m6A Regulator Information
General Information of the m6A Regulator (ID: REG00026)
| Regulator Name | Zinc finger CCCH domain-containing protein 13 (ZC3H13) | ||||
|---|---|---|---|---|---|
| Synonyms |
KIAA0853
Click to Show/Hide
|
||||
| Gene Name | ZC3H13 | ||||
| Sequence |
MSKIRRKVTVENTKTISDSTSRRPSVFERLGPSTGSTAETQCRNWLKTGNCLYGNTCRFV
HGPSPRGKGYSSNYRRSPERPTGDLRERMKNKRQDVDTEPQKRNTEESSSPVRKESSRGR HREKEDIKITKERTPESEEENVEWETNRDDSDNGDINYDYVHELSLEMKRQKIQRELMKL EQENMEKREEIIIKKEVSPEVVRSKLSPSPSLRKSSKSPKRKSSPKSSSASKKDRKTSAV SSPLLDQQRNSKTNQSKKKGPRTPSPPPPIPEDIALGKKYKEKYKVKDRIEEKTRDGKDR GRDFERQREKRDKPRSTSPAGQHHSPISSRHHSSSSQSGSSIQRHSPSPRRKRTPSPSYQ RTLTPPLRRSASPYPSHSLSSPQRKQSPPRHRSPMREKGRHDHERTSQSHDRRHERREDT RGKRDREKDSREEREYEQDQSSSRDHRDDREPRDGRDRRDARDTRDRRELRDSRDMRDSR EMRDYSRDTKESRDPRDSRSTRDAHDYRDREGRDTHRKEDTYPEESRSYGRNHLREESSR TEIRNESRNESRSEIRNDRMGRSRGRVPELPEKGSRGSRGSQIDSHSSNSNYHDSWETRS SYPERDRYPERDNRDQARDSSFERRHGERDRRDNRERDQRPSSPIRHQGRNDELERDERR EERRVDRVDDRRDERARERDRERERDRERERERERERDREREKERELERERARERERERE KERDRERDRDRDHDRERERERERDREKEREREREERERERERERERERERERERERARER DKERERQRDWEDKDKGRDDRREKREEIREDRNPRDGHDERKSKKRYRNEGSPSPRQSPKR RREHSPDSDAYNSGDDKNEKHRLLSQVVRPQESRSLSPSHLTEDRQGRWKEEDRKPERKE SSRRYEEQELKEKVSSVDKQREQTEILESSRMRAQDIIGHHQSEDRETSDRAHDENKKKA KIQKKPIKKKKEDDVGIERGNIETTSEDGQVFSPKKGQKKKSIEKKRKKSKGDSDISDEE AAQQSKKKRGPRTPPITTKEELVEMCNGKNGILEDSQKKEDTAFSDWSDEDVPDRTEVTE AEHTATATTPGSTPSPLSSLLPPPPPVATATATTVPATLAATTAAAATSFSTSAITISTS ATPTNTTNNTFANEDSHRKCHRTRVEKVETPHVTIEDAQHRKPMDQKRSSSLGSNRSNRS HTSGRLRSPSNDSAHRSGDDQSGRKRVLHSGSRDREKTKSLEITGERKSRIDQLKRGEPS RSTSSDRQDSRSHSSRRSSPESDRQVHSRSGSFDSRDRLQERDRYEHDRERERERRDTRQ REWDRDADKDWPRNRDRDRLRERERERERDKRRDLDRERERLISDSVERDRDRDRDRTFE SSQIESVKRCEAKLEGEHERDLESTSRDSLALDKERMDKDLGSVQGFEETNKSERTESLE GDDESKLDDAHSLGSGAGEGYEPISDDELDEILAGDAEKREDQQDEEKMPDPLDVIDVDW SGLMPKHPKEPREPGAALLKFTPGAVMLRVGISKKLAGSELFAKVKETCQRLLEKPKDAD NLFEHELGALNMAALLRKEERASLLSNLGPCCKALCFRRDSAIRKQLVKNEKGTIKQAYT SAPMVDNELLRLSLRLFKRKTTCHAPGHEKTEDNKLSQSSIQQELCVS Click to Show/Hide
|
||||
| Family | ZC3H13 family | ||||
| Function |
Associated component of the WMM complex, a complex that mediates N6-methyladenosine (m6A) methylation of RNAs, a modification that plays a role in the efficiency of mRNA splicing and RNA processing . Acts as a key regulator of m6A methylation by promoting m6A methylation of mRNAs at the 3'-UTR (By similarity). Controls embryonic stem cells (ESCs) pluripotency via its role in m6A methylation (By similarity). In the WMM complex, anchors component of the MACOM subcomplex in the nucleus (By similarity). Also required for bridging WTAP to the RNA-binding component RBM15 (RBM15 or RBM15B) (By similarity).
