3569609; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147965 | ||
| mod ID: M6ASITE006937 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499832-65499833:+ | [27] | |
| Sequence | GCTAAGGGCAAAATGTACAAACTTAGAAGAAAATTGGAAGA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; H1299; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000619449.2; ENST00000534336.1; rmsk_3569609 | ||
| External Link | RMBase: m6A_site_147966 | ||
| mod ID: M6ASITE006938 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499858-65499859:+ | [27] | |
| Sequence | AAGAAAATTGGAAGATAGAAACAAGATAGAAAATGAAAATA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; H1299; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000534336.1; rmsk_3569609; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147967 | ||
| mod ID: M6ASITE006939 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499909-65499910:+ | [27] | |
| Sequence | TTTCAGATAGAAAATGAAAAACAAGCTAAGACAAGTATTGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; Huh7; iSLK; TIME; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000544868.2; rmsk_3569609 | ||
| External Link | RMBase: m6A_site_147968 | ||
| mod ID: M6ASITE006940 | Click to Show/Hide the Full List | ||
| mod site | chr11:65499919-65499920:+ | [27] | |
| Sequence | AAAATGAAAAACAAGCTAAGACAAGTATTGGAGAAGTATAG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; hNPCs; hESCs; fibroblasts; A549; LCLs; Huh7; iSLK; TIME; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | rmsk_3569609; ENST00000544868.2; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147969 | ||
| mod ID: M6ASITE006941 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500096-65500097:+ | [27] | |
| Sequence | AGATGAGGGTGTTTACGTAGACCAGAACCAATTTAGAAGAA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; U2OS; hNPCs; hESCs; fibroblasts; A549; GM12878; LCLs; Huh7; iSLK; MSC; TIME; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147970 | ||
| mod ID: M6ASITE006942 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500102-65500103:+ | [27] | |
| Sequence | GGGTGTTTACGTAGACCAGAACCAATTTAGAAGAATACTTG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; BGC823; HepG2; U2OS; hNPCs; hESCs; fibroblasts; A549; GM12878; LCLs; Huh7; iSLK; MSC; TIME; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000544868.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147971 | ||
| mod ID: M6ASITE006943 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500174-65500175:+ | [27] | |
| Sequence | ATCAAAAAGCTACTAAAAGGACTGGTGTAATTTAAAAAAAA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; BGC823; Brain; U2OS; hNPCs; hESCs; fibroblasts; A549; GM12878; LCLs; CD8T; MM6; Huh7; Jurkat; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000544868.2 | ||
| External Link | RMBase: m6A_site_147972 | ||
| mod ID: M6ASITE006944 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500194-65500195:+ | [27] | |
| Sequence | ACTGGTGTAATTTAAAAAAAACTAAGGCAGAAGGCTTTTGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; BGC823; Brain; U2OS; hNPCs; hESCs; fibroblasts; A549; GM12878; LCLs; CD8T; MM6; Huh7; Jurkat; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000544868.2; ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147973 | ||
| mod ID: M6ASITE006945 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500275-65500276:+ | [27] | |
| Sequence | TAGTTTGAAAAATGTGAAGGACTTTCGTAACGGAAGTAATT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; BGC823; Brain; U2OS; hNPCs; hESCs; fibroblasts; A549; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000610481.1; ENST00000544868.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147974 | ||
| mod ID: M6ASITE006946 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500284-65500285:+ | [30] | |
| Sequence | AAATGTGAAGGACTTTCGTAACGGAAGTAATTCAAGATCAA | ||
| Motif Score | 2.142029762 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000534336.1; ENST00000619449.2; ENST00000610481.1 | ||
| External Link | RMBase: m6A_site_147975 | ||
| mod ID: M6ASITE006947 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500317-65500318:+ | [30] | |
| Sequence | AAGATCAAGAGTAATTACCAACTTAATGTTTTTGCATTGGA | ||
| Motif Score | 2.595904762 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000610481.1; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147976 | ||
| mod ID: M6ASITE006948 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500337-65500338:+ | [27] | |
| Sequence | ACTTAATGTTTTTGCATTGGACTTTGAGTTAAGATTATTTT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; Brain; U2OS; H1B; H1A; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000544868.2; ENST00000534336.1; ENST00000610481.1 | ||
| External Link | RMBase: m6A_site_147977 | ||
| mod ID: M6ASITE006949 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500371-65500372:+ | [27] | |
| Sequence | TTATTTTTTAAATCCTGAGGACTAGCATTAATTGACAGCTG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; Brain; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000544868.2; ENST00000534336.1; ENST00000610481.1 | ||
