m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00169)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
ACLY
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1) [READER]
| Representative RNA-seq result indicating the expression of this target gene regulated by HNRNPA2B1 | ||
| Cell Line | HeLa cell line | Homo sapiens |
|
Treatment: HNRNPA2B1 knockdown HeLa cells
Control: HeLa cells
|
GSE70061 | |
| Regulation |
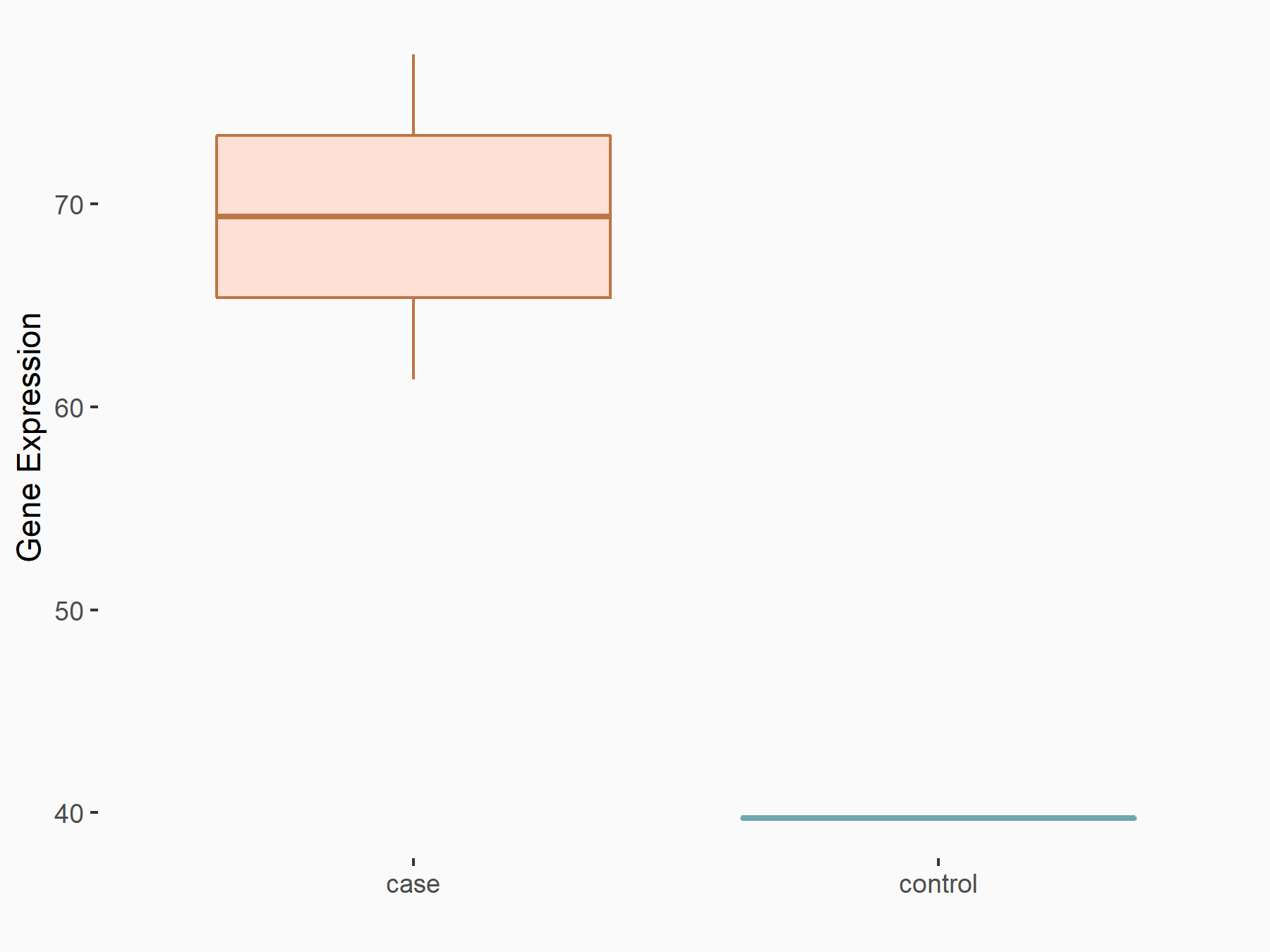 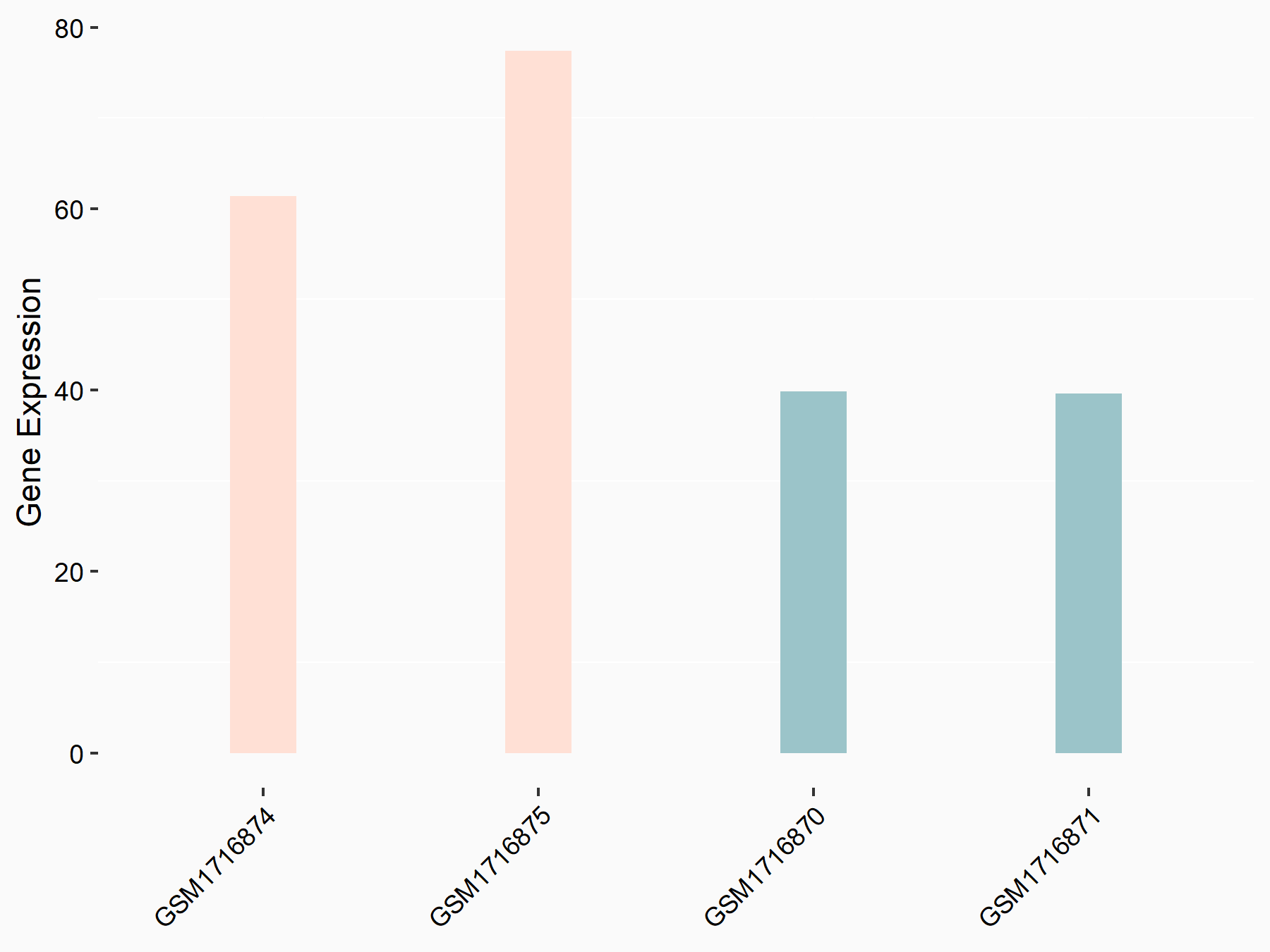 |
logFC: 7.79E-01 p-value: 2.27E-02 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | The levels of m6A and its regulator HNRNPA2B1 were significantly increased in cancerous tissues of esophageal cancer(ESCA), and overexpression of HNRNPA2B1 promotes ESCA progression via up-regulation of de novo fatty acid synthetic enzymes ATP-citrate synthase (ACLY) and ACC1. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Esophageal cancer | ICD-11: 2B70 | ||
| Pathway Response | Metabolic pathways | hsa01100 | ||
| Cell Process | Fatty acid synthesis | |||
| In-vitro Model | TE-10 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1760 |
| Eca-109 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
Methyltransferase-like 14 (METTL14) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL14 | ||
| Cell Line | MDA-MB-231 | Homo sapiens |
|
Treatment: siMETTL14 MDA-MB-231 cells
Control: MDA-MB-231 cells
|
GSE81164 | |
| Regulation |
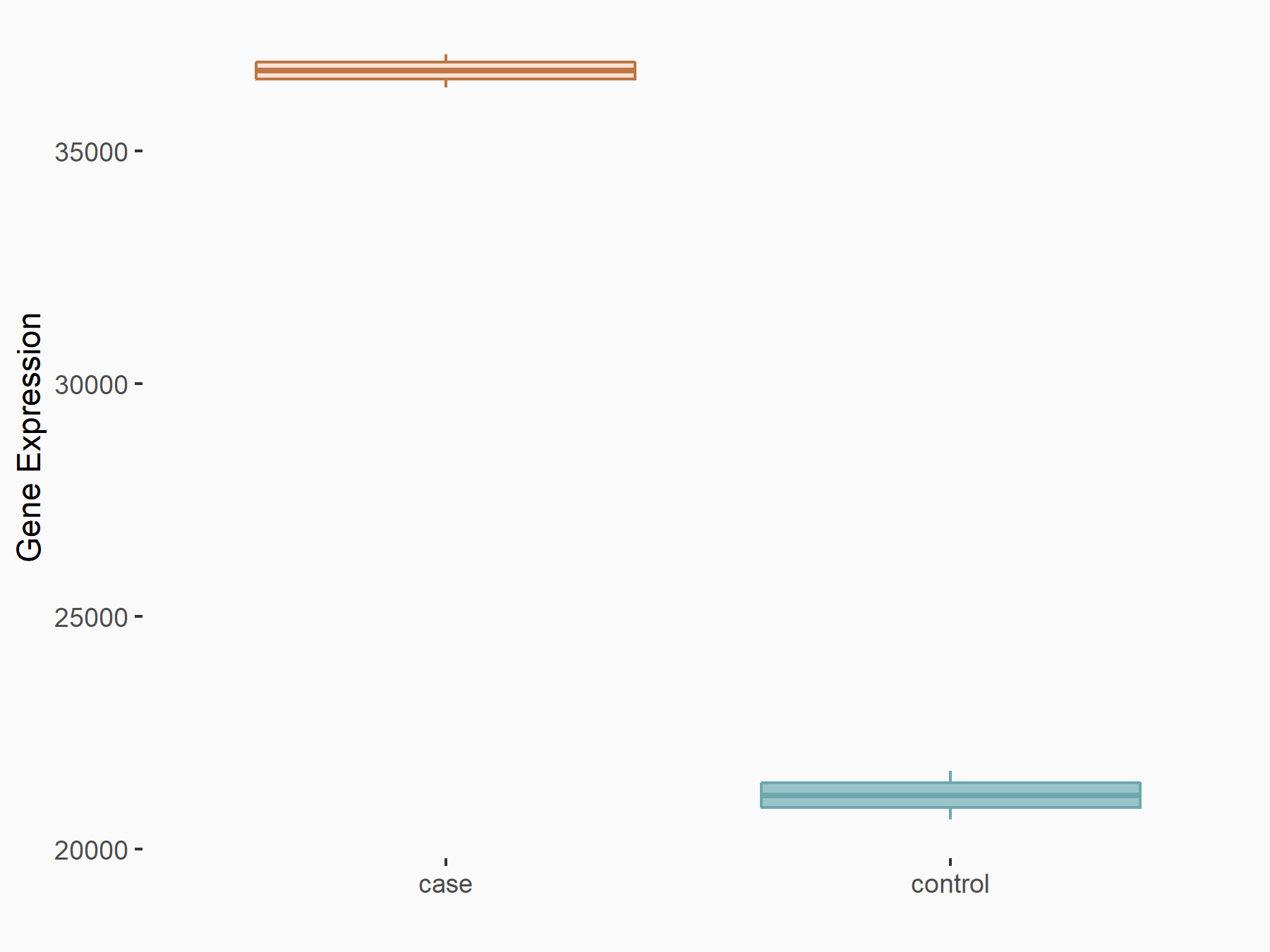 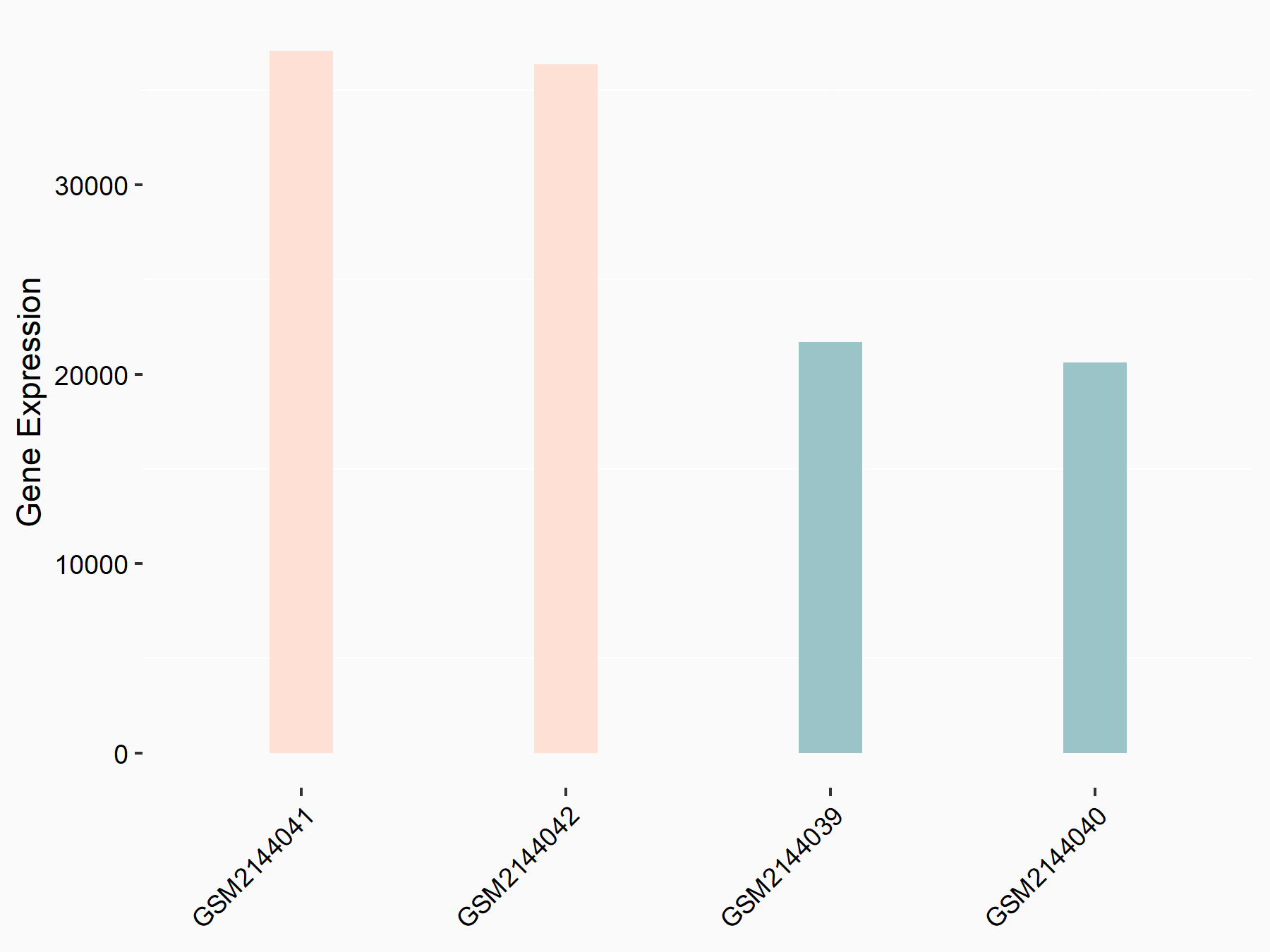 |
logFC: 7.95E-01 p-value: 2.66E-12 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | Targeting METTL3/14 in vitro increases protein level of ATP-citrate synthase (ACLY) and SCD1 as well as triglyceride and cholesterol production and accumulation of lipid droplets. These findings demonstrate a new NAFLD mouse model that provides a study platform for DM2-related NAFLD and reveals a unique epitranscriptional regulating mechanism for lipid metabolism via m6A-modified protein expression of ACLY and SCD1. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-alcoholic fatty liver disease | ICD-11: DB92 | ||
| Pathway Response | Glycerolipid metabolism | hsa00561 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | LM3 | Malignant neoplasms | Mus musculus | CVCL_D269 |
| MHCC97-H | Adult hepatocellular carcinoma | Homo sapiens | CVCL_4972 | |
| In-vivo Model | Mice with a Tmem30a deletion specifically in pancreatic beta cells were generated as previously described. Mice developed with NAFLD were named for Tmem30a-associated NAFLD (TAN) mice. The littermate mice with genotypes of Tmem30aloxP/loxP were used as controls. | |||
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | Liver | Mus musculus |
|
Treatment: Mettl3 knockout liver
Control: Wild type liver cells
|
GSE198513 | |
| Regulation |
  |
logFC: -6.16E-01 p-value: 1.78E-18 |
| More Results | Click to View More RNA-seq Results | |
| In total 2 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | Type 2 diabetes (T2D) is characterized by lack of insulin, insulin resistance and high blood sugar. METTL3 silence decreased the m6A methylated and total mRNA level of Fatty acid synthase (Fasn), subsequently inhibited fatty acid metabolism. The expression of Acc1, ATP-citrate synthase (ACLY), Dgat2, Ehhadh, Fasn, Foxo, Pgc1a and Sirt1, which are critical to the regulation of fatty acid synthesis and oxidation were dramatically decreased in livers of hepatocyte-specific METTL3 knockout mice. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Type 2 diabetes mellitus | ICD-11: 5A11 | ||
| Pathway Response | Insulin resistance | hsa04931 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| In-vivo Model | Hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were generated by crossing mice with TBG-Cre Tg mice. METTL3 flox (METTL3 fl/fl) and hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were used for experiments. | |||
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | Targeting METTL3/14 in vitro increases protein level of ATP-citrate synthase (ACLY) and SCD1 as well as triglyceride and cholesterol production and accumulation of lipid droplets. These findings demonstrate a new NAFLD mouse model that provides a study platform for DM2-related NAFLD and reveals a unique epitranscriptional regulating mechanism for lipid metabolism via m6A-modified protein expression of ACLY and SCD1. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Non-alcoholic fatty liver disease | ICD-11: DB92 | ||
| Pathway Response | Glycerolipid metabolism | hsa00561 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | LM3 | Malignant neoplasms | Mus musculus | CVCL_D269 |
| MHCC97-H | Adult hepatocellular carcinoma | Homo sapiens | CVCL_4972 | |
| In-vivo Model | Mice with a Tmem30a deletion specifically in pancreatic beta cells were generated as previously described. Mice developed with NAFLD were named for Tmem30a-associated NAFLD (TAN) mice. The littermate mice with genotypes of Tmem30aloxP/loxP were used as controls. | |||
Esophageal cancer [ICD-11: 2B70]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | The levels of m6A and its regulator HNRNPA2B1 were significantly increased in cancerous tissues of esophageal cancer(ESCA), and overexpression of HNRNPA2B1 promotes ESCA progression via up-regulation of de novo fatty acid synthetic enzymes ATP-citrate synthase (ACLY) and ACC1. | |||
| Responsed Disease | Esophageal cancer [ICD-11: 2B70] | |||
| Target Regulator | Heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Metabolic pathways | hsa01100 | ||
| Cell Process | Fatty acid synthesis | |||
| In-vitro Model | TE-10 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1760 |
| Eca-109 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_6898 | |
Type 2 diabetes mellitus [ICD-11: 5A11]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [3] | |||
| Response Summary | Type 2 diabetes (T2D) is characterized by lack of insulin, insulin resistance and high blood sugar. METTL3 silence decreased the m6A methylated and total mRNA level of Fatty acid synthase (Fasn), subsequently inhibited fatty acid metabolism. The expression of Acc1, ATP-citrate synthase (ACLY), Dgat2, Ehhadh, Fasn, Foxo, Pgc1a and Sirt1, which are critical to the regulation of fatty acid synthesis and oxidation were dramatically decreased in livers of hepatocyte-specific METTL3 knockout mice. | |||
| Responsed Disease | Type 2 diabetes mellitus [ICD-11: 5A11] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Insulin resistance | hsa04931 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 |
| In-vivo Model | Hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were generated by crossing mice with TBG-Cre Tg mice. METTL3 flox (METTL3 fl/fl) and hepatocyte-specific METTL3 knockout mice (TBG-Cre, METTL3 fl/fl) were used for experiments. | |||