Click to Show/Hide
|
||||
| Gene ID | 23091 | ||||
| Uniprot ID | |||||
| Regulator Type | WRITER ERASER READER | ||||
| Mechanism Diagram | Click to View the Original Diagram | ||||
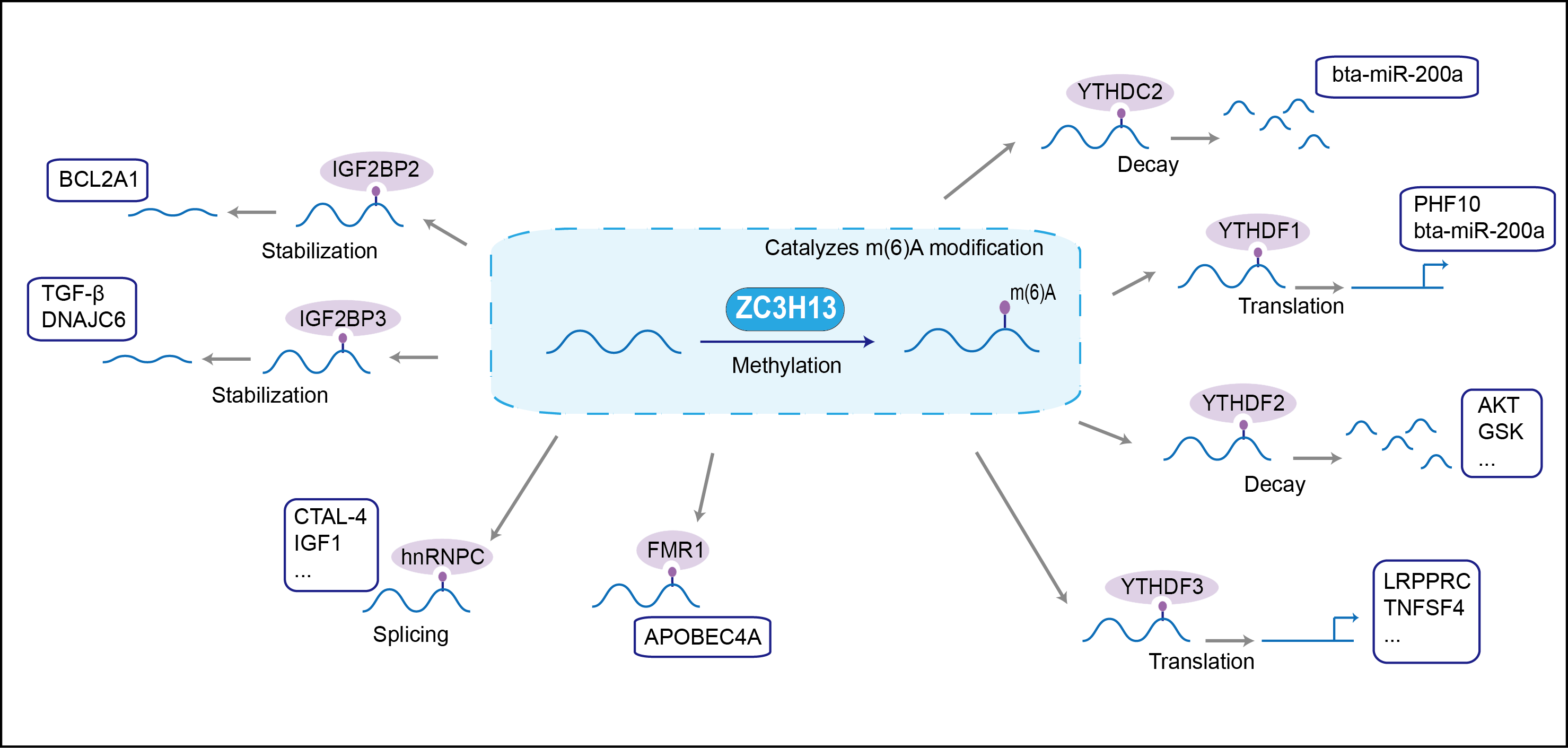
|
|||||
| Target Genes | Click to View Potential Target Genes of This Regulator | ||||
Full List of Target Gene(s) of This m6A Regulator and Corresponding Disease/Drug Response(s)
ZC3H13 can regulate the m6A methylation of following target genes, and result in corresponding disease/drug response(s). You can browse corresponding disease or drug response(s) resulted from the regulation of certain target gene.