| External Link | RMBase: m6A_site_147978 | ||
| mod ID: M6ASITE006950 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500385-65500386:+ | [31] | |
| Sequence | CTGAGGACTAGCATTAATTGACAGCTGACCCAGGTGCTACA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | A549; AML | ||
| Seq Type List | m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000544868.2; ENST00000619449.2; ENST00000534336.1; ENST00000610481.1 | ||
| External Link | RMBase: m6A_site_147979 | ||
| mod ID: M6ASITE006951 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500392-65500393:+ | [31] | |
| Sequence | CTAGCATTAATTGACAGCTGACCCAGGTGCTACACAGAAGT | ||
| Motif Score | 2.839113095 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000610481.1; ENST00000534336.1; ENST00000619449.2; ENST00000544868.2 | ||
| External Link | RMBase: m6A_site_147980 | ||
| mod ID: M6ASITE006952 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500434-65500435:+ | [27] | |
| Sequence | GATTCAGTGAATCTAGGAAGACAGCAGCAGACAGGATTCCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; Brain; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000544868.2; ENST00000619449.2; ENST00000610481.1; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147981 | ||
| mod ID: M6ASITE006953 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500444-65500445:+ | [27] | |
| Sequence | ATCTAGGAAGACAGCAGCAGACAGGATTCCAGGAACCAGTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; Brain; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000544868.2; ENST00000534336.1; ENST00000610481.1 | ||
| External Link | RMBase: m6A_site_147982 | ||
| mod ID: M6ASITE006954 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500458-65500459:+ | [27] | |
| Sequence | CAGCAGACAGGATTCCAGGAACCAGTGTTTGATGAAGCTAG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; Brain; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000610481.1; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147983 | ||
| mod ID: M6ASITE006955 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500480-65500481:+ | [27] | |
| Sequence | CAGTGTTTGATGAAGCTAGGACTGAGGAGCAAGCGAGCAAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; BGC823; Brain; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000534336.1; ENST00000544868.2; ENST00000610481.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147984 | ||
| mod ID: M6ASITE006956 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500585-65500586:+ | [31] | |
| Sequence | GGAAGAAGGAAGGAGCGCTAACGATTTGGTGGTGAAGCTAG | ||
| Motif Score | 2.142029762 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000544868.2; ENST00000610481.1; ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147985 | ||
| mod ID: M6ASITE006957 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500718-65500719:+ | [27] | |
| Sequence | TTGTGCGTAGAGGATCCTAGACCAGCATGCCAGTGTGCCAA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; A549; HEK293T; U2OS; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000544868.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147986 | ||
| mod ID: M6ASITE006958 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500803-65500804:+ | [27] | |
| Sequence | CAATATGTTGTTTTTCTGGAACTTACTTATGGTAACCTTTT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; U2OS; hNPCs; fibroblasts; GM12878; LCLs; A549; H1299; Huh7; peripheral-blood; iSLK; MSC; TIME; TREX; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000544868.2; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147987 | ||
| mod ID: M6ASITE006959 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500807-65500808:+ | [30] | |
| Sequence | ATGTTGTTTTTCTGGAACTTACTTATGGTAACCTTTTATTT | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000544868.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147988 | ||
| mod ID: M6ASITE006960 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500817-65500818:+ | [31] | |
| Sequence | TCTGGAACTTACTTATGGTAACCTTTTATTTATTTTCTAAT | ||
| Motif Score | 2.147452381 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000534336.1; ENST00000544868.2; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147989 | ||
| mod ID: M6ASITE006961 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500856-65500857:+ | [30] | |
| Sequence | ATATAATGGGGGAGTTTCGTACTGAGGTGTAAAGGGATTTA | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000544868.2; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147990 | ||
| mod ID: M6ASITE006962 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500966-65500967:+ | [27] | |
| Sequence | TCTGAAGCTTTTGAGGGCAGACTGCCAAGTCCTGGAGAAAT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; U2OS; fibroblasts; LCLs; Huh7; iSLK; MSC; TIME; TREX; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000544868.2; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147991 | ||
| mod ID: M6ASITE006963 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501037-65501038:+ | [27] | |
| Sequence | TTTACACGAATTTGAGGAAAACCAAATGAATTTGATAGCCA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; U2OS; LCLs; iSLK; MSC; TIME; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147992 | ||