Non-alcoholic fatty liver disease [ICD-11: DB92]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | Targeting METTL3/14 in vitro increases protein level of ATP-citrate synthase (ACLY) and SCD1 as well as triglyceride and cholesterol production and accumulation of lipid droplets. These findings demonstrate a new NAFLD mouse model that provides a study platform for DM2-related NAFLD and reveals a unique epitranscriptional regulating mechanism for lipid metabolism via m6A-modified protein expression of ACLY and SCD1. | |||
| Responsed Disease | Non-alcoholic fatty liver disease [ICD-11: DB92] | |||
| Target Regulator | Methyltransferase-like 14 (METTL14) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Glycerolipid metabolism | hsa00561 | ||
| Cell Process | Lipid metabolism | |||
| In-vitro Model | LM3 | Malignant neoplasms | Mus musculus | CVCL_D269 |
| MHCC97-H | Adult hepatocellular carcinoma | Homo sapiens | CVCL_4972 | |
| In-vivo Model | Mice with a Tmem30a deletion specifically in pancreatic beta cells were generated as previously described. Mice developed with NAFLD were named for Tmem30a-associated NAFLD (TAN) mice. The littermate mice with genotypes of Tmem30aloxP/loxP were used as controls. | |||
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00169)
| In total 2 m6A sequence/site(s) in this target gene | |||
| mod ID: AC4SITE000222 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892361-41892362:- | ||
| Sequence | TGGCTGATGCCATGAGGAAGCATCCGGAGGTAGATGTGCTC | ||
| Cell/Tissue List | DLD1 | ||
| Seq Type List | acRIP-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000393896.6; ENST00000590151.5; ENST00000353196.5; ENST00000537919.5 | ||
| External Link | RMBase: ac4C_site_794 | ||
| mod ID: AC4SITE000223 | Click to Show/Hide the Full List | ||
| mod site | chr17:41913853-41913854:- | [4] | |
| Sequence | CATGTCGGCCAAGGCAATTTCAGAGCAGACGGGCAAAGAAC | ||
| Cell/Tissue List | H1 | ||
| Seq Type List | ac4C-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000590735.1; ENST00000592970.1; ENST00000393896.6; ENST00000590151.5; ENST00000590770.5; ENST00000352035.7; ENST00000353196.5 | ||
| External Link | RMBase: ac4C_site_795 | ||
Adenosine-to-Inosine editing (A-to-I)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE007997 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867853-41867854:- | [5] | |
| Sequence | AGGGGCTGTATCGTCATCCGTGGGATGATATTTCATATGTT | ||
| Transcript ID List | ENST00000537919.5; ENST00000393896.6; ENST00000353196.5; ENST00000588779.1; ENST00000352035.7; ENST00000590151.5 | ||
| External Link | RMBase: RNA-editing_site_57495 | ||
2'-O-Methylation (2'-O-Me)
| In total 2 m6A sequence/site(s) in this target gene | |||
| mod ID: 2OMSITE000151 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867760-41867761:- | [6] | |
| Sequence | TGAAGACAAGATCTCTTCCCCCAAGAAAAAGTGTACAGACA | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000588779.1; ENST00000590151.5; ENST00000353196.5; ENST00000352035.7; ENST00000537919.5 | ||
| External Link | RMBase: Nm_site_2334 | ||
| mod ID: 2OMSITE000152 | Click to Show/Hide the Full List | ||
| mod site | chr17:41913825-41913826:- | [6] | |
| Sequence | ACGGGCAAAGAACTCCTTTACAAGTTCATCTGTACCACCTC | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | Nm-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000592970.1; ENST00000537919.5; ENST00000352035.7; ENST00000590770.5; ENST00000353196.5; ENST00000590735.1; ENST00000590151.5 | ||
| External Link | RMBase: Nm_site_2335 | ||
5-methylcytidine (m5C)
| In total 4 m6A sequence/site(s) in this target gene | |||
| mod ID: M5CSITE001118 | Click to Show/Hide the Full List | ||
| mod site | chr17:41870836-41870837:- | ||
| Sequence | CCACGTGCAGGCTCTTAAAGCCTTACATGATTCACCTTCAC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000393896.6; ENST00000537919.5; ENST00000352035.7; ENST00000353196.5 | ||
| External Link | RMBase: m5C_site_18891 | ||
| mod ID: M5CSITE001119 | Click to Show/Hide the Full List | ||
| mod site | chr17:41871834-41871835:- | [7] | |
| Sequence | ACACGCACTTGTTTCTCCTCCAGGGGGATCGGTTTGGGGGT | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000393896.6; ENST00000353196.5; ENST00000537919.5 | ||
| External Link | RMBase: m5C_site_18892 | ||
| mod ID: M5CSITE001120 | Click to Show/Hide the Full List | ||
| mod site | chr17:41886313-41886314:- | [7] | |
| Sequence | CCTGATTGTGGTTTCTGCCCCCACAGGTTGGAGGCATCAAG | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000353196.5; ENST00000590151.5; ENST00000537919.5; ENST00000352035.7 | ||
| External Link | RMBase: m5C_site_18893 | ||
| mod ID: M5CSITE001121 | Click to Show/Hide the Full List | ||
| mod site | chr17:41887709-41887710:- | [7] | |
| Sequence | CAGGAAGGGACTTTTGTCATCCTCTAGATCCGGACCATCGC | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000393896.6; ENST00000537919.5; ENST00000353196.5; ENST00000590151.5 | ||
| External Link | RMBase: m5C_site_18894 | ||
N6-methyladenosine (m6A)
| In total 115 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE032456 | Click to Show/Hide the Full List | ||
| mod site | chr17:41866928-41866929:- | [8] | |
| Sequence | TTTAAATAAACTATATAGTAACAATGAAGGCATACTGCTGG | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362812 | ||
| mod ID: M6ASITE032457 | Click to Show/Hide the Full List | ||
| mod site | chr17:41866939-41866940:- | [9] | |
| Sequence | TCATAGTCTGGTTTAAATAAACTATATAGTAACAATGAAGG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362813 | ||
| mod ID: M6ASITE032458 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867004-41867005:- | [8] | |
| Sequence | CTACTTTGCATCTGTAATCCACAAAGATTCTGGGCAGCTGC | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000353196.5; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362814 | ||
| mod ID: M6ASITE032459 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867032-41867033:- | [9] | |
| Sequence | ACTGAAGTGTGGGTCCAAGGACTCCTAACTACTTTGCATCT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362815 | ||
| mod ID: M6ASITE032460 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867052-41867053:- | [10] | |
| Sequence | AGCTGATTTGTCTGTCTGTAACTGAAGTGTGGGTCCAAGGA | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362816 | ||
| mod ID: M6ASITE032461 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867137-41867138:- | [8] | |
| Sequence | TGTAAAGTTGTAACATACATACATTTAAAATGGAAATGCAG | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000352035.7; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362817 | ||
| mod ID: M6ASITE032462 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867145-41867146:- | [8] | |
| Sequence | ACTTAATATGTAAAGTTGTAACATACATACATTTAAAATGG | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000352035.7; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362818 | ||
