m6A Regulator Information
General Information of the m6A Regulator (ID: REG00002)
| Regulator Name | ELAV-like protein 1 (ELAVL1) | ||||
|---|---|---|---|---|---|
| Synonyms |
Hu-antigen R; HuR; HUR
Click to Show/Hide
|
||||
| Gene Name | ELAVL1 | ||||
| Sequence |
MSNGYEDHMAEDCRGDIGRTNLIVNYLPQNMTQDELRSLFSSIGEVESAKLIRDKVAGHS
LGYGFVNYVTAKDAERAINTLNGLRLQSKTIKVSYARPSSEVIKDANLYISGLPRTMTQK DVEDMFSRFGRIINSRVLVDQTTGLSRGVAFIRFDKRSEAEEAITSFNGHKPPGSSEPIT VKFAANPNQNKNVALLSQLYHSPARRFGGPVHHQAQRFRFSPMGVDHMSGLSGVNVPGNA SSGWCIFIYNLGQDADEGILWQMFGPFGAVTNVKVIRDFNTNKCKGFGFVTMTNYEEAAM AIASLNGYRLGDKILQVSFKTNKSHK Click to Show/Hide
|
||||
| Family | RRM elav family | ||||
| Function |
RNA-binding protein that binds to the 3'-UTR region of mRNAs and increases their stability. Involved in embryonic stem cells (ESCs) differentiation: preferentially binds mRNAs that are not methylated by N6-methyladenosine (m6A), stabilizing them, promoting ESCs differentiation (By similarity). Binds to poly-U elements and AU-rich elements (AREs) in the 3'-UTR of target mRNAs. Binds avidly to the AU-rich element in FOS and IL3/interleukin-3 mRNAs. In the case of the FOS AU-rich element, binds to a core element of 27 nucleotides that contain AUUUA, AUUUUA, and AUUUUUA motifs. Binds preferentially to the 5'-UUUU[AG]UUU-3' motif in vitro. With ZNF385A, binds the 3'-UTR of p53/TP53 mRNA to control their nuclear export induced by CDKN2A. Hence, may regulate p53/TP53 expression and mediate in part the CDKN2A anti-proliferative activity. May also bind with ZNF385A the CCNB1 mRNA (By similarity). Increases the stability of the leptin mRNA harboring an AU-rich element (ARE) in its 3' UTR.
Click to Show/Hide
|
||||
| Gene ID | 1994 | ||||
| Uniprot ID | |||||
| Regulator Type | WRITER ERASER READER | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
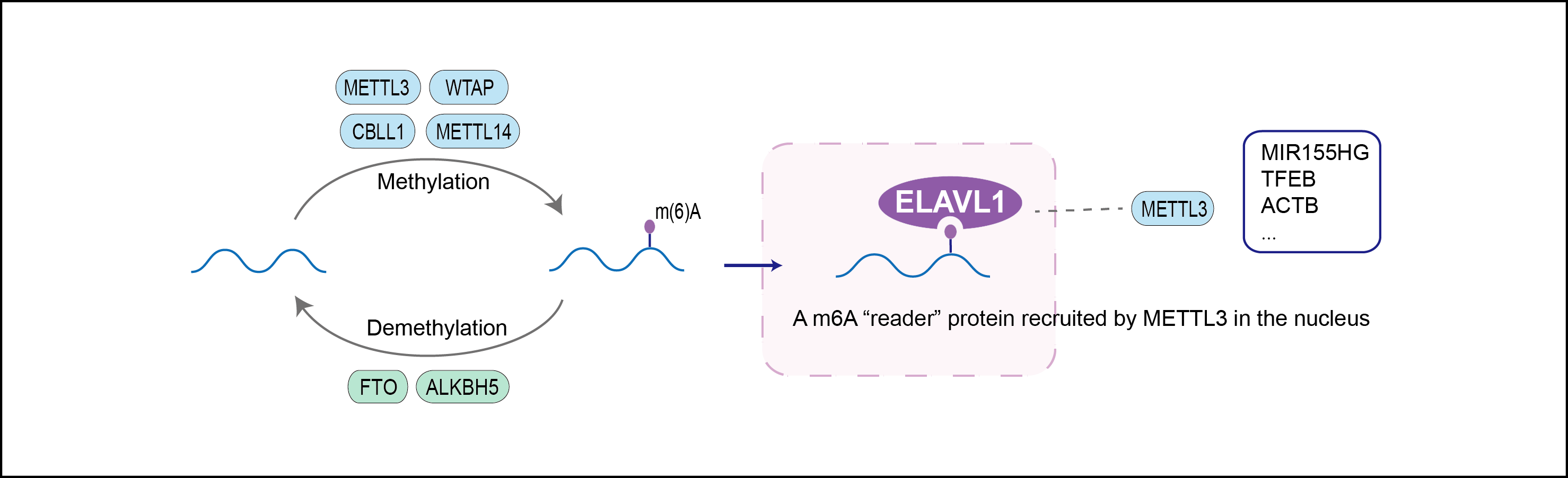
|
|||||
Full List of Target Gene(s) of This m6A Regulator and Corresponding Disease/Drug Response(s)
ELAVL1 can regulate the m6A methylation of following target genes, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulted from the regulation of certain target gene.