Browse Target Gene related Disease
Browse Target Gene related Drug
Kinesin-like protein KIF26B (KIF26B)
| Representative RNA-seq result indicating the expression of this target gene regulated by ZC3H13 | ||
| Cell Line | mouse embryonic stem cells | Mus musculus |
|
Treatment: ZC3H13-/- ESCs
Control: Wild type ESCs
|
GSE145309 | |
| Regulation |
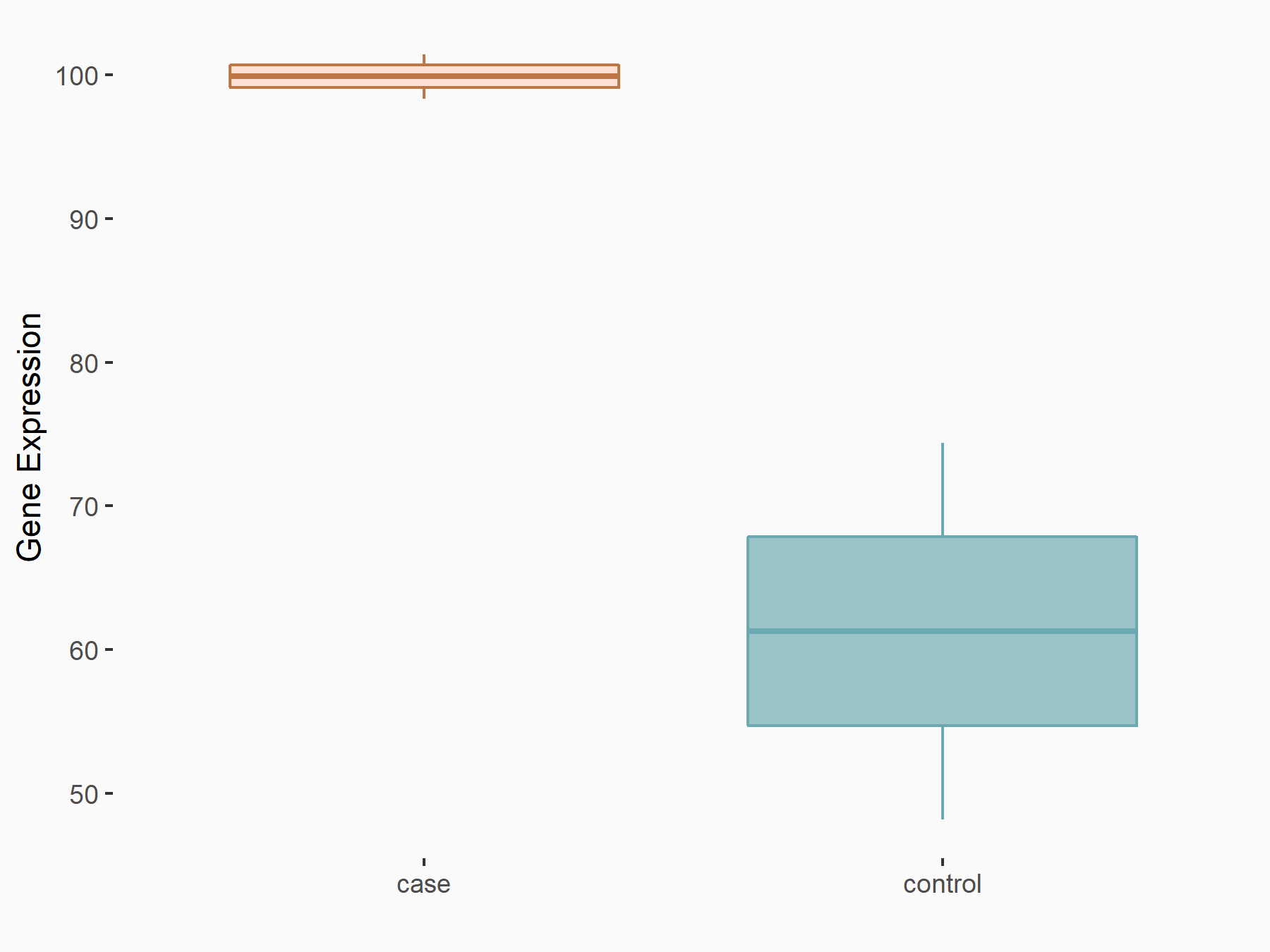  |
logFC: 7.11E-01 p-value: 3.34E-02 |
| More Results | Click to View More RNA-seq Results | |
Solid tumour/cancer [ICD-11: 2A00-2F9Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [1] | |||
| Responsed Disease | Solid tumour/cancer [ICD-11: 2A00-2F9Z] | |||