| mod ID: M6ASITE032463 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867165-41867166:- | [10] | |
| Sequence | ACAAAATATGTGGAGTAGTAACTTAATATGTAAAGTTGTAA | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362819 | ||
| mod ID: M6ASITE032464 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867185-41867186:- | [10] | |
| Sequence | TTGCATCCTTAGCCATTGTCACAAAATATGTGGAGTAGTAA | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362820 | ||
| mod ID: M6ASITE032465 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867239-41867240:- | [9] | |
| Sequence | TGAAATGTTCTGTGTTGCAGACCAGAGTCTGTTTATGTCAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362821 | ||
| mod ID: M6ASITE032466 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867288-41867289:- | [10] | |
| Sequence | TAAAGATCTTTTTGTATGTTACGTGTTAAGGGCTTGTTTGG | ||
| Motif Score | 2.046785714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362822 | ||
| mod ID: M6ASITE032467 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867329-41867330:- | [9] | |
| Sequence | TTCCTTATATAACATGAAGAACATTGTATTAATCTGATTTT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HEK293T; hESC-HEK293T | ||
| Seq Type List | m6A-seq; DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362823 | ||
| mod ID: M6ASITE032468 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867338-41867339:- | [11] | |
| Sequence | CCTTTCTTCTTCCTTATATAACATGAAGAACATTGTATTAA | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | HEK293; hESC-HEK293T | ||
| Seq Type List | m6A-REF-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000352035.7; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362824 | ||
| mod ID: M6ASITE032469 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867363-41867364:- | [10] | |
| Sequence | GTCTCTCTTTTTTTTTTTTTACTTCCCTTTCTTCTTCCTTA | ||
| Motif Score | 2.494845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000353196.5; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362825 | ||
| mod ID: M6ASITE032470 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867402-41867403:- | [8] | |
| Sequence | TTATGGCCTCAAGATACTATACATTTAAAGCACCCCAATGT | ||
| Motif Score | 2.110482143 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362826 | ||
| mod ID: M6ASITE032471 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867407-41867408:- | [10] | |
| Sequence | AACATTTATGGCCTCAAGATACTATACATTTAAAGCACCCC | ||
| Motif Score | 2.53247619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362827 | ||
| mod ID: M6ASITE032472 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867426-41867427:- | [9] | |
| Sequence | CTTTGGAGGAAGGGAATGAAACATTTATGGCCTCAAGATAC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000353196.5; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362828 | ||
| mod ID: M6ASITE032473 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867459-41867460:- | [9] | |
| Sequence | TTTCTTTTTCCATTAGTTAAACATTTTTTTCTCCTTTGGAG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T; hESC-HEK293T | ||
| Seq Type List | m6A-seq; DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000590151.5; ENST00000352035.7; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362829 | ||
| mod ID: M6ASITE032474 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867525-41867526:- | [10] | |
| Sequence | TTATATGGAAGCATCTAAGTACTGTCAGGATGGGGTCTTCC | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000590151.5; ENST00000537919.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362830 | ||
| mod ID: M6ASITE032475 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867559-41867560:- | [10] | |
| Sequence | ATTTGTGACTTTGCTCTGCTACCTGCTGTATTTATTATATG | ||
| Motif Score | 2.05802381 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000352035.7; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362831 | ||
| mod ID: M6ASITE032476 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867572-41867573:- | [10] | |
| Sequence | AAACCAAGCCAATATTTGTGACTTTGCTCTGCTACCTGCTG | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | HEK293T; CD8T; A549 | ||
| Seq Type List | DART-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000353196.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362832 | ||
| mod ID: M6ASITE032477 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867590-41867591:- | [10] | |
| Sequence | TTATAAGCATAGAAATAAAAACCAAGCCAATATTTGTGACT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HEK293T; HepG2 | ||
| Seq Type List | DART-seq; m6A-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000352035.7; ENST00000353196.5; ENST00000590151.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362833 | ||
| mod ID: M6ASITE032478 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867667-41867668:- | [9] | |
| Sequence | CTGGGGTACAGGCACCGAAGACCAACATCCACAGGCTAACA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; hESCs; fibroblasts; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000352035.7; ENST00000393896.6; ENST00000590151.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362835 | ||
| mod ID: M6ASITE032479 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867694-41867695:- | [9] | |
| Sequence | AGCAGGGGCCTGGAATGTAAACAGCCACTGGGGTACAGGCA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293; HepG2; hESCs; fibroblasts; endometrial | ||
| Seq Type List | m6A-seq; m6A-REF-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000353196.5; ENST00000590151.5; ENST00000393896.6; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362836 | ||
| mod ID: M6ASITE032480 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867742-41867743:- | [9] | |
| Sequence | CCCCAAGAAAAAGTGTACAGACAGCTGGCAGTGGAGCCTGC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; hESCs; fibroblasts; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000590151.5; ENST00000352035.7; ENST00000353196.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362837 | ||
| mod ID: M6ASITE032481 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867775-41867776:- | [9] | |
| Sequence | CCTACTGCAGTAAACTGAAGACAAGATCTCTTCCCCCAAGA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; hESCs; A549 | ||
| Seq Type List | m6A-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000588779.1; ENST00000352035.7; ENST00000393896.6; ENST00000590151.5; ENST00000537919.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362838 | ||
| mod ID: M6ASITE032482 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867782-41867783:- | [9] | |
| Sequence | CAGGAACCCTACTGCAGTAAACTGAAGACAAGATCTCTTCC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; hESCs | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000393896.6; ENST00000588779.1; ENST00000352035.7; ENST00000537919.5; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362839 | ||
| mod ID: M6ASITE032483 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867797-41867798:- | [9] | |