Browse Target Gene related Disease
Cyclin-dependent kinase 4 (CDK4)
Liver cancer [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [1] | |||
| Responsed Disease | Liver cancer [ICD-11: 2C12] | |||
| Target Regulation | Up regulation | |||
Developmentally-regulated GTP-binding protein 1 (DRG1)
Osteosarcoma [ICD-11: 2B51]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [2] | |||
| Responsed Disease | Osteosarcoma [ICD-11: 2B51] | |||
| Target Regulation | Up regulation | |||
| Cell Process | Cell cycle | |||
| Cell apoptosis | ||||
In-vitro Model |
143B | Osteosarcoma | Homo sapiens | CVCL_2270 |
| hFOB 1.19 | Normal | Homo sapiens | CVCL_3708 | |
| MG-63 | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| SaOS-2 | Osteosarcoma | Homo sapiens | CVCL_0548 | |
| U2OS | Osteosarcoma | Homo sapiens | CVCL_0042 | |
| Response Summary | ELAVL1 knockdown impaired the stability of DRG1 mRNA, thereby reducing both the mRNA and protein levels of Developmentally-regulated GTP-binding protein 1 (DRG1). In all, DRG1 exerted tumorigenic effects in osteosarcoma, and the up-regulation of DRG1 in OS was induced by METTL3 and ELAVL1 in an m6A-dependent manner. | |||
DNA (cytosine-5)-methyltransferase 3B (DNMT3B)
Acute ischemic stroke [ICD-11: 8B11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [3] | |||
| Responsed Disease | Acute ischemic stroke [ICD-11: 8B11] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
PC12 | Rat adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 |
| In-vivo Model | The left internal carotid artery of the rats was isolated. Then the ligation of middle cerebral artery was performed by a 4/0 surgical nylon monofilament to occlude the blood flow. 2 h later, we removed the filament to restore the blood reperfusion for 24 h. During the entire operation, they were kept at 37.0 ± 0.5 °C. The laser Doppler flowmetry (Transonic Systems, Ithaca, NY, USA) was applied to confirm the regional ischemia and reperfusion. Rats were decapitated after 24 h of reperfusion. | |||
Hepatoma-derived growth factor (HDGF)
Bladder cancer [ICD-11: 2C94]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [4] | |||
| Responsed Disease | Bladder cancer [ICD-11: 2C94] | |||
| Target Regulation | Up regulation | |||
| Cell Process | mRNA stability | |||
Zinc finger E-box-binding homeobox 1 (ZEB1)
Triple-negative breast cancer [ICD-11: 2C6Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [5] | |||
| Responsed Disease | Triple-negative breast cancer [ICD-11: 2C6Z] | |||
In-vitro Model |
MDA-MB-453 | Breast adenocarcinoma | Homo sapiens | CVCL_0418 |
| MDA-MB-436 | Invasive breast carcinoma of no special type | Homo sapiens | CVCL_0623 | |
14-3-3 protein zeta/delta (YWHAZ)
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [6] | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
MGC-803 | Gastric mucinous adenocarcinoma | Homo sapiens | CVCL_5334 |
| AGS | Gastric adenocarcinoma | Homo sapiens | CVCL_0139 | |
| HGC-27 | Gastric carcinoma | Homo sapiens | CVCL_1279 | |
| MKN28 | Gastric tubular adenocarcinoma | Homo sapiens | CVCL_1416 | |
| MKN45 | Gastric adenocarcinoma | Homo sapiens | CVCL_0434 | |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| BGC-823 | Gastric carcinoma | Homo sapiens | CVCL_3360 | |
| GES-1 | Normal | Homo sapiens | CVCL_EQ22 | |
Anoctamin-7 (ANO7)
Prostate cancer [ICD-11: 2C82]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Prostate cancer [ICD-11: 2C82] | |||
| Target Regulation | Up regulation | |||
In-vitro Model |
WPMY-1 | Normal | Homo sapiens | CVCL_3814 |
| LNCaP | Prostate carcinoma | Homo sapiens | CVCL_0395 | |
| 22Rv1 | Prostate carcinoma | Homo sapiens | CVCL_1045 | |
| DU145 | Prostate carcinoma | Homo sapiens | CVCL_0105 | |
| LNCaP C4-2B | Prostate carcinoma | Homo sapiens | CVCL_4784 | |
| PC-3 | Prostate carcinoma | Homo sapiens | CVCL_0035 | |
