m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00259)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
FOXO1
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Methyltransferase-like 14 (METTL14) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL14 | ||
| Cell Line | MDA-MB-231 | Homo sapiens |
|
Treatment: siMETTL14 MDA-MB-231 cells
Control: MDA-MB-231 cells
|
GSE81164 | |
| Regulation |
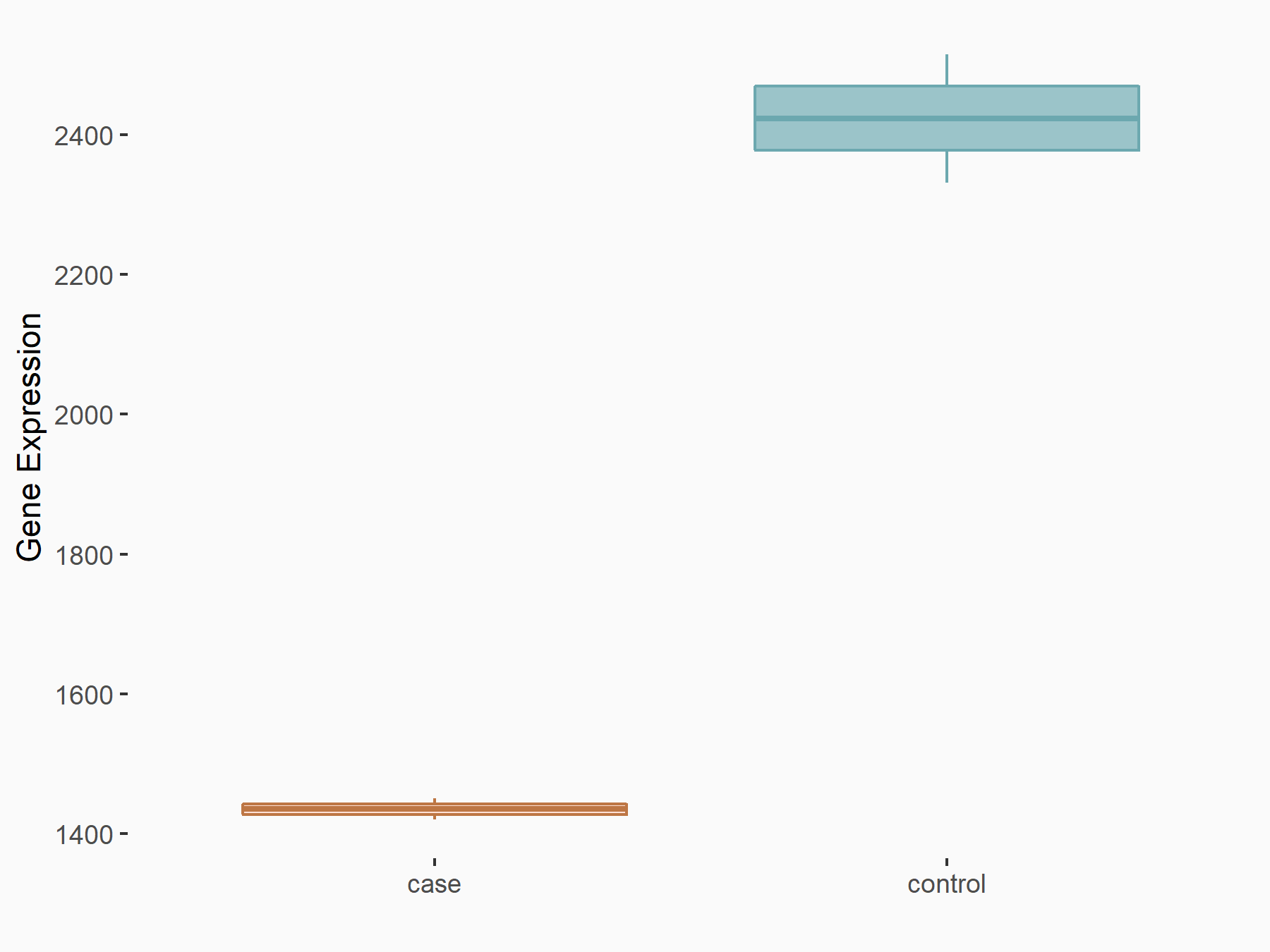  |
logFC: -7.56E-01 p-value: 2.27E-07 |
| More Results | Click to View More RNA-seq Results | |
| In total 2 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | METTL14 promotes Forkhead box protein O1 (FOXO1) expression by enhancing its m6A modification and inducing endothelial cell inflammatory response as well as atherosclerotic plaque formation. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Herpes infection | ICD-11: 1F00 | ||
| Pathway Response | FoxO signaling pathway | hsa04068 | ||
| In-vitro Model | HUVEC-C | Normal | Homo sapiens | CVCL_2959 |
| In-vivo Model | METTL14+/- mice are generated by mating wild-type mice (C57/BL6 background) with METTL14+/- mice. METTL14+/-/APOE-/- healthy offspring mice are produced by heterozygous METTL14+/- mice and heterozygous APOE-/- mice by Mendelian ratios. APOE-/- mice and C57/BL6 mice were purchased from Model Animal Research Center of Nanjing (Nanjing, Jiangsu, China). All mice were housed in the Laboratory Animals Center of the Henan Provincial People's Hospital, with controlled temperature and humidity and a 12:12-hour dark-light cycle, and were provided water and mouse chow ad libitum. | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | METTL14 promotes Forkhead box protein O1 (FOXO1) expression by enhancing its m6A modification and inducing endothelial cell inflammatory response as well as atherosclerotic plaque formation. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Atherosclerosis | ICD-11: BD40.Z | ||
| Pathway Response | FoxO signaling pathway | hsa04068 | ||
| In-vitro Model | HUVEC-C | Normal | Homo sapiens | CVCL_2959 |
| In-vivo Model | Mettl14-/+ mice are generated by mating wild-type mice (C57/BL6 background) with Mettl14-/+ mice. Mettl14-/+/APOE-/- healthy offspring mice are produced by heterozygous Mettl14-/+ mice and heterozygous APOE-/- mice by Mendelian ratios. APOE-/- mice and C57/BL6 mice were purchased from Model Animal Research Center of Nanjing (Nanjing, Jiangsu, China). All mice were housed in the Laboratory Animals Center of the Henan Provincial People's Hospital, with controlled temperature and humidity and a 12:12-hour dark-light cycle, and were provided water and mouse chow ad libitum. | |||
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | mouse embryonic stem cells | Mus musculus |
|
Treatment: METTL3-/- ESCs
Control: Wild type ESCs
|
GSE145309 | |
| Regulation |
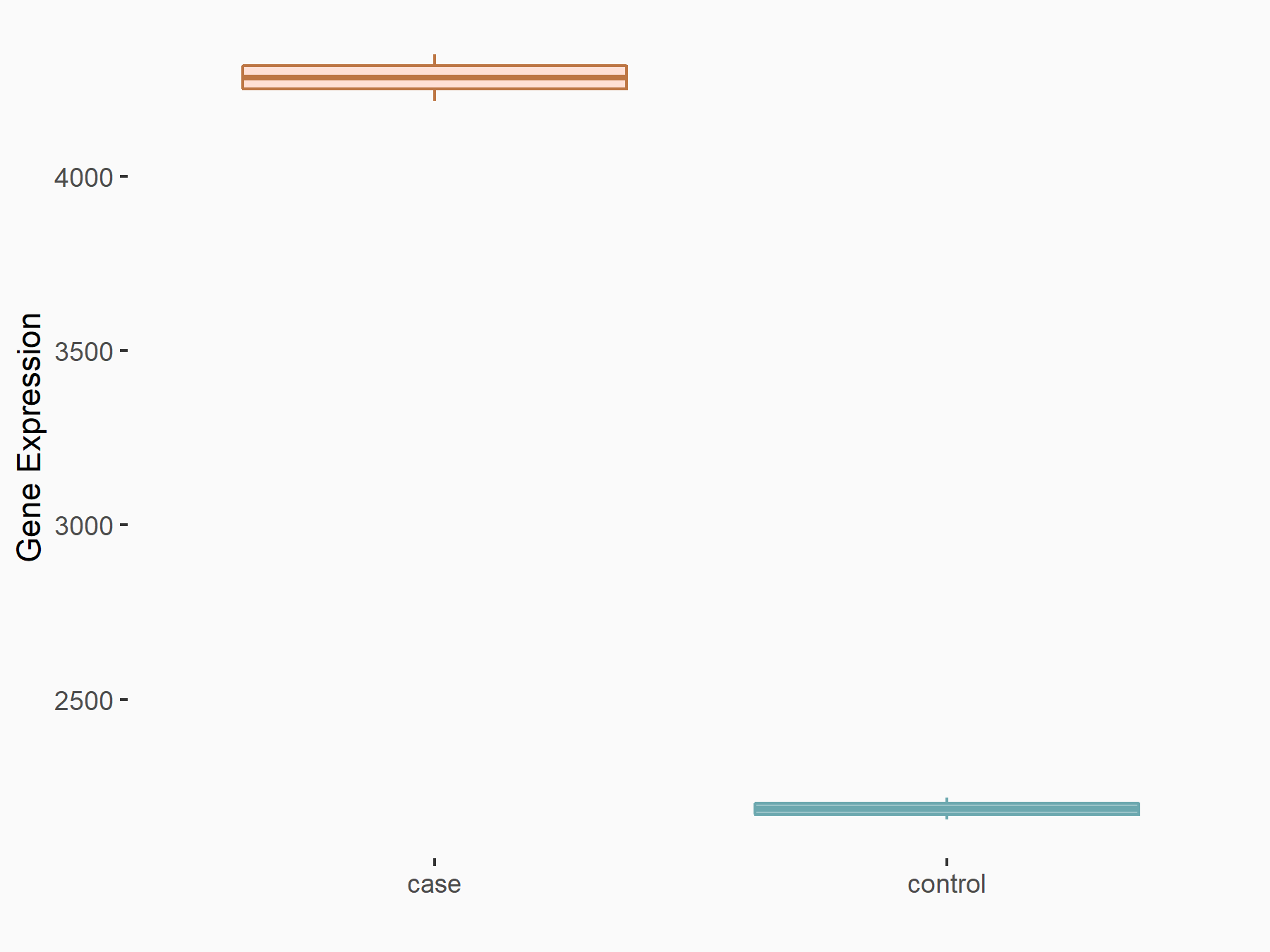  |
logFC: 9.70E-01 p-value: 7.07E-55 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | Type 2 diabetes (T2D) is characterized by lack of insulin, insulin resistance and high blood sugar. METTL3 silence decreased the m6A methylated and total mRNA level of Fatty acid synthase (Fasn), subsequently inhibited fatty acid metabolism. The expression of Acc1, Acly, Dgat2, Ehhadh, Fasn, Forkhead box protein O1 (FOXO1), Pgc1a and Sirt1, which are critical to the regulation of fatty acid synthesis and oxidation were dramatically decreased in livers of hepatocyte-specific METTL3 knockout mice. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Type 2 diabetes mellitus | ICD-11: 5A11 | ||
| Pathway Response | Insulin resistance | hsa04931 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| In-vivo Model | Hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were generated by crossing mice with TBG-Cre Tg mice. METTL3 flox (METTL3 fl/fl) and hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were used for experiments. | |||
Fat mass and obesity-associated protein (FTO) [ERASER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | Glucose Is Involved in the Dynamic Regulation of m6A in Patients with Type 2 Diabetes. High-glucose stimulation enhances FTO expression, which leads to decreased m6A, and the lower m6A induces methyltransferase upregulation; FTO then triggers the mRNA expression of Forkhead box protein O1 (FOXO1), FASN, G6PC, and DGAT2, and these four genes were correlated with glucose and lipid metabolism. | |||