| Sequence | AGCATGTAACAGAGCCAGGAACCCTACTGCAGTAAACTGAA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; hESCs | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000588779.1; ENST00000352035.7; ENST00000537919.5; ENST00000590151.5; ENST00000353196.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362840 | ||
| mod ID: M6ASITE032484 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867809-41867810:- | [11] | |
| Sequence | CCGGAACACATGAGCATGTAACAGAGCCAGGAACCCTACTG | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000352035.7; ENST00000537919.5; ENST00000353196.5; ENST00000588779.1; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362841 | ||
| mod ID: M6ASITE032485 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867824-41867825:- | [9] | |
| Sequence | ATTTCATATGTTCTTCCGGAACACATGAGCATGTAACAGAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000588779.1; ENST00000352035.7; ENST00000393896.6; ENST00000590151.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362842 | ||
| mod ID: M6ASITE032486 | Click to Show/Hide the Full List | ||
| mod site | chr17:41867902-41867903:- | [8] | |
| Sequence | TGTGTTTGCTTCATTTTAGGACACTATCTTGATCAGAAGAG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000353196.5; ENST00000590151.5; ENST00000393896.6; ENST00000588779.1; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362843 | ||
| mod ID: M6ASITE032487 | Click to Show/Hide the Full List | ||
| mod site | chr17:41868761-41868762:- | [10] | |
| Sequence | GGAAGCTGATGAATATATTGACATTGGAGCCCTCAATGGCA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T; hESC-HEK293T | ||
| Seq Type List | DART-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000588779.1; ENST00000352035.7; ENST00000393896.6; ENST00000590151.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362844 | ||
| mod ID: M6ASITE032488 | Click to Show/Hide the Full List | ||
| mod site | chr17:41869060-41869061:- | [9] | |
| Sequence | ATTTGTAGACATGCTTAGAAACTGTGGGTCCTTTACTCGGT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000352035.7; ENST00000537919.5; ENST00000588779.1; ENST00000590151.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362845 | ||
| mod ID: M6ASITE032489 | Click to Show/Hide the Full List | ||
| mod site | chr17:41869072-41869073:- | [9] | |
| Sequence | CATCGGAGTCGCATTTGTAGACATGCTTAGAAACTGTGGGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000352035.7; ENST00000588779.1; ENST00000590151.5; ENST00000537919.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362846 | ||
| mod ID: M6ASITE032490 | Click to Show/Hide the Full List | ||
| mod site | chr17:41869573-41869574:- | [9] | |
| Sequence | TTTCTAGATAAACAACCCAGACATGCGAGTGCAGATCCTCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; MT4 | ||
| Seq Type List | m6A-seq; MAZTER-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000393896.6; ENST00000588779.1; ENST00000353196.5; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362847 | ||
| mod ID: M6ASITE032491 | Click to Show/Hide the Full List | ||
| mod site | chr17:41869582-41869583:- | [9] | |
| Sequence | TTGGGTTCATTTCTAGATAAACAACCCAGACATGCGAGTGC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; MT4 | ||
| Seq Type List | m6A-seq; MAZTER-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000588779.1; ENST00000393896.6; ENST00000352035.7; ENST00000590151.5; ENST00000537919.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362848 | ||
| mod ID: M6ASITE032492 | Click to Show/Hide the Full List | ||
| mod site | chr17:41870367-41870368:- | [9] | |
| Sequence | TGAGCAAGGACCAGCTCAGAACCAAGCAGGGAAGGTGGGTG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000588779.1; ENST00000537919.5; ENST00000352035.7; ENST00000590151.5; rmsk_4693671; ENST00000393896.6; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362849 | ||
| mod ID: M6ASITE032493 | Click to Show/Hide the Full List | ||
| mod site | chr17:41870378-41870379:- | [9] | |
| Sequence | AGGTTGCTTGATGAGCAAGGACCAGCTCAGAACCAAGCAGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000588779.1; ENST00000353196.5; rmsk_4693671; ENST00000590151.5; ENST00000537919.5; ENST00000393896.6; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362850 | ||
| mod ID: M6ASITE032494 | Click to Show/Hide the Full List | ||
| mod site | chr17:41870426-41870427:- | [9] | |
| Sequence | ACCACCTTCTAGGCAGCCAAACACTTGGCCATCTCAGGTGA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000393896.6; rmsk_4693671; ENST00000353196.5; ENST00000590151.5; ENST00000352035.7; ENST00000588779.1 | ||
| External Link | RMBase: m6A_site_362851 | ||
| mod ID: M6ASITE032495 | Click to Show/Hide the Full List | ||
| mod site | chr17:41870448-41870449:- | [9] | |
| Sequence | GGTCATGCATGTGATTCAGGACACCACCTTCTAGGCAGCCA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000393896.6; ENST00000537919.5; rmsk_4693671; ENST00000352035.7; ENST00000588779.1 | ||
| External Link | RMBase: m6A_site_362852 | ||
| mod ID: M6ASITE032496 | Click to Show/Hide the Full List | ||
| mod site | chr17:41870691-41870692:- | [9] | |
| Sequence | AAGGACAGTAAATATTTGAAACAGTTTATATAGTCTCACAC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000588779.1; ENST00000537919.5; ENST00000353196.5; ENST00000393896.6; ENST00000352035.7; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362853 | ||
| mod ID: M6ASITE032497 | Click to Show/Hide the Full List | ||
| mod site | chr17:41870707-41870708:- | [9] | |
| Sequence | GGAGAGACCAGTGGGAAAGGACAGTAAATATTTGAAACAGT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000590151.5; ENST00000588779.1; ENST00000537919.5; ENST00000352035.7; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362854 | ||
| mod ID: M6ASITE032498 | Click to Show/Hide the Full List | ||
| mod site | chr17:41871743-41871744:- | [9] | |
| Sequence | TATCCCCATGGAGTTTGTGAACAAGATGAAGAAGGAAGGGA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; HepG2 | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000352035.7; ENST00000393896.6; ENST00000353196.5; rmsk_4693674; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362855 | ||
| mod ID: M6ASITE032499 | Click to Show/Hide the Full List | ||
| mod site | chr17:41871773-41871774:- | [11] | |
| Sequence | GATGTTCAGTAAAGCCTTTGACAGTGGCATTATCCCCATGG | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | rmsk_4693674; ENST00000353196.5; ENST00000537919.5; ENST00000393896.6; ENST00000352035.7; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362856 | ||
| mod ID: M6ASITE032500 | Click to Show/Hide the Full List | ||
| mod site | chr17:41872068-41872069:- | [9] | |
| Sequence | TTGTGCGCGAGCTGGGAAAGACCTGGTCTCCAGCCTCACCT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000353196.5; ENST00000393896.6; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362857 | ||
| mod ID: M6ASITE032501 | Click to Show/Hide the Full List | ||
| mod site | chr17:41872098-41872099:- | [8] | |