| mod ID: M6ASITE006964 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501065-65501066:+ | [27] | |
| Sequence | AATTTGATAGCCAAATTGAGACAATTTCAGCAAATCTGTAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; U2OS; LCLs; iSLK; MSC; TREX; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147993 | ||
| mod ID: M6ASITE006965 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501140-65501141:+ | [32] | |
| Sequence | TTCAGTTTTGTGAATAGATGACCTGTTTTTACTTCCTCACC | ||
| Motif Score | 2.839113095 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147994 | ||
| mod ID: M6ASITE006966 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501198-65501199:+ | [32] | |
| Sequence | ATGTAGAGTTTGGATGTGTAACTGAGGCGGGGGGGAGTTTT | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147995 | ||
| mod ID: M6ASITE006967 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501304-65501305:+ | [30] | |
| Sequence | TAGAATCCTAAAGGCAAATGACTCAAGGTGTAACAGAAAAC | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | HEK293T; AML | ||
| Seq Type List | DART-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147996 | ||
| mod ID: M6ASITE006968 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501323-65501324:+ | [27] | |
| Sequence | GACTCAAGGTGTAACAGAAAACAAGAAAATCCAATATCAGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; fibroblasts; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_147997 | ||
| mod ID: M6ASITE006969 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501352-65501353:+ | [27] | |
| Sequence | TCCAATATCAGGATAATCAGACCACCACAGGTTTACAGTTT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; fibroblasts; MT4; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147998 | ||
| mod ID: M6ASITE006970 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501379-65501380:+ | [27] | |
| Sequence | CAGGTTTACAGTTTATAGAAACTAGAGCAGTTCTCACGTTG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; fibroblasts; MT4; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_147999 | ||
| mod ID: M6ASITE006972 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501473-65501474:+ | [27] | |
| Sequence | CCCCCCACCCCCTTAATCAGACTTTAAAAGTGCTTAACCCC | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; fibroblasts; MT4; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; DART-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148000 | ||
| mod ID: M6ASITE006973 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501498-65501499:+ | [27] | |
| Sequence | AAAAGTGCTTAACCCCTTAAACTTGTTATTTTTTACTTGAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; fibroblasts; MT4; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148001 | ||
| mod ID: M6ASITE006974 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501538-65501539:+ | [33] | |
| Sequence | AGCATTTTGGGATGGTCTTAACAGGGAAGAGAGAGGGTGGG | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | HEK293; AML | ||
| Seq Type List | m6A-REF-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148002 | ||
| mod ID: M6ASITE006975 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501609-65501610:+ | [32] | |
| Sequence | CAGATGCTATAGTACTATTGACAAACTGGGTTAGAGAAGGA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148003 | ||
| mod ID: M6ASITE006976 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501613-65501614:+ | [27] | |
| Sequence | TGCTATAGTACTATTGACAAACTGGGTTAGAGAAGGAGTGT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; fibroblasts; MT4; endometrial; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148004 | ||
| mod ID: M6ASITE006977 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501655-65501656:+ | [27] | |
| Sequence | CCGCTGTGCTGTTGGCACGAACACCTTCAGGGACTGGAGCT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; fibroblasts; MT4; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148005 | ||
| mod ID: M6ASITE006978 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501667-65501668:+ | [27] | |
| Sequence | TGGCACGAACACCTTCAGGGACTGGAGCTGCTTTTATCCTT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; fibroblasts; MT4; endometrial; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148006 | ||
| mod ID: M6ASITE006979 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501763-65501764:+ | [27] | |
| Sequence | TATTCAGTCATCTCAGGAGAACTTCAGAAGAGCTTGAGTAG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; MT4; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148007 | ||
| mod ID: M6ASITE006980 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501939-65501940:+ | [27] | |
| Sequence | TAGTTTATGATTGCAGATAAACTCATGCCAGAGAACTTAAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; MT4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000534336.1; ENST00000508832.2; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148008 | ||
| mod ID: M6ASITE006981 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501953-65501954:+ | [27] | |
| Sequence | AGATAAACTCATGCCAGAGAACTTAAAGTCTTAGAATGGAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000508832.2 | ||