| Responsed Disease | Diabetes | ICD-11: 5A10-5A14 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
Herpes infection [ICD-11: 1F00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | METTL14 promotes Forkhead box protein O1 (FOXO1) expression by enhancing its m6A modification and inducing endothelial cell inflammatory response as well as atherosclerotic plaque formation. | |||
| Responsed Disease | Herpes infection [ICD-11: 1F00] | |||
| Target Regulator | Methyltransferase-like 14 (METTL14) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | FoxO signaling pathway | hsa04068 | ||
| In-vitro Model | HUVEC-C | Normal | Homo sapiens | CVCL_2959 |
| In-vivo Model | METTL14+/- mice are generated by mating wild-type mice (C57/BL6 background) with METTL14+/- mice. METTL14+/-/APOE-/- healthy offspring mice are produced by heterozygous METTL14+/- mice and heterozygous APOE-/- mice by Mendelian ratios. APOE-/- mice and C57/BL6 mice were purchased from Model Animal Research Center of Nanjing (Nanjing, Jiangsu, China). All mice were housed in the Laboratory Animals Center of the Henan Provincial People's Hospital, with controlled temperature and humidity and a 12:12-hour dark-light cycle, and were provided water and mouse chow ad libitum. | |||
Diabetes [ICD-11: 5A10-5A14]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [3] | |||
| Response Summary | Glucose Is Involved in the Dynamic Regulation of m6A in Patients with Type 2 Diabetes. High-glucose stimulation enhances FTO expression, which leads to decreased m6A, and the lower m6A induces methyltransferase upregulation; FTO then triggers the mRNA expression of Forkhead box protein O1 (FOXO1), FASN, G6PC, and DGAT2, and these four genes were correlated with glucose and lipid metabolism. | |||
| Responsed Disease | Diabetes [ICD-11: 5A10-5A14] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
Type 2 diabetes mellitus [ICD-11: 5A11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | Type 2 diabetes (T2D) is characterized by lack of insulin, insulin resistance and high blood sugar. METTL3 silence decreased the m6A methylated and total mRNA level of Fatty acid synthase (Fasn), subsequently inhibited fatty acid metabolism. The expression of Acc1, Acly, Dgat2, Ehhadh, Fasn, Forkhead box protein O1 (FOXO1), Pgc1a and Sirt1, which are critical to the regulation of fatty acid synthesis and oxidation were dramatically decreased in livers of hepatocyte-specific METTL3 knockout mice. | |||
| Responsed Disease | Type 2 diabetes mellitus [ICD-11: 5A11] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Insulin resistance | hsa04931 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| In-vivo Model | Hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were generated by crossing mice with TBG-Cre Tg mice. METTL3 flox (METTL3 fl/fl) and hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were used for experiments. | |||
Atherosclerosis [ICD-11: BD40]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | METTL14 promotes Forkhead box protein O1 (FOXO1) expression by enhancing its m6A modification and inducing endothelial cell inflammatory response as well as atherosclerotic plaque formation. | |||
| Responsed Disease | Atherosclerosis [ICD-11: BD40.Z] | |||
| Target Regulator | Methyltransferase-like 14 (METTL14) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | FoxO signaling pathway | hsa04068 | ||
| In-vitro Model | HUVEC-C | Normal | Homo sapiens | CVCL_2959 |
| In-vivo Model | Mettl14-/+ mice are generated by mating wild-type mice (C57/BL6 background) with Mettl14-/+ mice. Mettl14-/+/APOE-/- healthy offspring mice are produced by heterozygous Mettl14-/+ mice and heterozygous APOE-/- mice by Mendelian ratios. APOE-/- mice and C57/BL6 mice were purchased from Model Animal Research Center of Nanjing (Nanjing, Jiangsu, China). All mice were housed in the Laboratory Animals Center of the Henan Provincial People's Hospital, with controlled temperature and humidity and a 12:12-hour dark-light cycle, and were provided water and mouse chow ad libitum. | |||