| Sequence | AGCCGTCTCTGGAGCCCACAACACCATCATTTGTGCGCGAG | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000393896.6; ENST00000353196.5; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362858 | ||
| mod ID: M6ASITE032502 | Click to Show/Hide the Full List | ||
| mod site | chr17:41873905-41873906:- | [9] | |
| Sequence | AGCATCTGCGATGAGCGAGGACAGGAGCTCATCTACGCGGG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000590151.5; ENST00000353196.5; ENST00000352035.7; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362859 | ||
| mod ID: M6ASITE032503 | Click to Show/Hide the Full List | ||
| mod site | chr17:41873944-41873945:- | [9] | |
| Sequence | GAGCTTGGTTTGATCCGCAAACCTGCCTCGTTCATGACCAG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000353196.5; ENST00000393896.6; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362860 | ||
| mod ID: M6ASITE032504 | Click to Show/Hide the Full List | ||
| mod site | chr17:41878118-41878119:- | [9] | |
| Sequence | GCCCCCAACCGTGCCCATGGACTACTCCTGGGCCAGGGTAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000537919.5; ENST00000590151.5; ENST00000353196.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362861 | ||
| mod ID: M6ASITE032505 | Click to Show/Hide the Full List | ||
| mod site | chr17:41878862-41878863:- | [9] | |
| Sequence | TGAAACTGCAGTAGCCAAGAACCAGGCTTTGAAGGAAGCAG | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000353196.5; ENST00000352035.7; ENST00000590151.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362862 | ||
| mod ID: M6ASITE032506 | Click to Show/Hide the Full List | ||
| mod site | chr17:41878878-41878879:- | [9] | |
| Sequence | GTGCCAACCAGGCTTCTGAAACTGCAGTAGCCAAGAACCAG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000537919.5; ENST00000393896.6; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362863 | ||
| mod ID: M6ASITE032507 | Click to Show/Hide the Full List | ||
| mod site | chr17:41884229-41884230:- | [9] | |
| Sequence | TCATGTGTTACGCTATCAGGACACTCCAGGAGTCAAAATGA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; HepG2; MT4 | ||
| Seq Type List | m6A-seq; MAZTER-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000537919.5; ENST00000353196.5; ENST00000352035.7; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362864 | ||
| mod ID: M6ASITE032508 | Click to Show/Hide the Full List | ||
| mod site | chr17:41886114-41886115:- | [9] | |
| Sequence | GGGCGTGGCCATTGGTGGGGACAGGTAATCAGCTGGGAAGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; kidney; HepG2; Huh7 | ||
| Seq Type List | m6A-seq; m6A-REF-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000537919.5; ENST00000590151.5; ENST00000353196.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362865 | ||
| mod ID: M6ASITE032509 | Click to Show/Hide the Full List | ||
| mod site | chr17:41886154-41886155:- | [9] | |
| Sequence | TCAACAATATCATCTCTCGGACCACGGATGGCGTCTATGAG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000590151.5; ENST00000353196.5; ENST00000352035.7; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362866 | ||
| mod ID: M6ASITE032510 | Click to Show/Hide the Full List | ||
| mod site | chr17:41886171-41886172:- | [8] | |
| Sequence | AGGCATGTCCAACGAGCTCAACAATATCATCTCTCGGACCA | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000393896.6; ENST00000590151.5; ENST00000353196.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362867 | ||
| mod ID: M6ASITE032511 | Click to Show/Hide the Full List | ||
| mod site | chr17:41886233-41886234:- | [9] | |
| Sequence | GACAACATCCTGGCCTCCAAACTGTACCGCCCAGGCAGCGT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000537919.5; ENST00000393896.6; ENST00000352035.7; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362868 | ||
| mod ID: M6ASITE032512 | Click to Show/Hide the Full List | ||
| mod site | chr17:41886249-41886250:- | [8] | |
| Sequence | CACAGGTGGGATGCTGGACAACATCCTGGCCTCCAAACTGT | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000353196.5; ENST00000393896.6; ENST00000352035.7; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362869 | ||
| mod ID: M6ASITE032513 | Click to Show/Hide the Full List | ||
| mod site | chr17:41886252-41886253:- | [9] | |
| Sequence | CAACACAGGTGGGATGCTGGACAACATCCTGGCCTCCAAAC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000352035.7; ENST00000537919.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362870 | ||
| mod ID: M6ASITE032514 | Click to Show/Hide the Full List | ||
| mod site | chr17:41886270-41886271:- | [8] | |
| Sequence | TGGGTGCTTTAAGATTGGCAACACAGGTGGGATGCTGGACA | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000590151.5; ENST00000393896.6; ENST00000537919.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362871 | ||
| mod ID: M6ASITE032515 | Click to Show/Hide the Full List | ||
| mod site | chr17:41887607-41887608:- | [9] | |
| Sequence | AAGGGAGTGACCATCATCGGACCTGCCACTGTGAGCTTCCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000393896.6; ENST00000537919.5; ENST00000352035.7; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362872 | ||
| mod ID: M6ASITE032516 | Click to Show/Hide the Full List | ||
| mod site | chr17:41887632-41887633:- | [9] | |
| Sequence | AAAGCTGATCAAGAAGGCGGACCAGAAGGGAGTGACCATCA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000352035.7; ENST00000393896.6; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362873 | ||
| mod ID: M6ASITE032517 | Click to Show/Hide the Full List | ||
| mod site | chr17:41887696-41887697:- | [9] | |
| Sequence | TTGTCATCCTCTAGATCCGGACCATCGCCATCATAGCTGAA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000537919.5; ENST00000352035.7; ENST00000393896.6; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362874 | ||
| mod ID: M6ASITE032518 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892288-41892289:- | [9] | |
| Sequence | CAGCACCATGGAGACCATGAACTATGCCCAGGTAGATCACT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000537919.5; ENST00000590151.5; ENST00000352035.7; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362875 | ||
| mod ID: M6ASITE032519 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892295-41892296:- | [9] | |
| Sequence | CCTATGACAGCACCATGGAGACCATGAACTATGCCCAGGTA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000352035.7; ENST00000353196.5; ENST00000537919.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362876 | ||
| mod ID: M6ASITE032520 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892309-41892310:- | [11] | |
| Sequence | CTCTCTCCGCTCTGCCTATGACAGCACCATGGAGACCATGA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000353196.5; ENST00000393896.6; ENST00000590151.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362877 | ||
| mod ID: M6ASITE032521 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892384-41892385:- | [9] | |