| LNCaP C4-2 | Prostate carcinoma | Homo sapiens | CVCL_4782 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| In-vivo Model | Ten mice were randomly divided into two groups, and C4-2-pZXE-circDDIT4 or C4-2-pZXE-NC cell suspension was concentrated to 5 × 106 cells/100 μL PBS and then injected into the flanks of the nude mice. After 20 days, the mice were euthanized, and the xenograft tumors were harvested, weighed, and photographed. | |||
Cysteine methyltransferase DNMT3A (DNMT3A)
Chronic obstructive pulmonary disease [ICD-11: CA22]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [8] | |||
| Responsed Disease | Chronic obstructive pulmonary disease [ICD-11: CA22] | |||
| Target Regulation | Up regulation | |||
| In-vivo Model | Male BALB/c mice (SJA Laboratory Animal Company, Hunan, China) with age of 6-8 weeks were used in this study to establish COPD model. Mice were housed in individually ventilated cages under a pathogen-free condition, with ad libitum access to food and water. Animal welfare was monitored daily, and all efforts were made to minimize suffering. All animal procedures were conducted in accordance with the guidelines for use of laboratory animals, with approval from the Institutional Animal Care and Use Committee at Jiangxi Provincial People's Hospital (The First Affiliated Hospital of Nanchang Medical College). | |||
ZEB1 antisense RNA 1 (ZEB1-AS1)
Triple-negative breast cancer [ICD-11: 2C6Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [5] | |||
| Responsed Disease | Triple-negative breast cancer [ICD-11: 2C6Z] | |||
In-vitro Model |
MDA-MB-453 | Breast adenocarcinoma | Homo sapiens | CVCL_0418 |
| MDA-MB-436 | Invasive breast carcinoma of no special type | Homo sapiens | CVCL_0623 | |
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
RNA modification
m6A Target: Hepatoma-derived growth factor (HDGF)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00073 | ||
| Epigenetic Regulator | Y-box-binding protein 1 (YBX1) | |
| Regulated Target | Hepatoma-derived growth factor (HDGF) | |
| Crosstalk relationship | m5C → m6A | |
| Disease | Bladder cancer | |
| Crosstalk ID: M6ACROT00074 | ||
| Epigenetic Regulator | RNA cytosine C(5)-methyltransferase NSUN2 (NSUN2) | |
| Regulated Target | Hepatoma-derived growth factor (HDGF) | |
| Crosstalk relationship | m5C → m6A | |
| Disease | Bladder cancer | |
m6A Target: Cyclin-dependent kinase 6 (CDK6)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00455 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 149 (MIR149) | |
| Crosstalk relationship | A-to-I → m6A | |
| Crosstalk ID: M6ACROT00456 | ||
| Epigenetic Regulator | Methyltransferase-like protein 1 (METTL1) | |
| Regulated Target | MicroRNA 149 (MIR149) | |
| Crosstalk relationship | m7G → m6A | |
m6A Target: High mobility group protein HMGI-C (HMGA2)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00465 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 149 (MIR149) | |
| Crosstalk relationship | A-to-I → m6A | |
| Crosstalk ID: M6ACROT00466 | ||
| Epigenetic Regulator | Methyltransferase-like protein 1 (METTL1) | |
| Regulated Target | MicroRNA 149 (MIR149) | |
| Crosstalk relationship | m7G → m6A | |
m6A Target: NAD-dependent protein deacetylase sirtuin-1 (SIRT1)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00478 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 155 (MIR155) | |
| Crosstalk relationship | A-to-I → m6A | |
| Crosstalk ID: M6ACROT00517 | ||
| Epigenetic Regulator | Double-stranded RNA-specific editase 1 (ADARB1) | |
| Regulated Target | MicroRNA 221 (MIR221) | |
| Crosstalk relationship | A-to-I → m6A | |
m6A Target: Caspase-2 (CASP2)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00480 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 155 (MIR155) | |
| Crosstalk relationship | A-to-I → m6A | |