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
Histone modification
m6A Regulator: RNA demethylase ALKBH5 (ALKBH5)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT03370 | ||
| Epigenetic Regulator | Histone-lysine N-methyltransferase SETDB1 (SETDB1) | |
| Regulated Target | Polo like kinase 3 (PLK3) | |
| Crosstalk relationship | Histone modification → m6A | |
| Disease | Triple-negative breast cancer | |
| Drug | Doxil | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00259)
| In total 9 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE006072 | Click to Show/Hide the Full List | ||
| mod site | chr13:40511221-40511222:- | [7] | |
| Sequence | CTCAGAAACCTAGAACCAGAAATATCATTTGACCCAGCAAT | ||
| Transcript ID List | ENST00000636651.1; rmsk_3995433; ENST00000615137.1 | ||
| External Link | RMBase: RNA-editing_site_34887 | ||
| mod ID: A2ISITE006073 | Click to Show/Hide the Full List | ||
| mod site | chr13:40535091-40535092:- | [7] | |
| Sequence | CCTCCTGAGTAGCTGGGATTACAGGCATGTGCCACCACGCC | ||
| Transcript ID List | ENST00000615137.1; ENST00000636651.1 | ||
| External Link | RMBase: RNA-editing_site_34888 | ||
| mod ID: A2ISITE006074 | Click to Show/Hide the Full List | ||
| mod site | chr13:40536508-40536509:- | [7] | |
| Sequence | ATCACTTGAGGTCAGGAATTAGAGACCAGCCTGGGAAACAT | ||
| Transcript ID List | rmsk_3995493; ENST00000636651.1 | ||
| External Link | RMBase: RNA-editing_site_34889 | ||
| mod ID: A2ISITE006075 | Click to Show/Hide the Full List | ||
| mod site | chr13:40546413-40546414:- | [7] | |
| Sequence | GAGGGTCTGTTTTCCATTGGAAATGGGCCTCTCTTTTCTTA | ||
| Transcript ID List | ENST00000636651.1 | ||
| External Link | RMBase: RNA-editing_site_34890 | ||
| mod ID: A2ISITE006076 | Click to Show/Hide the Full List | ||
| mod site | chr13:40566678-40566679:- | [7] | |
| Sequence | TGAGATAAAAAATTTAAAGTAGGCCAGGCGCAGTGGCTCAC | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: RNA-editing_site_34891 | ||
| mod ID: A2ISITE006077 | Click to Show/Hide the Full List | ||
| mod site | chr13:40646501-40646502:- | [7] | |
| Sequence | TTTTATAATATTCACCTTTTAGAAGTCACAGTAGTTGGCTG | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: RNA-editing_site_34892 | ||
| mod ID: A2ISITE006078 | Click to Show/Hide the Full List | ||
| mod site | chr13:40646730-40646731:- | [8] | |
| Sequence | CGTGGTGGCACATGTCTGTAATCCCGGCTACTTGGGAGGCT | ||
| Transcript ID List | ENST00000379561.6; rmsk_3995670 | ||
| External Link | RMBase: RNA-editing_site_34893 | ||
| mod ID: A2ISITE006079 | Click to Show/Hide the Full List | ||
| mod site | chr13:40653172-40653173:- | [7] | |
| Sequence | AGTTCGAGACAAGCCTGGCAACATGGTGAAACCCTGCCTCT | ||
| Transcript ID List | rmsk_3995681; ENST00000379561.6 | ||
| External Link | RMBase: RNA-editing_site_34894 | ||
| mod ID: A2ISITE006080 | Click to Show/Hide the Full List | ||
| mod site | chr13:40655072-40655073:- | [7] | |
| Sequence | AAAGTGCTGGGATCACTGGCATGAGCCCCCACGCCCTGCCG | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: RNA-editing_site_34895 | ||
5-methylcytidine (m5C)
| In total 15 m6A sequence/site(s) in this target gene | |||
| mod ID: M5CSITE000156 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665903-40665904:- | [9] | |
| Sequence | GCGGCCGCCGCGGCCGCCACCGGGGGGCTGTGCGGGGACTT | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11797 | ||
| mod ID: M5CSITE000157 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665904-40665905:- | [9] | |
| Sequence | GGCGGCCGCCGCGGCCGCCACCGGGGGGCTGTGCGGGGACT | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11798 | ||
| mod ID: M5CSITE000158 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665906-40665907:- | [9] | |
| Sequence | GCGGCGGCCGCCGCGGCCGCCACCGGGGGGCTGTGCGGGGA | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11799 | ||
| mod ID: M5CSITE000160 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665907-40665908:- | [9] | |
| Sequence | GGCGGCGGCCGCCGCGGCCGCCACCGGGGGGCTGTGCGGGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11800 | ||
| mod ID: M5CSITE000161 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665909-40665910:- | [9] | |
| Sequence | GCGGCGGCGGCCGCCGCGGCCGCCACCGGGGGGCTGTGCGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11801 | ||