| External Link | RMBase: m6A_site_148009 | ||
| mod ID: M6ASITE006982 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502010-65502011:+ | [32] | |
| Sequence | AACTTCCAAGTTGGCAAGTAACTCCCAATGATTTAGTTTTT | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000616527.4; ENST00000508832.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148010 | ||
| mod ID: M6ASITE006983 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502182-65502183:+ | [27] | |
| Sequence | TTGGGGAAGGAAAGTATTGAACTGGGGGTTGGTCTGGCCTA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; HepG2; AML | ||
| Seq Type List | m6A-seq; DART-seq; miCLIP | ||
| Transcript ID List | ENST00000508832.2; ENST00000534336.1; ENST00000619449.2; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148011 | ||
| mod ID: M6ASITE006984 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502211-65502212:+ | [32] | |
| Sequence | TGGTCTGGCCTACTGGGCTGACATTAACTACAATTATGGGA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000616527.4; ENST00000508832.2 | ||
| External Link | RMBase: m6A_site_148012 | ||
| mod ID: M6ASITE006985 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502310-65502311:+ | [27] | |
| Sequence | TGATTTAATAATAATTTAAAACTACTATAGAAACTGCAGAG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000508832.2; ENST00000616527.4; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148013 | ||
| mod ID: M6ASITE006986 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502313-65502314:+ | [30] | |
| Sequence | TTTAATAATAATTTAAAACTACTATAGAAACTGCAGAGCAA | ||
| Motif Score | 2.500660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000508832.2; ENST00000619449.2; ENST00000616527.4; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148014 | ||
| mod ID: M6ASITE006987 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502322-65502323:+ | [27] | |
| Sequence | AATTTAAAACTACTATAGAAACTGCAGAGCAAAGGAAGTGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000508832.2; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148015 | ||
| mod ID: M6ASITE006988 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502473-65502474:+ | [27] | |
| Sequence | TTCCATTGTTTAACTGCAAAACAAGATGTTAAGGTATGCTT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000616527.4; ENST00000534336.1; ENST00000508832.2; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148016 | ||
| mod ID: M6ASITE006989 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502522-65502523:+ | [27] | |
| Sequence | TTGTAAATTGTTTATTTTAAACTTATCTGTTTGTAAATTGT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000508832.2; ENST00000619449.2; rmsk_3569611; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148017 | ||
| mod ID: M6ASITE006990 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502617-65502618:+ | [27] | |
| Sequence | TTGTTGATGAGGGAGGGGAAACTTTTTTTTTTTCTATAGAC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000508832.2; ENST00000616527.4; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148018 | ||
| mod ID: M6ASITE006991 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502636-65502637:+ | [27] | |
| Sequence | AACTTTTTTTTTTTCTATAGACTTTTTTCAGATAACATCTT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000508832.2; ENST00000534336.1; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148019 | ||
| mod ID: M6ASITE006992 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502667-65502668:+ | [32] | |
| Sequence | ATAACATCTTCTGAGTCATAACCAGCCTGGCAGTATGATGG | ||
| Motif Score | 2.147452381 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000508832.2; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148020 | ||
| mod ID: M6ASITE006993 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502704-65502705:+ | [27] | |
| Sequence | ATGGCCTAGATGCAGAGAAAACAGCTCCTTGGTGAATTGAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; HepG2; hNPCs; fibroblasts; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000508832.2; ENST00000534336.1; ENST00000616527.4; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148021 | ||
| mod ID: M6ASITE006994 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502777-65502778:+ | [31] | |
| Sequence | TCCATTGGGGAATAAGCATAACCCTGAGATTCTTACTACTG | ||
| Motif Score | 2.147452381 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000534336.1; ENST00000508832.2; ENST00000618925.1; ENST00000619449.2; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148022 | ||
| mod ID: M6ASITE006995 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502804-65502805:+ | [27] | |
| Sequence | GATTCTTACTACTGATGAGAACATTATCTGCATATGCCAAA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; brain; kidney; liver; HEK293T; HepG2; hNPCs; fibroblasts; CD8T; A549; iSLK; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; m6A-REF-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000616527.4; ENST00000534336.1; ENST00000508832.2; ENST00000619449.2; ENST00000618925.1 | ||
| External Link | RMBase: m6A_site_148023 | ||
| mod ID: M6ASITE006996 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503116-65503117:+ | [27] | |