m6A Target: Cyclin-dependent kinase inhibitor 1B (CDKN1B/p27)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00503 | ||
| Epigenetic Regulator | Double-stranded RNA-specific editase 1 (ADARB1) | |
| Regulated Target | MicroRNA 222 (MIR222) | |
| Crosstalk relationship | A-to-I → m6A | |
| Crosstalk ID: M6ACROT00504 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 222 (MIR222) | |
| Crosstalk relationship | A-to-I → m6A | |
m6A Target: Cyclic-AMP-dependent transcription factor ATF-3 (ATF3)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00505 | ||
| Epigenetic Regulator | Double-stranded RNA-specific editase 1 (ADARB1) | |
| Regulated Target | MicroRNA 222 (MIR222) | |
| Crosstalk relationship | A-to-I → m6A | |
| Crosstalk ID: M6ACROT00506 | ||
| Epigenetic Regulator | Interferon-inducible protein 4 (ADAR1) | |
| Regulated Target | MicroRNA 222 (MIR222) | |
| Crosstalk relationship | A-to-I → m6A | |
m6A Target: ZEB1 antisense RNA 1 (ZEB1-AS1)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00551 | ||
| Epigenetic Regulator | Y-box-binding protein 1 (YBX1) | |
| Regulated Target | MicroRNA 205 (MIR205) | |
| Crosstalk relationship | m6A → m5C | |
DNA modification
m6A Target: Cysteine methyltransferase DNMT3A (DNMT3A)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT02070 | ||
| Epigenetic Regulator | Cysteine methyltransferase DNMT3A (DNMT3A) | |
| Regulated Target | Dachshund family transcription factor 1 (DACH1) | |
| Crosstalk relationship | m6A → DNA modification | |
| Disease | Chronic obstructive pulmonary disease | |
m6A Target: DNA (cytosine-5)-methyltransferase 3B (DNMT3B)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT02072 | ||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | |
| Crosstalk relationship | m6A → DNA modification | |
| Drug | Cisplatin | |
| Crosstalk ID: M6ACROT02073 | ||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | |
| Regulated Target | Serine/threonine-protein kinase PINK1, mitochondrial (PINK1) | |
| Crosstalk relationship | m6A → DNA modification | |
| Disease | Acute ischemic stroke | |
Non-coding RNA
m6A Target: 14-3-3 protein zeta/delta (YWHAZ)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05002 | ||
| Epigenetic Regulator | Small nucleolar RNA host gene 12 (SNHG12) | |
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Gastric cancer | |
m6A Target: Cyclin-dependent kinase 4 (CDK4)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05003 | ||
| Epigenetic Regulator | VPS9D1 antisense RNA 1 (VPS9D1-AS1) | |
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Liver cancer | |
m6A Target: Zinc finger E-box-binding homeobox 1 (ZEB1)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05023 | ||
| Epigenetic Regulator | ZEB1 antisense RNA 1 (ZEB1-AS1) | |
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Triple-negative breast cancer | |
| Crosstalk ID: M6ACROT05887 | ||
| Epigenetic Regulator | ZEB1 antisense RNA 1 (ZEB1-AS1) | |
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Triple-negative breast cancer | |
m6A Target: Anoctamin-7 (ANO7)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05078 | ||
| Epigenetic Regulator | Circ_DDIT4 | |
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Prostate cancer | |
m6A Target: Long intergenic non-protein coding RNA 336 (LINC00336)
| In total 2 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05785 | ||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 336 (LINC00336) | |
| Regulated Target | MicroRNA 6852 (MIR6852) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Lung cancer | |
| Crosstalk ID: M6ACROT05890 | ||
| Epigenetic Regulator | MicroRNA 6852 (MIR6852) | |
| Regulated Target | Cystathionine beta-synthase (CBS) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Lung cancer | |
m6A Target: Alpha-(1,3)-fucosyltransferase 4 (FUT4)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05888 | ||