In-vitro Model |
SK-LMS-1 | Vulvar leiomyosarcoma | Homo sapiens | CVCL_0628 |
| MDA-MB-157 | Breast carcinoma | Homo sapiens | CVCL_0618 | |
| MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| In-vivo Model | BALB/c nude mice (5 weeks old) were purchased from the Beijing HFK Bioscience Co. Ltd. 2×106 cells (50 uL) were mixed with 50 uL Matrigel (BD Biosciences, San Jose, CA, USA) were injected subcutaneously in the rear flank fat pad of the nude mice (N = 6, per group). | |||
| Response Summary | Ginsenoside Rh2 reduces m6A RNA methylation via downregulating KIF26B expression in some cancer cells. KIF26B elevates m6A RNA methylation via enhancing ZC3H13/CBLL1 nuclear localization. KIF26B-SRF forms a positive feedback loop facilitating tumor growth. | |||
PHD finger protein 10 (PHF10)
| Representative RNA-seq result indicating the expression of this target gene regulated by ZC3H13 | ||
| Cell Line | mouse embryonic stem cells | Mus musculus |
|
Treatment: ZC3H13-/- ESCs
Control: Wild type ESCs
|
GSE145309 | |
| Regulation |
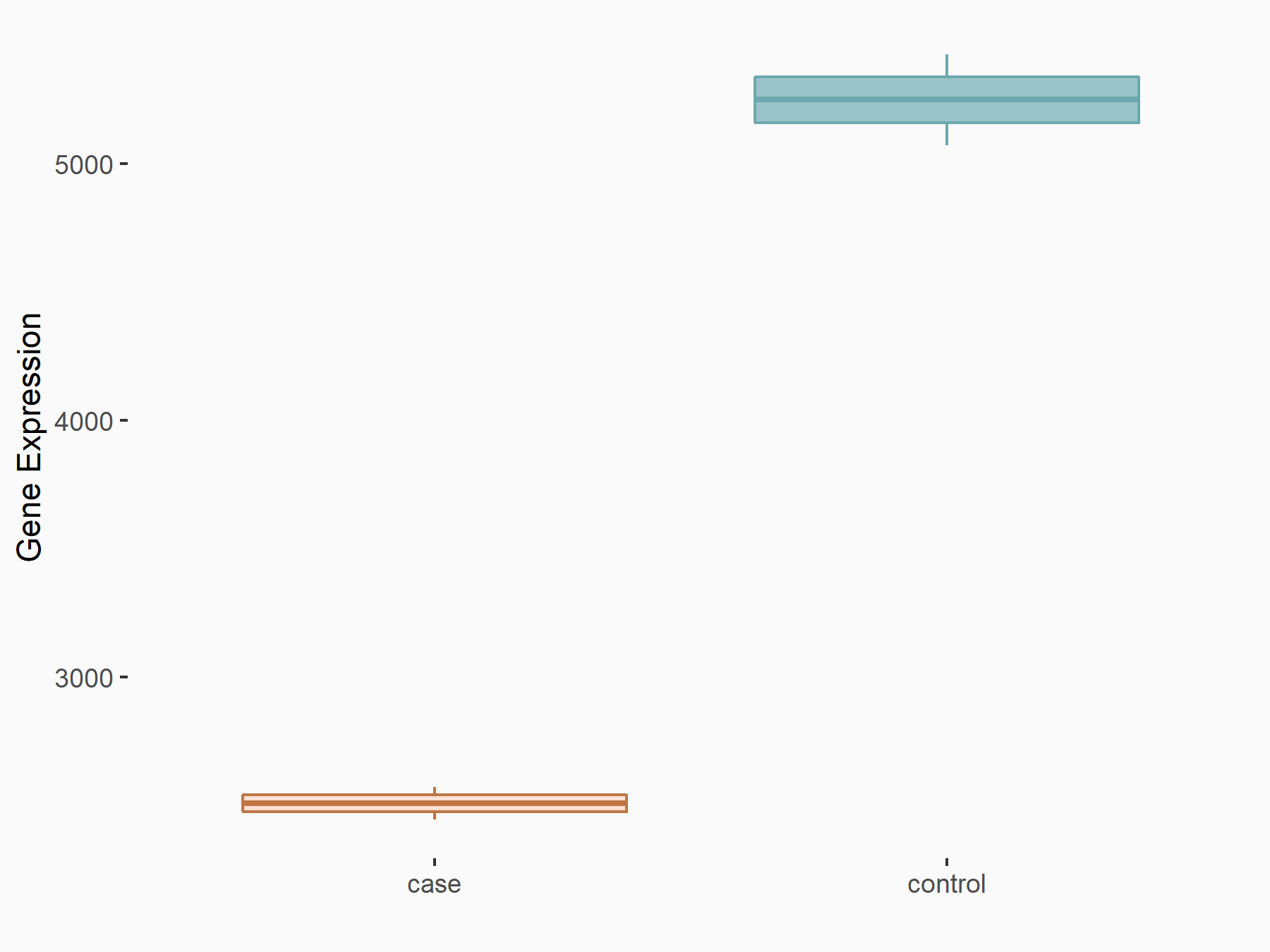 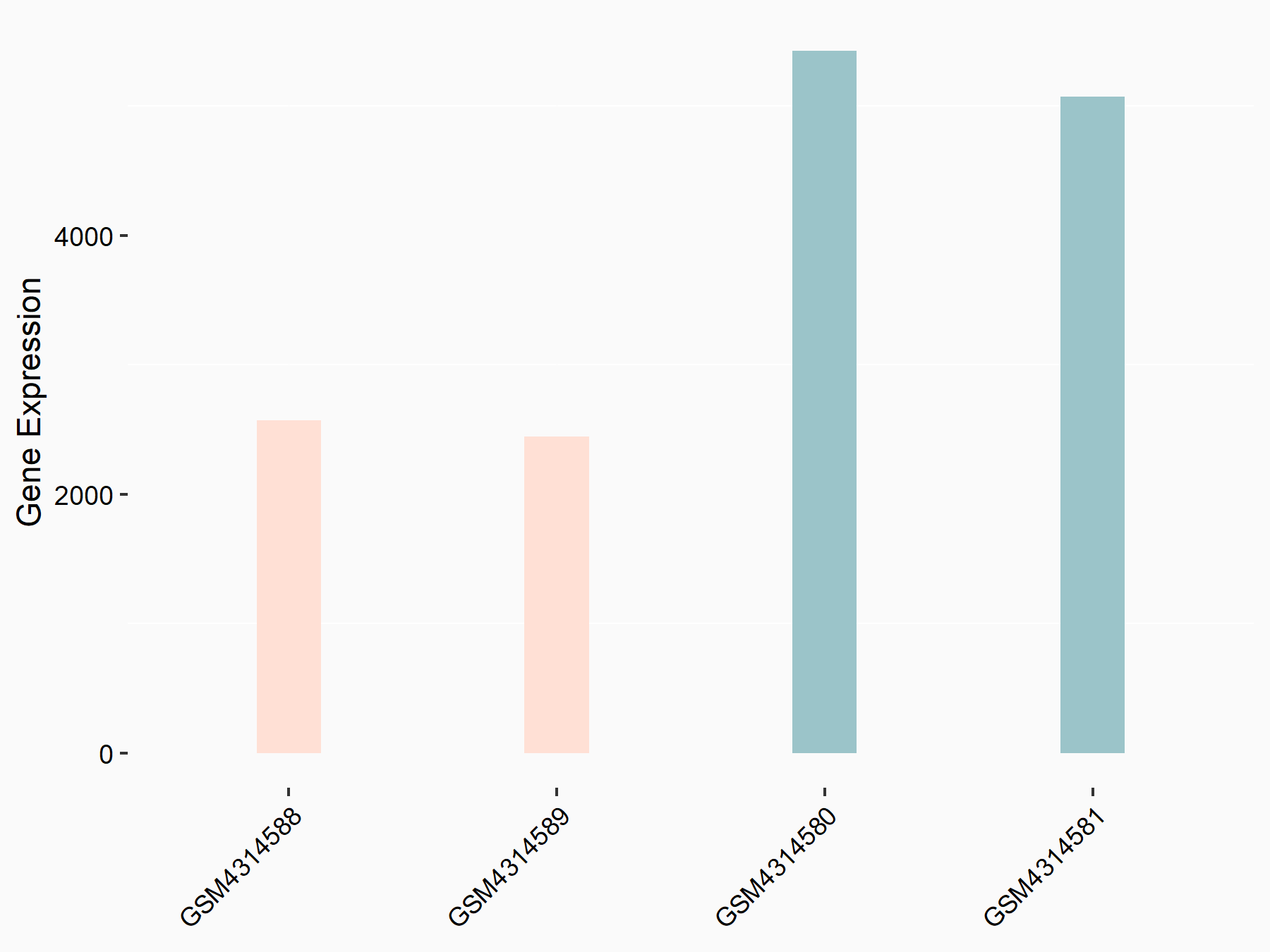 |
logFC: -1.07E+00 p-value: 1.29E-67 |
| More Results | Click to View More RNA-seq Results | |
Pancreatic cancer [ICD-11: 2C10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [2] | |||