| Sequence | CAATGTTTCGTTTGCCTCAGACAGGTATCTCTTCGTTATCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HEK293; hNPCs; fibroblasts; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; m6A-REF-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000508832.2; ENST00000618925.1; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148024 | ||
| mod ID: M6ASITE006997 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503170-65503171:+ | [27] | |
| Sequence | ATTTCATCTGGGAGCAGAAAACAGCAGGCAGCTGTTAACAG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000618925.1; ENST00000534336.1; ENST00000616527.4; ENST00000508832.2; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148025 | ||
| mod ID: M6ASITE006998 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503354-65503355:+ | [27] | |
| Sequence | AGCCTTTTTAAAATTGTAGGACTTGTTCCTGTGGGCTTCAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000616527.4; ENST00000619449.2; ENST00000618925.1; ENST00000508832.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148026 | ||
| mod ID: M6ASITE006999 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503583-65503584:+ | [27] | |
| Sequence | TGATGTAAATTGTGTAGAAAACCATTAAATCATTCAAAATA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000616527.4; ENST00000618925.1; ENST00000534336.1; ENST00000508832.2 | ||
| External Link | RMBase: m6A_site_148027 | ||
| mod ID: M6ASITE007000 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503608-65503609:+ | [27] | |
| Sequence | TAAATCATTCAAAATAATAAACTATTTTTATTAGAGAATGT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000618925.1; ENST00000508832.2; ENST00000534336.1; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148028 | ||
| mod ID: M6ASITE007001 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503730-65503731:+ | [27] | |
| Sequence | TCTTCTCTAATCTTTCAGAAACTTTGTCTGCGAACACTCTT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000618925.1; ENST00000619449.2; ENST00000612781.1; ENST00000620465.4; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148029 | ||
| mod ID: M6ASITE007002 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503743-65503744:+ | [27] | |
| Sequence | TTCAGAAACTTTGTCTGCGAACACTCTTTAATGGACCAGAT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000618925.1; ENST00000534336.1; ENST00000620465.4; ENST00000616527.4; ENST00000612781.1 | ||
| External Link | RMBase: m6A_site_148030 | ||
| mod ID: M6ASITE007003 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503757-65503758:+ | [27] | |
| Sequence | CTGCGAACACTCTTTAATGGACCAGATCAGGATTTGAGCGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000612781.1; ENST00000620465.4; ENST00000618925.1; ENST00000619449.2; ENST00000534336.1; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148031 | ||
| mod ID: M6ASITE007004 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503808-65503809:+ | [27] | |
| Sequence | GTAACTTTAAGGCAGGAAAGACAAATTTTATTCTTCATAAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000612781.1; ENST00000616527.4; ENST00000620465.4 | ||
| External Link | RMBase: m6A_site_148032 | ||
| mod ID: M6ASITE007005 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503901-65503902:+ | [27] | |
| Sequence | AGGAATAAATAACCTCTTAGACAGGTGGGAGATTATGATCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000616527.4; ENST00000612781.1; ENST00000620465.4 | ||
| External Link | RMBase: m6A_site_148033 | ||
| mod ID: M6ASITE007006 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503976-65503977:+ | [32] | |
| Sequence | AGTCAGGGGTCTATAAATTGACAGTGATTAGAGTAATACTT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000616527.4; ENST00000612781.1; ENST00000534336.1; ENST00000619449.2; ENST00000620465.4 | ||
| External Link | RMBase: m6A_site_148034 | ||
| mod ID: M6ASITE007007 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504037-65504038:+ | [30] | |
| Sequence | CATGTTAACTTTAAATGCTTACAATCTTAGAGTGGTAGGCA | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000618132.1; ENST00000620465.4; ENST00000616527.4; ENST00000612781.1 | ||
| External Link | RMBase: m6A_site_148035 | ||
| mod ID: M6ASITE007008 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504219-65504220:+ | [27] | |
| Sequence | TGTTCTAGTGAGTGTATGAGACCTTGCAGTGAGTTTATCAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000620465.4; ENST00000616527.4; ENST00000619449.2; ENST00000534336.1; ENST00000618132.1; ENST00000612781.1 | ||
| External Link | RMBase: m6A_site_148036 | ||
| mod ID: M6ASITE007009 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504325-65504326:+ | [27] | |
| Sequence | AGCTTTTTTTTTTTTTACAGACTTCACAGAGAATGCAGTTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000618132.1; ENST00000620465.4; ENST00000612781.1; ENST00000534336.1; ENST00000616527.4 | ||
| External Link | RMBase: m6A_site_148037 | ||
| mod ID: M6ASITE007010 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504633-65504634:+ | [27] | |
| Sequence | ATTTCTGGTGGTGGGAGGGGACTGAAGCCTTTAGTCTTTTC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000610851.1; ENST00000613376.1; ENST00000612781.1; ENST00000618132.1; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148038 | ||