| Epigenetic Regulator | HOXB cluster antisense RNA 1 (HOXB-AS1) | |
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Multiple myeloma | |
Xenobiotics Compound(s) Regulating the m6A Methylation Regulator
| Compound Name | 1-(Benzenesulfonyl)-3-phenylindole-4,5-dione | Investigative |
|---|---|---|
| Synonyms |
CHEMBL4075458; SCHEMBL19737490; BDBM50270003
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 12.8 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-7-(4-methoxyphenyl)sulfanyl-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4064932; BDBM50269995
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 15 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-7-(4-methoxyphenyl)-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4095060; SCHEMBL22130724; BDBM50269992
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 41 nM
|
[9] |
| Compound Name | 1-(4-Fluorophenyl)sulfonyl-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4100171; SCHEMBL21222486; BDBM50269999
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 48 nM
|
[9] |
| Compound Name | Dihydrotanshinone I | Investigative |
| Synonyms |
Dihydrotanshinone I; 87205-99-0; 15,16-dihydrotanshinone I; UNII-562G9360V6; (-)-Dihydrotanshinone I; (1R)-1,6-dimethyl-1,2-dihydronaphtho[1,2-g][1]benzofuran-10,11-dione; DIHYDROTANSHINONE; 562G9360V6; DihydrotanshinoneI; Dihydrotanshinone-I; Tanshinone I, dihydro-; SR-05000002191; HSDB 8105; DHTS; 4m0e; CHEMBL227075; SCHEMBL13049977; DTXSID20236187; CHEBI:149872; HY-N0360; ZINC2585546; BDBM50423877; MFCD28016070; s9020; AKOS032962078; CCG-208567; Dihydrotanshinone I, >=98% (HPLC); NCGC00163651-01; NCGC00163651-06; D5379; N1844; A862726; SR-05000002191-2; SR-05000002191-3; Q21099654; (1R)-1,6-dimethyl-1,2-dihydrophenanthro[1,2-b]furan-10,11-dione; (R)-1,6-dimethyl-1,2-dihydrophenanthro[1,2-b]furan-10,11-dione; (-)-1,2-Dihydro-1,6-dimethylphenanthro[1,2-b]furan-10,11-dione;1,6-Dimethyl-1,2,10,11-tetrahydrophenanthro[1,2-b]furan-10,11-dione;4,17-Dimethyl-15-oxagona-1,3,5(10),6,8,13-hexene-11,12-dione;15,16-Dihydrotanshine I;1,6-DiMethyl-1,2-dihydrophenanthro[1,2-b]furan-10,11-dione
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 50 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-7-(3-methoxyphenyl)-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4105230; BDBM50269991
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 55 nM
|
[9] |
| Compound Name | 1-Methylsulfonyl-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4073526; SCHEMBL19737481; BDBM50270012
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 56 nM
|
[9] |
| Compound Name | N-[4-(4,5-dioxo-3-phenylindol-1-yl)sulfonylphenyl]acetamide | Investigative |
| Synonyms |
CHEMBL4094161; SCHEMBL22130699; BDBM50269996
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 81 nM
|
[9] |
| Compound Name | 1-(3-Nitrophenyl)sulfonyl-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4081086; SCHEMBL19737379; BDBM50270013
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 100 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-7-(4-nitrophenyl)-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4067653; BDBM50270007
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 100 nM
|
[9] |
| Compound Name | 1-(3-Fluorophenyl)sulfonyl-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4092808; SCHEMBL19737426; BDBM50269997
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 100 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-3-[4-(dimethylamino)phenyl]indole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4076484; SCHEMBL19737562; BDBM50270014
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 100 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-3,7-diphenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4102819; SCHEMBL21222570; BDBM50269994