| Sequence | CCTGATCCCTGTCTTCAAGAACATGGCTGATGCCATGAGGA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000352035.7; ENST00000393896.6; ENST00000590151.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362878 | ||
| mod ID: M6ASITE032522 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892414-41892415:- | [11] | |
| Sequence | GCAGAAGTTTTACTGGGGGCACAAAGAGATCCTGATCCCTG | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000393896.6; ENST00000537919.5; ENST00000352035.7; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362879 | ||
| mod ID: M6ASITE032523 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892438-41892439:- | [11] | |
| Sequence | ACTTTCTCACAGTGGGGACCACAAGCAGAAGTTTTACTGGG | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | kidney; hESC-HEK293T | ||
| Seq Type List | m6A-REF-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000590151.5; ENST00000352035.7; ENST00000393896.6; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362880 | ||
| mod ID: M6ASITE032524 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892441-41892442:- | [9] | |
| Sequence | TTTACTTTCTCACAGTGGGGACCACAAGCAGAAGTTTTACT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HEK293T; endometrial; NB4 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000393896.6; ENST00000353196.5; ENST00000537919.5; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362881 | ||
| mod ID: M6ASITE032525 | Click to Show/Hide the Full List | ||
| mod site | chr17:41893092-41893093:- | [9] | |
| Sequence | GGCCGTGCAAGGCATGCTGGACTTTGACTATGTCTGCTCCC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; peripheral-blood; HEK293T; endometrial; NB4 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000393896.6; ENST00000352035.7; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362882 | ||
| mod ID: M6ASITE032526 | Click to Show/Hide the Full List | ||
| mod site | chr17:41893117-41893118:- | [9] | |
| Sequence | CCATTGTGTGGGGCATGCAGACCCGGGCCGTGCAAGGCATG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; peripheral-blood; HEK293T; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000353196.5; ENST00000352035.7; ENST00000590151.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362883 | ||
| mod ID: M6ASITE032527 | Click to Show/Hide the Full List | ||
| mod site | chr17:41893146-41893147:- | [8] | |
| Sequence | CACCACCCTCTTCAGCCGCCACACCAAGGCCATTGTGTGGG | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000590151.5; ENST00000352035.7; ENST00000537919.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362884 | ||
| mod ID: M6ASITE032528 | Click to Show/Hide the Full List | ||
| mod site | chr17:41897752-41897753:- | [8] | |
| Sequence | AAGGCCAAGCCTGCCATGCCACAAGGTAACTCCCTGAGGCT | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000590151.5; ENST00000352035.7; ENST00000353196.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362885 | ||
| mod ID: M6ASITE032529 | Click to Show/Hide the Full List | ||
| mod site | chr17:41897820-41897821:- | [9] | |
| Sequence | AGACGCCAGCCCCCAGCAGGACAGCATCTTTTTCTGAGTCC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; kidney; HepG2; MT4; Huh7; peripheral-blood; endometrial; NB4 | ||
| Seq Type List | m6A-seq; m6A-REF-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000393896.6; ENST00000352035.7; ENST00000590770.5; ENST00000353196.5; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362886 | ||
| mod ID: M6ASITE032530 | Click to Show/Hide the Full List | ||
| mod site | chr17:41898661-41898662:- | [9] | |
| Sequence | CACAGCGGCCCACACTGCAAACTTCCTCCTCAACGCCAGCG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; MT4; Huh7; peripheral-blood; endometrial; NB4; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; miCLIP | ||
| Transcript ID List | ENST00000590770.5; ENST00000353196.5; ENST00000537919.5; ENST00000352035.7; ENST00000590151.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362887 | ||
| mod ID: M6ASITE032531 | Click to Show/Hide the Full List | ||
| mod site | chr17:41898739-41898740:- | [8] | |
| Sequence | TGTCTTTGGCACAGAGACTCACATGACGGCCATTGTGGGCA | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000353196.5; ENST00000393896.6; ENST00000590770.5; ENST00000590151.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362888 | ||
| mod ID: M6ASITE032532 | Click to Show/Hide the Full List | ||
| mod site | chr17:41898743-41898744:- | [9] | |
| Sequence | TCCATGTCTTTGGCACAGAGACTCACATGACGGCCATTGTG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; MT4; Huh7; peripheral-blood; endometrial; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000393896.6; ENST00000590770.5; ENST00000537919.5; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362889 | ||
| mod ID: M6ASITE032533 | Click to Show/Hide the Full List | ||
| mod site | chr17:41898749-41898750:- | [11] | |
| Sequence | TCCCCATCCATGTCTTTGGCACAGAGACTCACATGACGGCC | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000590770.5; ENST00000590151.5; ENST00000352035.7; ENST00000353196.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362890 | ||
| mod ID: M6ASITE032534 | Click to Show/Hide the Full List | ||
| mod site | chr17:41898779-41898780:- | [9] | |
| Sequence | TTCCTTCCCTTGCAGGGAAGACCACTGGGATCCCCATCCAT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; peripheral-blood; endometrial; NB4 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000352035.7; ENST00000590770.5; ENST00000353196.5; ENST00000590151.5; ENST00000393896.6 | ||
| External Link | RMBase: m6A_site_362891 | ||
| mod ID: M6ASITE032535 | Click to Show/Hide the Full List | ||
| mod site | chr17:41901758-41901759:- | [8] | |
| Sequence | CCCTGAAGGAGCACGAAGTCACAATCTTTGTCCGAAGAGGT | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000590770.5; ENST00000590151.5; ENST00000352035.7; ENST00000353196.5; ENST00000393896.6; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362892 | ||
| mod ID: M6ASITE032536 | Click to Show/Hide the Full List | ||
| mod site | chr17:41904449-41904450:- | [9] | |
| Sequence | CTGCTTTCTTCCTTCTCAGGACCAATGCTTTGGAGCACTGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000393896.6; ENST00000588547.1; ENST00000352035.7; ENST00000353196.5; ENST00000590151.5; ENST00000590770.5 | ||
| External Link | RMBase: m6A_site_362893 | ||
| mod ID: M6ASITE032537 | Click to Show/Hide the Full List | ||
| mod site | chr17:41904507-41904508:- | [9] | |
| Sequence | GCAAGCAGCTGCTACGCTGAACTATAACTCTCCCACCTGAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; MT4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000588547.1; ENST00000352035.7; ENST00000393896.6; ENST00000590770.5; ENST00000590151.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362894 | ||
| mod ID: M6ASITE032538 | Click to Show/Hide the Full List | ||
| mod site | chr17:41904561-41904562:- | [9] | |
| Sequence | GGGGTGGAGAAGACACTGGGACAGGAGCTGCCCATCTGCGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000588547.1; ENST00000393896.6; ENST00000590770.5; ENST00000590151.5; ENST00000537919.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362895 | ||