| mod ID: M6ASITE007011 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504677-65504678:+ | [32] | |
| Sequence | ATGCAACCTTAAAATCAGTGACAAGAAACATTCCAAACAAG | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000613376.1; ENST00000618132.1; ENST00000612781.1; ENST00000534336.1; ENST00000610851.1 | ||
| External Link | RMBase: m6A_site_148039 | ||
| mod ID: M6ASITE007013 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504684-65504685:+ | [27] | |
| Sequence | CTTAAAATCAGTGACAAGAAACATTCCAAACAAGCAACAGT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000612781.1; ENST00000534336.1; ENST00000613376.1; ENST00000619449.2; ENST00000610851.1; ENST00000618132.1 | ||
| External Link | RMBase: m6A_site_148040 | ||
| mod ID: M6ASITE007014 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504693-65504694:+ | [27] | |
| Sequence | AGTGACAAGAAACATTCCAAACAAGCAACAGTCTTCAAGAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000613376.1; ENST00000610851.1; ENST00000618132.1; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148041 | ||
| mod ID: M6ASITE007015 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504719-65504720:+ | [27] | |
| Sequence | AACAGTCTTCAAGAAATTAAACTGGCAAGTGGAAATGTTTA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610851.1; ENST00000619449.2; ENST00000618132.1; ENST00000534336.1; ENST00000613376.1 | ||
| External Link | RMBase: m6A_site_148042 | ||
| mod ID: M6ASITE007016 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504741-65504742:+ | [27] | |
| Sequence | TGGCAAGTGGAAATGTTTAAACAGTTCAGTGATCTTTAGTG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613376.1; ENST00000619449.2; ENST00000534336.1; ENST00000610851.1; ENST00000618132.1 | ||
| External Link | RMBase: m6A_site_148043 | ||
| mod ID: M6ASITE007017 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504821-65504822:+ | [27] | |
| Sequence | CTTAATTCTTACATGCAGGAACACTCAGCAGACACACGTAT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000613376.1; ENST00000619449.2; ENST00000610851.1; ENST00000618132.1; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148044 | ||
| mod ID: M6ASITE007018 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504832-65504833:+ | [27] | |
| Sequence | CATGCAGGAACACTCAGCAGACACACGTATGCGAAGGGCCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000613376.1; ENST00000618132.1; ENST00000610851.1 | ||
| External Link | RMBase: m6A_site_148045 | ||
| mod ID: M6ASITE007019 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504863-65504864:+ | [27] | |
| Sequence | CGAAGGGCCAGAGAAGCCAGACCCAGTAAGAAAAAATAGCC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610851.1; ENST00000618227.1; ENST00000619449.2; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148046 | ||
| mod ID: M6ASITE007020 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504900-65504901:+ | [27] | |
| Sequence | AGCCTATTTACTTTAAATAAACCAAACATTCCATTTTAAAT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610851.1; ENST00000619449.2; ENST00000534336.1; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148047 | ||
| mod ID: M6ASITE007021 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504905-65504906:+ | [27] | |
| Sequence | ATTTACTTTAAATAAACCAAACATTCCATTTTAAATGTGGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000618227.1; ENST00000610851.1 | ||
| External Link | RMBase: m6A_site_148048 | ||
| mod ID: M6ASITE007022 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504934-65504935:+ | [27] | |
| Sequence | TTTAAATGTGGGGATTGGGAACCACTAGTTCTTTCAGATGG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000618227.1; ENST00000610851.1; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148049 | ||
| mod ID: M6ASITE007024 | Click to Show/Hide the Full List | ||
| mod site | chr11:65504965-65504966:+ | [27] | |
| Sequence | TTTCAGATGGTATTCTTCAGACTATAGAAGGAGCTTCCAGT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000618227.1; ENST00000534336.1; ENST00000610851.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148050 | ||
| mod ID: M6ASITE007025 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505001-65505002:+ | [27] | |
| Sequence | CCAGTTGAATTCACCAGTGGACAAAATGAGGAAAACAGGTG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000618227.1; ENST00000619449.2; ENST00000534336.1; ENST00000610851.1 | ||
| External Link | RMBase: m6A_site_148051 | ||
| mod ID: M6ASITE007026 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505015-65505016:+ | [27] | |
| Sequence | CAGTGGACAAAATGAGGAAAACAGGTGAACAAGCTTTTTCT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000610851.1; ENST00000618227.1; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148052 | ||
| mod ID: M6ASITE007027 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505023-65505024:+ | [27] | |
| Sequence | AAAATGAGGAAAACAGGTGAACAAGCTTTTTCTGTATTTAC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000610851.1; ENST00000619449.2; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148053 | ||