| mod ID: M5CSITE000162 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665910-40665911:- | [9] | |
| Sequence | GGCGGCGGCGGCCGCCGCGGCCGCCACCGGGGGGCTGTGCG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11802 | ||
| mod ID: M5CSITE000163 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665913-40665914:- | [9] | |
| Sequence | GGTGGCGGCGGCGGCCGCCGCGGCCGCCACCGGGGGGCTGT | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11803 | ||
| mod ID: M5CSITE000164 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665915-40665916:- | [9] | |
| Sequence | GCGGTGGCGGCGGCGGCCGCCGCGGCCGCCACCGGGGGGCT | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11804 | ||
| mod ID: M5CSITE000165 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665916-40665917:- | [9] | |
| Sequence | GGCGGTGGCGGCGGCGGCCGCCGCGGCCGCCACCGGGGGGC | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11805 | ||
| mod ID: M5CSITE000166 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665918-40665919:- | [9] | |
| Sequence | GCGGCGGTGGCGGCGGCGGCCGCCGCGGCCGCCACCGGGGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11806 | ||
| mod ID: M5CSITE000167 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665919-40665920:- | [9] | |
| Sequence | GGCGGCGGTGGCGGCGGCGGCCGCCGCGGCCGCCACCGGGG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11807 | ||
| mod ID: M5CSITE000168 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665922-40665923:- | ||
| Sequence | GGCGGCGGCGGTGGCGGCGGCGGCCGCCGCGGCCGCCACCG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11808 | ||
| mod ID: M5CSITE000169 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665925-40665926:- | [9] | |
| Sequence | CGTGGCGGCGGCGGTGGCGGCGGCGGCCGCCGCGGCCGCCA | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11809 | ||
| mod ID: M5CSITE000171 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665928-40665929:- | ||
| Sequence | CTCCGTGGCGGCGGCGGTGGCGGCGGCGGCCGCCGCGGCCG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11810 | ||
| mod ID: M5CSITE000172 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665934-40665935:- | ||
| Sequence | GCCCGGCTCCGTGGCGGCGGCGGTGGCGGCGGCGGCCGCCG | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m5C_site_11811 | ||
N6-methyladenosine (m6A)
| In total 66 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE017005 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556020-40556021:- | [10] | |
| Sequence | ATGTATTGTGGTTTATGCGAACAGACCAACCTGGCATTACA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225778 | ||
| mod ID: M6ASITE017006 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556194-40556195:- | [11] | |
| Sequence | TCCAACGATATACAAATTGGACTTGTTCAACTGCTGGATAT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HEK293T; H1A; H1B; hESCs; fibroblasts; GM12878; CD8T; A549; Huh7; HEK293A-TOA; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225779 | ||
| mod ID: M6ASITE017007 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556203-40556204:- | [12] | |
| Sequence | AAATCATTCTCCAACGATATACAAATTGGACTTGTTCAACT | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225780 | ||
| mod ID: M6ASITE017008 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556258-40556259:- | [11] | |
| Sequence | GTGAGCAGTAAATCAATGGAACATCCCAAGAAGAGGATAAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HEK293T; hESC-HEK293T; H1A; H1B; hESCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225781 | ||
| mod ID: M6ASITE017009 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556311-40556312:- | [11] | |
| Sequence | GGAAAGTTCTGCTTTGACAAACTGACAAAGTCTAAATGAGC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T; hESCs; fibroblasts; Huh7; HEK293A-TOA | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225782 | ||
| mod ID: M6ASITE017010 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556597-40556598:- | [11] | |
| Sequence | CTGGTCTGTCTGGAAAGCAAACATTATGTGGCCTCTGGTAG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225783 | ||
| mod ID: M6ASITE017011 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556641-40556642:- | [11] | |
| Sequence | TCCTTGGTAGCTCTCTGAGAACAGTGAAGTCCAGGGAAAGG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225784 | ||