| mod ID: M6ASITE032539 | Click to Show/Hide the Full List | ||
| mod site | chr17:41904569-41904570:- | [9] | |
| Sequence | CTCTTAAAGGGGTGGAGAAGACACTGGGACAGGAGCTGCCC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000588547.1; ENST00000537919.5; ENST00000590770.5; ENST00000393896.6; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362896 | ||
| mod ID: M6ASITE032540 | Click to Show/Hide the Full List | ||
| mod site | chr17:41904756-41904757:- | [9] | |
| Sequence | CATTGGAGGCAGCATCGCAAACTTCACCAACGTGGCTGCCA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000352035.7; ENST00000590770.5; ENST00000353196.5; ENST00000393896.6; ENST00000588547.1; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362897 | ||
| mod ID: M6ASITE032541 | Click to Show/Hide the Full List | ||
| mod site | chr17:41905560-41905561:- | [9] | |
| Sequence | AGACCTATGACTATGCCAAGACTATCCTCTCCCTCATGACC | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000393896.6; ENST00000590151.5; ENST00000352035.7; ENST00000590770.5; ENST00000537919.5; ENST00000588547.1 | ||
| External Link | RMBase: m6A_site_362898 | ||
| mod ID: M6ASITE032542 | Click to Show/Hide the Full List | ||
| mod site | chr17:41905578-41905579:- | [9] | |
| Sequence | GCGCCCCCAGCGAGCAGCAGACCTATGACTATGCCAAGACT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000353196.5; ENST00000393896.6; ENST00000590770.5; ENST00000352035.7; ENST00000588547.1; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362899 | ||
| mod ID: M6ASITE032543 | Click to Show/Hide the Full List | ||
| mod site | chr17:41905616-41905617:- | [9] | |
| Sequence | GGGTGTCAACGAGCTGGCAAACTATGGGGAGTACTCAGGCG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T; HepG2; Huh7; endometrial | ||
| Seq Type List | m6A-seq; DART-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000590770.5; ENST00000353196.5; ENST00000537919.5; ENST00000352035.7; ENST00000588547.1; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362900 | ||
| mod ID: M6ASITE032544 | Click to Show/Hide the Full List | ||
| mod site | chr17:41906564-41906565:- | [9] | |
| Sequence | ACCCCAAAGGGAGGATCTGGACCATGGTGGCCGGGGGTGGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000353196.5; ENST00000590770.5; ENST00000393896.6; ENST00000537919.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362901 | ||
| mod ID: M6ASITE032545 | Click to Show/Hide the Full List | ||
| mod site | chr17:41906584-41906585:- | [9] | |
| Sequence | CCTGAAGCTGACCTTGCTGAACCCCAAAGGGAGGATCTGGA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000537919.5; ENST00000590770.5; ENST00000393896.6; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362902 | ||
| mod ID: M6ASITE032546 | Click to Show/Hide the Full List | ||
| mod site | chr17:41906629-41906630:- | [9] | |
| Sequence | CCAGGAAGCCTACATTGCAGACCTCGATGCCAAAAGTGGGG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000590735.1; ENST00000393896.6; ENST00000353196.5; ENST00000590151.5; ENST00000590770.5; ENST00000352035.7; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362903 | ||
| mod ID: M6ASITE032547 | Click to Show/Hide the Full List | ||
| mod site | chr17:41906638-41906639:- | [11] | |
| Sequence | GTCTGGTCCCCAGGAAGCCTACATTGCAGACCTCGATGCCA | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | kidney; hESC-HEK293T | ||
| Seq Type List | m6A-REF-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000353196.5; ENST00000590735.1; ENST00000393896.6; ENST00000590770.5; ENST00000590151.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362904 | ||
| mod ID: M6ASITE032548 | Click to Show/Hide the Full List | ||
| mod site | chr17:41907484-41907485:- | [8] | |
| Sequence | CTGCAAAGTGAAGTGGGGTGACATCGAGTTCCCTCCCCCCT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000590770.5; ENST00000590735.1; ENST00000393896.6; ENST00000537919.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362905 | ||
| mod ID: M6ASITE032549 | Click to Show/Hide the Full List | ||
| mod site | chr17:41907508-41907509:- | [8] | |
| Sequence | GGTGGACGCCACTGCCGACTACATCTGCAAAGTGAAGTGGG | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000393896.6; ENST00000590735.1; ENST00000537919.5; ENST00000590770.5; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362906 | ||
| mod ID: M6ASITE032550 | Click to Show/Hide the Full List | ||
| mod site | chr17:41909023-41909024:- | [9] | |
| Sequence | CCTCTTCAATTTCTACGAGGACTTGTACTTCACCTACCTCG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000537919.5; ENST00000590151.5; ENST00000393896.6; ENST00000590770.5; ENST00000590735.1; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362907 | ||
| mod ID: M6ASITE032551 | Click to Show/Hide the Full List | ||
| mod site | chr17:41909518-41909519:- | [9] | |
| Sequence | GTTGGTCCACGCCCCTGAAGACAAGAAAGAGTTGAGTGAGA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; endometrial | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000590735.1; ENST00000393896.6; ENST00000590151.5; ENST00000537919.5; ENST00000352035.7; ENST00000590770.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362908 | ||
| mod ID: M6ASITE032552 | Click to Show/Hide the Full List | ||
| mod site | chr17:41909544-41909545:- | [9] | |
| Sequence | AATCCTGAGGACATCAAAAAACACCTGTTGGTCCACGCCCC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; endometrial; AML | ||
| Seq Type List | m6A-seq; MAZTER-seq; miCLIP | ||
| Transcript ID List | ENST00000537919.5; ENST00000590770.5; ENST00000352035.7; ENST00000590151.5; ENST00000393896.6; ENST00000353196.5; ENST00000590735.1 | ||
| External Link | RMBase: m6A_site_362909 | ||
| mod ID: M6ASITE032553 | Click to Show/Hide the Full List | ||
| mod site | chr17:41909554-41909555:- | [9] | |
| Sequence | TGAGAAACTGAATCCTGAGGACATCAAAAAACACCTGTTGG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; endometrial | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000590735.1; ENST00000393896.6; ENST00000590770.5; ENST00000537919.5; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362910 | ||
| mod ID: M6ASITE032554 | Click to Show/Hide the Full List | ||
| mod site | chr17:41909568-41909569:- | [9] | |
| Sequence | CTTGTTGGCGTGGATGAGAAACTGAATCCTGAGGACATCAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000393896.6; ENST00000537919.5; ENST00000352035.7; ENST00000590770.5; ENST00000590735.1; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362911 | ||
| mod ID: M6ASITE032555 | Click to Show/Hide the Full List | ||
| mod site | chr17:41909656-41909657:- | [9] | |
| Sequence | CTATGCCACCCGAGAAGGGGACTACGTCCTGTTCCACCACG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000590735.1; ENST00000537919.5; ENST00000393896.6; ENST00000590770.5; ENST00000590151.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362912 | ||