| mod ID: M6ASITE007028 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505068-65505069:+ | [27] | |
| Sequence | AAAGTCAGATCAGTTATGGGACAATAGTATTGAATAGATTT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610851.1; ENST00000618227.1; ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148054 | ||
| mod ID: M6ASITE007029 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505123-65505124:+ | [27] | |
| Sequence | AGTAACTGGCATGTGAGCAAACTGTGTTGGCGTGGGGGTGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000618227.1; ENST00000619449.2; ENST00000610851.1 | ||
| External Link | RMBase: m6A_site_148055 | ||
| mod ID: M6ASITE007030 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505223-65505224:+ | [27] | |
| Sequence | CACCGAAGGCTTAAAGTAGGACAACCATGGAGCCTTCCTGT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000534336.1; ENST00000617489.1; ENST00000618227.1; ENST00000619449.2; ENST00000610851.1; ENST00000616691.1 | ||
| External Link | RMBase: m6A_site_148056 | ||
| mod ID: M6ASITE007031 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505254-65505255:+ | [27] | |
| Sequence | GCCTTCCTGTGGCAGGAGAGACAACAAAGCGCTATTATCCT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000617489.1; ENST00000618227.1; ENST00000619449.2; ENST00000616691.1; ENST00000610851.1 | ||
| External Link | RMBase: m6A_site_148057 | ||
| mod ID: M6ASITE007032 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505323-65505324:+ | [29] | |
| Sequence | GATTTTTATTAGTAATGAGGACTTGCCTCAACTCCCTCTTT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000617489.1; ENST00000618227.1; ENST00000610851.1; ENST00000534336.1; ENST00000619449.2; ENST00000616691.1 | ||
| External Link | RMBase: m6A_site_148058 | ||
| mod ID: M6ASITE007033 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505556-65505557:+ | [33] | |
| Sequence | CCACAAGCAACTTCTCTGCCACATCGCCACCCCGTGCCTTT | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000616691.1; ENST00000610851.1; ENST00000617489.1; ENST00000619449.2; ENST00000534336.1; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148059 | ||
| mod ID: M6ASITE007035 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505590-65505591:+ | [29] | |
| Sequence | TGCCTTTTGATCTAGCACAGACCCTTCACCCCTCACCTCGA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; HepG2; MT4; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000617489.1; ENST00000610851.1; ENST00000534336.1; ENST00000619449.2; ENST00000616691.1; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148060 | ||
| mod ID: M6ASITE007036 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505695-65505696:+ | [27] | |
| Sequence | CGAGGTCTTTGGTGGGTTGAACTATGTTAGAAAAGGCCATT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000610851.1; ENST00000534336.1; ENST00000617489.1; ENST00000619449.2; ENST00000618227.1; ENST00000616691.1 | ||
| External Link | RMBase: m6A_site_148061 | ||
| mod ID: M6ASITE007037 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505736-65505737:+ | [32] | |
| Sequence | AATTTGCCTGCAAATTGTTAACAGAAGGGTATTAAAACCAC | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000618227.1; ENST00000610851.1; ENST00000617489.1; ENST00000616691.1 | ||
| External Link | RMBase: m6A_site_148062 | ||
| mod ID: M6ASITE007038 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505752-65505753:+ | [27] | |
| Sequence | GTTAACAGAAGGGTATTAAAACCACAGCTAAGTAGCTCTAT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000618227.1; ENST00000616691.1; ENST00000619449.2; ENST00000617489.1; ENST00000610851.1; ENST00000534336.1 | ||
| External Link | RMBase: m6A_site_148063 | ||
| mod ID: M6ASITE007039 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505791-65505792:+ | [32] | |
| Sequence | ATTATAATACTTATCCAGTGACTAAAACCAACTTAAACCAG | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | AML | ||
| Seq Type List | miCLIP | ||
| Transcript ID List | ENST00000617489.1; ENST00000619449.2; ENST00000534336.1; ENST00000610851.1; ENST00000616691.1; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148064 | ||
| mod ID: M6ASITE007040 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505797-65505798:+ | [27] | |
| Sequence | ATACTTATCCAGTGACTAAAACCAACTTAAACCAGTAAGTG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000617489.1; ENST00000618227.1; ENST00000619449.2; ENST00000610851.1; ENST00000534336.1; ENST00000616691.1 | ||
| External Link | RMBase: m6A_site_148065 | ||
| mod ID: M6ASITE007041 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505807-65505808:+ | [27] | |
| Sequence | AGTGACTAAAACCAACTTAAACCAGTAAGTGGAGAAATAAC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000616691.1; ENST00000534336.1; ENST00000618227.1; ENST00000619449.2; ENST00000610851.1; ENST00000617489.1 | ||
| External Link | RMBase: m6A_site_148066 | ||
| mod ID: M6ASITE007042 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505838-65505839:+ | [27] | |
| Sequence | GAGAAATAACATGTTCAAGAACTGTAATGCTGGGTGGGAAC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000616691.1; ENST00000617489.1; ENST00000619449.2; ENST00000534336.1; ENST00000610851.1; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148067 | ||