| mod ID: M6ASITE017012 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556826-40556827:- | [13] | |
| Sequence | ACTGCATCATAATACAAGGAACCTCAGAGCCCCCATTTGTT | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225785 | ||
| mod ID: M6ASITE017013 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556833-40556834:- | [14] | |
| Sequence | TGCCATTACTGCATCATAATACAAGGAACCTCAGAGCCCCC | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225786 | ||
| mod ID: M6ASITE017014 | Click to Show/Hide the Full List | ||
| mod site | chr13:40556914-40556915:- | [13] | |
| Sequence | CCTCCCGGGTATGTAACTGAACTTGGTGCCAAAGTACTTGT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225787 | ||
| mod ID: M6ASITE017015 | Click to Show/Hide the Full List | ||
| mod site | chr13:40557015-40557016:- | [13] | |
| Sequence | TTTTTTTGAAGATTCATTGAACAGCCACCACTCTATCATCC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225788 | ||
| mod ID: M6ASITE017016 | Click to Show/Hide the Full List | ||
| mod site | chr13:40557178-40557179:- | [14] | |
| Sequence | AATTTTTCTGTATATAGCCCACATCACACTTGCTTTGTCTT | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225789 | ||
| mod ID: M6ASITE017017 | Click to Show/Hide the Full List | ||
| mod site | chr13:40557331-40557332:- | [15] | |
| Sequence | TTTCCAATTACCTGTAACTGACAGACCAAATTAATTGGCTT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293 | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225790 | ||
| mod ID: M6ASITE017018 | Click to Show/Hide the Full List | ||
| mod site | chr13:40557556-40557557:- | [13] | |
| Sequence | GGAGGGAATTTAAAAATGGGACTTGAGTGGTTTGTAGAATT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225791 | ||
| mod ID: M6ASITE017019 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558116-40558117:- | [14] | |
| Sequence | TGGATTAGTACTAATTTTATACATGCTTAACTGGTTTGTAC | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225792 | ||
| mod ID: M6ASITE017020 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558185-40558186:- | [11] | |
| Sequence | CAGCTTGTAAATTTTGTGGAACCACAGGTATTTGGGGCAGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225793 | ||
| mod ID: M6ASITE017021 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558230-40558231:- | [10] | |
| Sequence | TGTAATAATGTTTTCTTAAAACTAGAGTCTACTTTGTTACA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225794 | ||
| mod ID: M6ASITE017022 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558252-40558253:- | [10] | |
| Sequence | ATTTTTGGAATAGATATTGAACTGTAATAATGTTTTCTTAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225795 | ||
| mod ID: M6ASITE017023 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558274-40558275:- | [10] | |
| Sequence | GTTATTGTTGGGACTTAAGAACATTTTTGGAATAGATATTG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225796 | ||
| mod ID: M6ASITE017024 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558335-40558336:- | [10] | |
| Sequence | TTATTTGCAAATTTGTACAAACATTTAAATGGTTCTAATTT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HEK293T; Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225797 | ||
| mod ID: M6ASITE017025 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558424-40558425:- | [11] | |
| Sequence | TAAATTAATGGACTTGTTAAACTTTTGGAAAAAAAAAGATT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; H1A; H1B; hESCs; fibroblasts; GM12878; CD8T; Huh7; Jurkat; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225798 | ||
| mod ID: M6ASITE017026 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558433-40558434:- | [11] | |
| Sequence | TCAATCTGCTAAATTAATGGACTTGTTAAACTTTTGGAAAA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; H1A; H1B; hESCs; fibroblasts; GM12878; CD8T; Huh7; Jurkat; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225799 | ||
| mod ID: M6ASITE017027 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558469-40558470:- | [11] | |
| Sequence | GCCACAGAATTCACATGAGAACCAAGTAGCCTGTTATCAAT | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; H1A; H1B; hESCs; fibroblasts; A549; GM12878; Huh7; Jurkat; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225800 | ||
| mod ID: M6ASITE017028 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558477-40558478:- | [14] | |
| Sequence | TTCACGTTGCCACAGAATTCACATGAGAACCAAGTAGCCTG | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225801 | ||