| mod ID: M6ASITE032556 | Click to Show/Hide the Full List | ||
| mod site | chr17:41910228-41910229:- | [11] | |
| Sequence | GATCGAGCCCTTCGTCCCCCACAGTCAGGTATGTGTGAGGC | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000393896.6; ENST00000590770.5; ENST00000590735.1; ENST00000352035.7; ENST00000590151.5; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362913 | ||
| mod ID: M6ASITE032557 | Click to Show/Hide the Full List | ||
| mod site | chr17:41910255-41910256:- | [9] | |
| Sequence | GGCCACAGGCTTCCTCAAGAACTTTCTGATCGAGCCCTTCG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; Huh7; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000393896.6; ENST00000590770.5; ENST00000353196.5; ENST00000592970.1; ENST00000590151.5; ENST00000352035.7; ENST00000537919.5; ENST00000590735.1 | ||
| External Link | RMBase: m6A_site_362914 | ||
| mod ID: M6ASITE032558 | Click to Show/Hide the Full List | ||
| mod site | chr17:41912421-41912422:- | [11] | |
| Sequence | CACGGCTGGGACAGGAAGCCACAGTGAGTGGGCATGGGGTC | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000353196.5; ENST00000393896.6; ENST00000537919.5; ENST00000352035.7; ENST00000590770.5; ENST00000590735.1; ENST00000592970.1; ENST00000590151.5 | ||
| External Link | RMBase: m6A_site_362915 | ||
| mod ID: M6ASITE032559 | Click to Show/Hide the Full List | ||
| mod site | chr17:41912431-41912432:- | [9] | |
| Sequence | TGGCTGAAGCCACGGCTGGGACAGGAAGCCACAGTGAGTGG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000592970.1; ENST00000393896.6; ENST00000537919.5; ENST00000590770.5; ENST00000590151.5; ENST00000353196.5; ENST00000590735.1; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362916 | ||
| mod ID: M6ASITE032560 | Click to Show/Hide the Full List | ||
| mod site | chr17:41912497-41912498:- | [9] | |
| Sequence | CTGATCAAACGTCGTGGAAAACTTGGTCTCGTTGGGGTCAA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000590151.5; ENST00000590770.5; ENST00000592970.1; ENST00000537919.5; ENST00000353196.5; ENST00000352035.7; ENST00000393896.6; ENST00000590735.1 | ||
| External Link | RMBase: m6A_site_362917 | ||
| mod ID: M6ASITE032561 | Click to Show/Hide the Full List | ||
| mod site | chr17:41912522-41912523:- | [9] | |
| Sequence | GAACTTGGTAGTCAAGCCAGACCAGCTGATCAAACGTCGTG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000590770.5; ENST00000590151.5; ENST00000537919.5; ENST00000592970.1; ENST00000352035.7; ENST00000393896.6; ENST00000590735.1; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362918 | ||
| mod ID: M6ASITE032562 | Click to Show/Hide the Full List | ||
| mod site | chr17:41913736-41913737:- | [9] | |
| Sequence | CTGGGCCCGCTTGCTGCAGGACCACCCCTGGCTGCTCAGCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000590735.1; ENST00000590770.5; ENST00000393896.6; ENST00000590151.5; ENST00000537919.5; ENST00000592970.1; ENST00000352035.7; ENST00000353196.5 | ||
| External Link | RMBase: m6A_site_362919 | ||
| mod ID: M6ASITE032563 | Click to Show/Hide the Full List | ||
| mod site | chr17:41913757-41913758:- | [9] | |
| Sequence | TCGGGTCACTCCTGACACAGACTGGGCCCGCTTGCTGCAGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HepG2; peripheral-blood; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000592970.1; ENST00000590735.1; ENST00000590770.5; ENST00000353196.5; ENST00000590151.5; ENST00000393896.6; ENST00000537919.5; ENST00000352035.7 | ||
| External Link | RMBase: m6A_site_362920 | ||
| mod ID: M6ASITE032564 | Click to Show/Hide the Full List | ||
| mod site | chr17:41913763-41913764:- | [8] | |
| Sequence | GTATGCTCGGGTCACTCCTGACACAGACTGGGCCCGCTTGC | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000352035.7; ENST00000592970.1; ENST00000590151.5; ENST00000393896.6; ENST00000590735.1; ENST00000537919.5; ENST00000353196.5; ENST00000590770.5 | ||
| External Link | RMBase: m6A_site_362921 | ||
| mod ID: M6ASITE032565 | Click to Show/Hide the Full List | ||
| mod site | chr17:41913826-41913827:- | [8] | |
| Sequence | GACGGGCAAAGAACTCCTTTACAAGTTCATCTGTACCACCT | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000590770.5; ENST00000393896.6; ENST00000592970.1; ENST00000352035.7; ENST00000590151.5; ENST00000353196.5; ENST00000537919.5; ENST00000590735.1 | ||
| External Link | RMBase: m6A_site_362922 | ||
| mod ID: M6ASITE032566 | Click to Show/Hide the Full List | ||
| mod site | chr17:41913834-41913835:- | [9] | |
| Sequence | TCAGAGCAGACGGGCAAAGAACTCCTTTACAAGTTCATCTG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; CD4T; peripheral-blood; HEK293T; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000590735.1; ENST00000353196.5; ENST00000592970.1; ENST00000352035.7; ENST00000537919.5; ENST00000590151.5; ENST00000393896.6; ENST00000590770.5 | ||
| External Link | RMBase: m6A_site_362923 | ||
| mod ID: M6ASITE032567 | Click to Show/Hide the Full List | ||
| mod site | chr17:41918890-41918891:- | [9] | |
| Sequence | GGAAGAAGCGCCGCGCACGGACTTCGGCAGAGGTGAGCACG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; H1A; H1B; MM6; CD4T; peripheral-blood; GSC-11; HEK293T; HEK293A-TOA; MSC; TIME; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000590735.1; ENST00000352035.7; ENST00000353196.5; ENST00000590770.5; ENST00000592970.1; ENST00000590151.5; ENST00000393896.6; ENST00000537919.5 | ||
| External Link | RMBase: m6A_site_362924 | ||
| mod ID: M6ASITE032568 | Click to Show/Hide the Full List | ||
| mod site | chr17:41918981-41918982:- | [9] | |
| Sequence | AAAAGTCCGGCTGGGCCGGGACAAAAGCCGGATCCCGGGAA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; hESC-HEK293T; H1A; H1B; A549; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; MSC; TIME; TREX; iSLK; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MAZTER-seq; m6A-CLIP/IP; MeRIP-seq | ||
| Transcript ID List | ENST00000537919.5; ENST00000353196.5; ENST00000393896.6; ENST00000592970.1 | ||
| External Link | RMBase: m6A_site_362925 | ||
| mod ID: M6ASITE032569 | Click to Show/Hide the Full List | ||
| mod site | chr17:41930406-41930407:- | [9] | |
| Sequence | CTTGTAAGACAGCCAAGAAAACAGGAAGAGGGTTGGAGGCA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000592970.1 | ||
| External Link | RMBase: m6A_site_362926 | ||
| mod ID: M6ASITE032570 | Click to Show/Hide the Full List | ||
| mod site | chr17:41930418-41930419:- | [9] | |
| Sequence | TGAAAAGGCTCCCTTGTAAGACAGCCAAGAAAACAGGAAGA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000592970.1 | ||
| External Link | RMBase: m6A_site_362927 | ||
Pseudouridine (Pseudo)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: PSESITE000087 | Click to Show/Hide the Full List | ||
| mod site | chr17:41892334-41892335:- | [12] | |
| Sequence | AGGTAGATGTGCTCATCAACTTTGCCTCTCTCCGCTCTGCC | ||
| Transcript ID List | ENST00000352035.7; ENST00000590151.5; ENST00000393896.6; ENST00000537919.5; ENST00000353196.5 | ||
| External Link | RMBase: Pseudo_site_2013 | ||
References