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 100 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-7-methyl-3-phenylindole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4070465; SCHEMBL22130661; BDBM50269990
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 200 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-3-(3-methoxyphenyl)indole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4075620; SCHEMBL19737487; BDBM50270001
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 200 nM
|
[9] |
| Compound Name | 1-(Benzenesulfonyl)-3-(4-methoxyphenyl)indole-4,5-dione | Investigative |
| Synonyms |
CHEMBL4077265; SCHEMBL19737482; BDBM50270002
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 200 nM
|
[9] |
| Compound Name | 4-(Benzenesulfonyl)-6-phenyl-4-azatetracyclo[9.2.2.02,10.03,7]pentadeca-2(10),3(7),5,12-tetraene-8,9-dione | Investigative |
| Synonyms |
CHEMBL4073683; SCHEMBL19737542; BDBM50270004
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki > 300 nM
|
[9] |
| Compound Name | CMLD-2 | Investigative |
| Synonyms |
958843-91-9; 5,7-dimethoxy-8-(1-(4-methoxyphenyl)-3-oxo-3-(pyrrolidin-1-yl)propyl)-4-phenyl-2H-chromen-2-one; CMLD-2; MLS000879470; SMR000465530; 5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-oxo-3-pyrrolidin-1-ylpropyl]-4-phenylchromen-2-one; KUC101379N; 5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-oxo-3-(pyrrolidin-1-yl)propyl]-4-phenyl-2H-chromen-2-one; CHEMBL1499653; SCHEMBL20928323; BDBM50746; cid_16746438; HMS2210J15; AKOS037647737; NCGC00166452-01; AS-74611; HY-124828; CS-0087821; W15560; 8-[3-keto-1-(4-methoxyphenyl)-3-pyrrolidino-propyl]-5,7-dimethoxy-4-phenyl-coumarin; 5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-oxidanylidene-3-pyrrolidin-1-yl-propyl]-4-phenyl-chromen-2-one; 5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-oxo-3-(1-pyrrolidinyl)propyl]-4-phenyl-1-benzopyran-2-one
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 350 nM
|
[10] |
| Compound Name | 2-Amino-6-[2-(3,4-dihydroxyphenyl)-2-oxoethyl]sulfanyl-4-[4-(phenoxymethyl)phenyl]pyridine-3,5-dicarbonitrile | Investigative |
| Synonyms |
CHEMBL4061748
Click to Show/Hide
|
|
| External link | ||
| Activity |
IC50 = 380 nM
|
[10] |
| Compound Name | 3-(5,7-dimethoxy-2-oxo-4-phenylchromen-8-yl)-N,N-diethyl-3-(4-methoxyphenyl)propanamide | Investigative |
| Synonyms |
CHEMBL1522581; NCGC00166661-01
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 570 nM
|
[10] |
| Compound Name | 3-(5,7-dimethoxy-2-oxo-4-phenylchromen-8-yl)-N,N-diethyl-3-(3,4,5-trimethoxyphenyl)propanamide | Investigative |
| Synonyms |
MLS000879482; SMR000465537; KUC101391N; CHEMBL1330249; SCHEMBL20928107; BDBM50745; cid_16746297; HMS2220E05; HMS3331O06; NCGC00166568-01; 3-(5,7-dimethoxy-2-oxidanylidene-4-phenyl-chromen-8-yl)-N,N-diethyl-3-(3,4,5-trimethoxyphenyl)propanamide; 3-(5,7-dimethoxy-2-oxo-4-phenyl-1-benzopyran-8-yl)-N,N-diethyl-3-(3,4,5-trimethoxyphenyl)propanamide; 3-(5,7-dimethoxy-2-oxo-4-phenylchromen-8-yl)-N,N-diethyl-3-(3,4,5-trimethoxyphenyl)propanamide; N,N-diethyl-3-(2-keto-5,7-dimethoxy-4-phenyl-chromen-8-yl)-3-(3,4,5-trimethoxyphenyl)propionamide
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 590 nM
|
[10] |
| Compound Name | 8-[3-Keto-1-(4-methoxyphenyl)-3-morpholino-propyl]-5,7-dimethoxy-4-phenyl-coumarin | Investigative |
| Synonyms |
MLS000879474; SMR000465528; KUC101375N; CHEMBL1390568; BDBM51309; cid_16746461; HMS2212O19; HMS3348C12; NCGC00166769-01; 5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-morpholin-4-yl-3-oxopropyl]-4-phenylchromen-2-one; 8-[3-keto-1-(4-methoxyphenyl)-3-morpholino-propyl]-5,7-dimethoxy-4-phenyl-coumarin; 5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-(4-morpholinyl)-3-oxopropyl]-4-phenyl-1-benzopyran-2-one; 5,7-dimethoxy-8-[1-(4-methoxyphenyl)-3-morpholin-4-yl-3-oxidanylidene-propyl]-4-phenyl-chromen-2-one
Click to Show/Hide
|
|
| External link | ||
| Activity |
Ki = 800 nM
|
[10] |
References