| mod ID: M6ASITE017029 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558502-40558503:- | [11] | |
| Sequence | TCTCCATTGAACAGCCTTGGACCTGTTCACGTTGCCACAGA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; H1A; H1B; hESCs; fibroblasts; A549; GM12878; Huh7; Jurkat; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225802 | ||
| mod ID: M6ASITE017030 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558512-40558513:- | [11] | |
| Sequence | CCATTCTGCATCTCCATTGAACAGCCTTGGACCTGTTCACG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; H1A; H1B; hESCs; fibroblasts; A549; GM12878; Huh7; Jurkat; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225803 | ||
| mod ID: M6ASITE017031 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558541-40558542:- | [11] | |
| Sequence | GAACTGACGGATCACAAAGAACTGAATCTCCATTCTGCATC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; H1A; H1B; hESCs; fibroblasts; A549; GM12878; Huh7; Jurkat; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225804 | ||
| mod ID: M6ASITE017032 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558548-40558549:- | [14] | |
| Sequence | TTCTGCGGAACTGACGGATCACAAAGAACTGAATCTCCATT | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225805 | ||
| mod ID: M6ASITE017033 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558559-40558560:- | [11] | |
| Sequence | TACTACCTGTTTTCTGCGGAACTGACGGATCACAAAGAACT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; H1A; H1B; hESCs; fibroblasts; A549; GM12878; Huh7; Jurkat; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225806 | ||
| mod ID: M6ASITE017034 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558590-40558591:- | [11] | |
| Sequence | CTCATGTCTTGATAAGTTAAACTTTTGTTTGTACTACCTGT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T; HepG2; H1A; hESCs; fibroblasts; A549; GM12878; Huh7; HEK293A-TOA; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225807 | ||
| mod ID: M6ASITE017035 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558777-40558778:- | [11] | |
| Sequence | TCATTACAATGAAGTGCCAAACTCACTACACCATATAATTG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; Huh7; CD4T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225808 | ||
| mod ID: M6ASITE017036 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558815-40558816:- | [11] | |
| Sequence | ATGTGCTGCTGTAGATAAGGACTGTGCCATTGGAAATTTCA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; CD8T; Huh7; Jurkat; CD4T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225809 | ||
| mod ID: M6ASITE017037 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558877-40558878:- | [11] | |
| Sequence | CGTCAGACTTGGCAGCAAAGACATTTTTCCTGTACAGGATG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; kidney; A549; hESC-HEK293T; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; CD8T; Huh7; Jurkat; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-REF-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225810 | ||
| mod ID: M6ASITE017038 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558891-40558892:- | [11] | |
| Sequence | TCCTTTTTTCCTTTCGTCAGACTTGGCAGCAAAGACATTTT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; Huh7; Jurkat; CD4T; peripheral-blood; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225811 | ||
| mod ID: M6ASITE017039 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558915-40558916:- | [11] | |
| Sequence | AAAAAAAAACAAAAAAAAAAACCCTCCTTTTTTCCTTTCGT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; Huh7; Jurkat; CD4T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225812 | ||
| mod ID: M6ASITE017040 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558927-40558928:- | [11] | |
| Sequence | TTTCTTGGTTAAAAAAAAAAACAAAAAAAAAAACCCTCCTT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; Huh7; Jurkat; CD4T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225813 | ||
| mod ID: M6ASITE017041 | Click to Show/Hide the Full List | ||
| mod site | chr13:40558994-40558995:- | [11] | |
| Sequence | CAGATTGTCTGACAGCAGGAACTGAGAGAAGCAGTCCAAAG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; CD8T; Huh7; CD4T; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225814 | ||