| mod ID: M6ASITE007043 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505857-65505858:+ | [27] | |
| Sequence | AACTGTAATGCTGGGTGGGAACATGTAACTTGTAGACTGGA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000610851.1; ENST00000617489.1; ENST00000618227.1; ENST00000619449.2; ENST00000534336.1; ENST00000616691.1 | ||
| External Link | RMBase: m6A_site_148068 | ||
| mod ID: M6ASITE007044 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505872-65505873:+ | [27] | |
| Sequence | TGGGAACATGTAACTTGTAGACTGGAGAAGATAGGCATTTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000617489.1; ENST00000619449.2; ENST00000534336.1; ENST00000618227.1; ENST00000610851.1; ENST00000616691.1 | ||
| External Link | RMBase: m6A_site_148069 | ||
| mod ID: M6ASITE007046 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505941-65505942:+ | [27] | |
| Sequence | TGCAAAAATTCTCTGCTAAGACTTTTTCAGGTGAACATAAC | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000617489.1; ENST00000534336.1; ENST00000610851.1; ENST00000619449.2; ENST00000616691.1; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148070 | ||
| mod ID: M6ASITE007047 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505955-65505956:+ | [29] | |
| Sequence | GCTAAGACTTTTTCAGGTGAACATAACAGACTTGGCCAAGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34; HepG2; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000616691.1; ENST00000610851.1; ENST00000534336.1; ENST00000619449.2; ENST00000618227.1; ENST00000617489.1 | ||
| External Link | RMBase: m6A_site_148071 | ||
| mod ID: M6ASITE007048 | Click to Show/Hide the Full List | ||
| mod site | chr11:65505964-65505965:+ | [29] | |
| Sequence | TTTTCAGGTGAACATAACAGACTTGGCCAAGCTAGCATCTT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | CD34; HepG2; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000610851.1; ENST00000616691.1; ENST00000619449.2; ENST00000617489.1; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148072 | ||
| mod ID: M6ASITE007049 | Click to Show/Hide the Full List | ||
| mod site | chr11:65506047-65506048:+ | [27] | |
| Sequence | GTTTTTCTTTTCCTGAGAAAACAACACGTATTGTTTTCTCA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEC-1-A | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000616691.1; ENST00000534336.1; ENST00000617489.1; ENST00000610851.1; ENST00000618227.1 | ||
| External Link | RMBase: m6A_site_148073 | ||
| mod ID: M6ASITE007050 | Click to Show/Hide the Full List | ||
| mod site | chr11:65506148-65506149:+ | [31] | |
| Sequence | TGGCACTCCTGGTTTCCAGGACGGGGTTCAAATCCCTGCGG | ||
| Motif Score | 3.616982143 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000618227.1; ENST00000616691.1; ENST00000610851.1; ENST00000534336.1; ENST00000617489.1; ENST00000611300.1; ENST00000619449.2 | ||
| External Link | RMBase: m6A_site_148074 | ||
| mod ID: M6ASITE007051 | Click to Show/Hide the Full List | ||
| mod site | chr11:65506287-65506288:+ | [29] | |
| Sequence | TAACAGCACAATATCTTTGAACTATATACATCCTTGATGTA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2; ENST00000616691.1; ENST00000610851.1; ENST00000618227.1; ENST00000617489.1 | ||
| External Link | RMBase: m6A_site_148075 | ||
N7-methylguanosine (m7G)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: m7GSITE000009 | Click to Show/Hide the Full List | ||
| mod site | chr11:65500854-65500855:+ | [34] | |
| Sequence | TAATATAATGGGGGAGTTTCGTACTGAGGTGTAAAGGGATT | ||
| Cell/Tissue List | A549; HeLa; HepG2 | ||
| Seq Type List | BoRed-seq&m7G-RIP-seq; m7G-seq | ||
| Transcript ID List | ENST00000619449.2; ENST00000534336.1; ENST00000544868.2 | ||
| External Link | RMBase: m7G_site_167 | ||
Pseudouridine (Pseudo)
| In total 4 m6A sequence/site(s) in this target gene | |||
| mod ID: PSESITE000325 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501134-65501135:+ | [35] | |
| Sequence | AAGTATTTCAGTTTTGTGAATAGATGACCTGTTTTTACTTC | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: Pseudo_site_931 | ||
| mod ID: PSESITE000326 | Click to Show/Hide the Full List | ||
| mod site | chr11:65501280-65501281:+ | [36] | |
| Sequence | AGTTCTTTTTCCCTTAGGTCTGTCTAGAATCCTAAAGGCAA | ||
| Transcript ID List | ENST00000534336.1; ENST00000619449.2 | ||
| External Link | RMBase: Pseudo_site_932 | ||
| mod ID: PSESITE000327 | Click to Show/Hide the Full List | ||
| mod site | chr11:65502920-65502921:+ | [37] | |
| Sequence | ATAACCACAAAAATAATGAATTGATGAGAAATACAATGAAG | ||
| Transcript ID List | ENST00000619449.2; ENST00000508832.2; ENST00000534336.1; ENST00000616527.4; ENST00000618249.1; ENST00000618925.1 | ||
| External Link | RMBase: Pseudo_site_933 | ||
| mod ID: PSESITE000328 | Click to Show/Hide the Full List | ||
| mod site | chr11:65503350-65503351:+ | [37] | |
| Sequence | ATCGAGCCTTTTTAAAATTGTAGGACTTGTTCCTGTGGGCT | ||
| Transcript ID List | ENST00000534336.1; ENST00000618925.1; ENST00000616527.4; ENST00000508832.2; ENST00000619449.2 | ||
| External Link | RMBase: Pseudo_site_934 | ||
References