| mod ID: M6ASITE017042 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559551-40559552:- | [11] | |
| Sequence | GCTTCCCACACAGTGTCAAGACAACGACACATAGCTGGGTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225815 | ||
| mod ID: M6ASITE017043 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559628-40559629:- | [16] | |
| Sequence | GGAATCCATCATTCGGAATGACCTCATGGATGGAGATACAT | ||
| Motif Score | 2.839113095 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225816 | ||
| mod ID: M6ASITE017044 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559652-40559653:- | [14] | |
| Sequence | CATTGAGCGCTTAGACTGTGACATGGAATCCATCATTCGGA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225817 | ||
| mod ID: M6ASITE017045 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559658-40559659:- | [11] | |
| Sequence | CATGTTCATTGAGCGCTTAGACTGTGACATGGAATCCATCA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; CD8T; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225818 | ||
| mod ID: M6ASITE017046 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559688-40559689:- | [16] | |
| Sequence | CCAGGAGAAGCTCCCAAGTGACTTGGATGGCATGTTCATTG | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225819 | ||
| mod ID: M6ASITE017047 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559804-40559805:- | [14] | |
| Sequence | ACCCAAGTGAAGACACCTGTACAAGTGCCTCTGCCCCACCC | ||
| Motif Score | 2.856142857 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225820 | ||
| mod ID: M6ASITE017048 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559812-40559813:- | [11] | |
| Sequence | ACCGCCTGACCCAAGTGAAGACACCTGTACAAGTGCCTCTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; U2OS; hESCs; GM12878; MT4; Huh7; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225821 | ||
| mod ID: M6ASITE017049 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559832-40559833:- | [11] | |
| Sequence | GCCCCACACCTCGGGTATGAACCGCCTGACCCAAGTGAAGA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; U2OS; hESCs; GM12878; MT4; Huh7; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225822 | ||
| mod ID: M6ASITE017050 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559899-40559900:- | [11] | |
| Sequence | ACCCTGGACATGCTCAGCAGACATCTGCAGTTAACGGGCGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hESCs; GM12878; MT4; Huh7; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225823 | ||
| mod ID: M6ASITE017051 | Click to Show/Hide the Full List | ||
| mod site | chr13:40559912-40559913:- | [11] | |
| Sequence | AGCTCCCATACCCACCCTGGACATGCTCAGCAGACATCTGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hESCs; GM12878; MT4; Huh7; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225824 | ||
| mod ID: M6ASITE017052 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560110-40560111:- | [11] | |
| Sequence | CAGTATAACTGTGCGCCTGGACTCTTGAAGGAGTTGCTGAC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225825 | ||
| mod ID: M6ASITE017053 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560159-40560160:- | [11] | |
| Sequence | GCCTATACAAACACTTCAGGACAATAAGTCGAGTTATGGAG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; CD8T; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225826 | ||
| mod ID: M6ASITE017054 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560169-40560170:- | [11] | |
| Sequence | TGCCCCAGATGCCTATACAAACACTTCAGGACAATAAGTCG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1B; H1A; hNPCs; hESCs; fibroblasts; GM12878; LCLs; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225827 | ||
| mod ID: M6ASITE017055 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560231-40560232:- | [11] | |
| Sequence | TTTGAATTCACCCAGCCCAAACTACCAAAAATATACATATG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hESCs; fibroblasts; GM12878; LCLs; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225828 | ||
| mod ID: M6ASITE017056 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560258-40560259:- | [11] | |
| Sequence | CTACTCGTTTGCGCCACCAAACACCAGTTTGAATTCACCCA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hESCs; fibroblasts; GM12878; LCLs; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225829 | ||
| mod ID: M6ASITE017057 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560381-40560382:- | [11] | |
| Sequence | TGAGATAAGCAATCCCGAAAACATGGAAAATCTTTTGGATA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; hESC-HEK293T; hESCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; MSC; TIME; TREX; iSLK; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225830 | ||
| mod ID: M6ASITE017058 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560488-40560489:- | [11] | |
| Sequence | CTCTCACCCATTATGACCGAACAGGATGATCTTGGAGAAGG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; Huh7; Jurkat; CD4T; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225831 | ||
| mod ID: M6ASITE017059 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560509-40560510:- | [11] | |
| Sequence | GCTAGTACTATTAGTGGGAGACTCTCACCCATTATGACCGA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225832 | ||
| mod ID: M6ASITE017060 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560541-40560542:- | [11] | |
| Sequence | GGAGTACATTTCGCCCTCGAACTAGCTCAAATGCTAGTACT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225833 | ||
| mod ID: M6ASITE017061 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560633-40560634:- | [11] | |
| Sequence | TGGCCAGGAGGGTGCTGGGGACAGCCCTGGATCACAGTTTT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225834 | ||
| mod ID: M6ASITE017062 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560717-40560718:- | [11] | |
| Sequence | GAGAAGAGCTGCATCCATGGACAACAACAGTAAATTTGCTA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; H1A; H1B; hNPCs; hESCs; fibroblasts; A549; GM12878; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225835 | ||
| mod ID: M6ASITE017063 | Click to Show/Hide the Full List | ||
| mod site | chr13:40560799-40560800:- | [11] | |
| Sequence | TTCGTGTGCAGAATGAAGGAACTGGAAAAAGTTCTTGGTGG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; fibroblasts; GM12878; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000473775.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225836 | ||
| mod ID: M6ASITE017064 | Click to Show/Hide the Full List | ||
| mod site | chr13:40562727-40562728:- | [11] | |
| Sequence | TTCTGCTGAAACTTGGAGAGACCACCTCTGGCCCCTTTCAC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000379561.6; ENST00000473775.1; ENST00000636651.1 | ||
| External Link | RMBase: m6A_site_225837 | ||
| mod ID: M6ASITE017065 | Click to Show/Hide the Full List | ||
| mod site | chr13:40562737-40562738:- | [13] | |
| Sequence | GCTCTGGTGTTTCTGCTGAAACTTGGAGAGACCACCTCTGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000636651.1; ENST00000473775.1; ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225838 | ||
| mod ID: M6ASITE017066 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665886-40665887:- | [17] | |
| Sequence | CACCGGGGGGCTGTGCGGGGACTTCCAGGGCCCGGAGGCGG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HEK293T; HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225839 | ||
| mod ID: M6ASITE017067 | Click to Show/Hide the Full List | ||
| mod site | chr13:40665967-40665968:- | [17] | |
| Sequence | CTTGCTGGAGGAGAGCGAGGACTTCCCGCAGGCGCCCGGCT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HEK293T; HEK293A-TOA; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225840 | ||
| mod ID: M6ASITE017068 | Click to Show/Hide the Full List | ||
| mod site | chr13:40666174-40666175:- | [11] | |
| Sequence | GGTGGTGGAGATCGACCCGGACTTCGAGCCGCTGCCCCGGC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; H1A; H1B; GM12878; Jurkat; CD4T; GSC-11; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225841 | ||
| mod ID: M6ASITE017069 | Click to Show/Hide the Full List | ||
| mod site | chr13:40666303-40666304:- | [11] | |
| Sequence | TTCGTTCCCCCAAATCTCGGACCGTCCCTTCGCGCCCCCTC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; H1A; H1B; hESCs; GM12878; Jurkat; CD4T; GSC-11; HEK293A-TOA; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225842 | ||
| mod ID: M6ASITE017070 | Click to Show/Hide the Full List | ||
| mod site | chr13:40666429-40666430:- | [11] | |
| Sequence | CCGTCCAGTCCGTGCGGCGGACCCCGAGGAGCCTCGATGTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; H1B; CD4T; GSC-11; HEK293T; HEK293A-TOA; MSC; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000379561.6 | ||
| External Link | RMBase: m6A_site_225843 | ||
References