| Responsed Disease | Pancreatic cancer [ICD-11: 2C10] | |||
| Target Regulation | Down regulation | |||
| Pathway Response | Homologous recombination | hsa03440 | ||
| Cell Process | Homologous recombination | |||
| DNA double strand breaks | ||||
In-vitro Model |
PANC-1 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 |
| MIA PaCa-2 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0428 | |
| hTERT-HPNE | Normal | Homo sapiens | CVCL_C466 | |
| DR-GFP-U2OS (DR-GFP-U2OS cells used for HR assay were generously provided by Huang Lab (Zhejiang University)) | ||||
| BxPC-3 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | |
| Response Summary | DNA damage repair is a major barrier for chemotherapy efficacy of pancreatic cancer, it's the first time that PHD finger protein 10 (PHF10) was found and involved in the DNA damage response. ZC3H13 knockdown downregulated the m6A methylation of PHF10 and decreased PHF10 translation in a YTHDF1-dependent manner. | |||
Pyruvate kinase PKM (PKM2/PKM)
| Representative RNA-seq result indicating the expression of this target gene regulated by ZC3H13 | ||
| Cell Line | Human umbilical vein endothelial cells | Homo sapiens |
|
Treatment: siKIAA0853 HUVECs
Control: siControl HUVECs
|
GSE167067 | |
| Regulation |
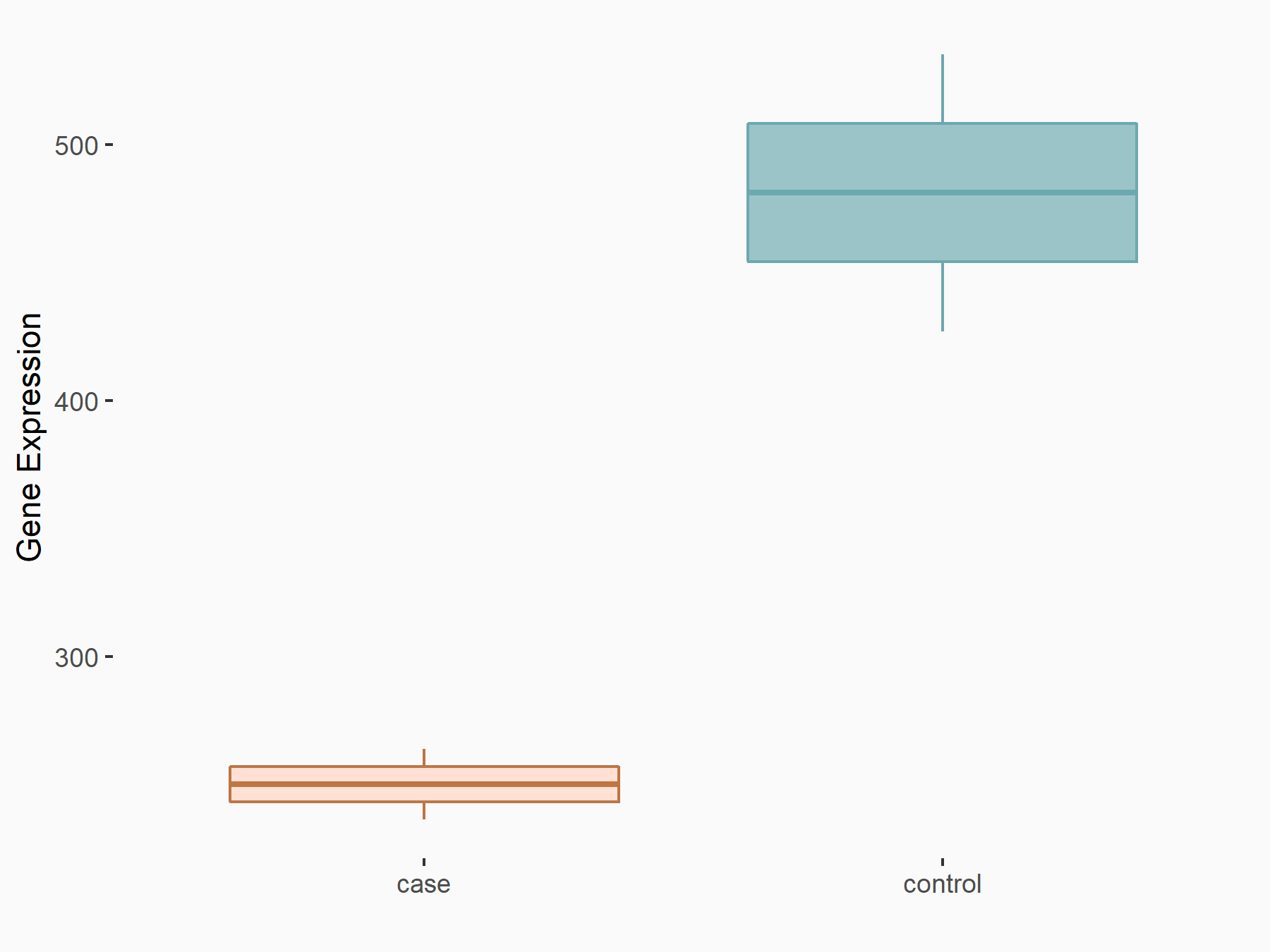 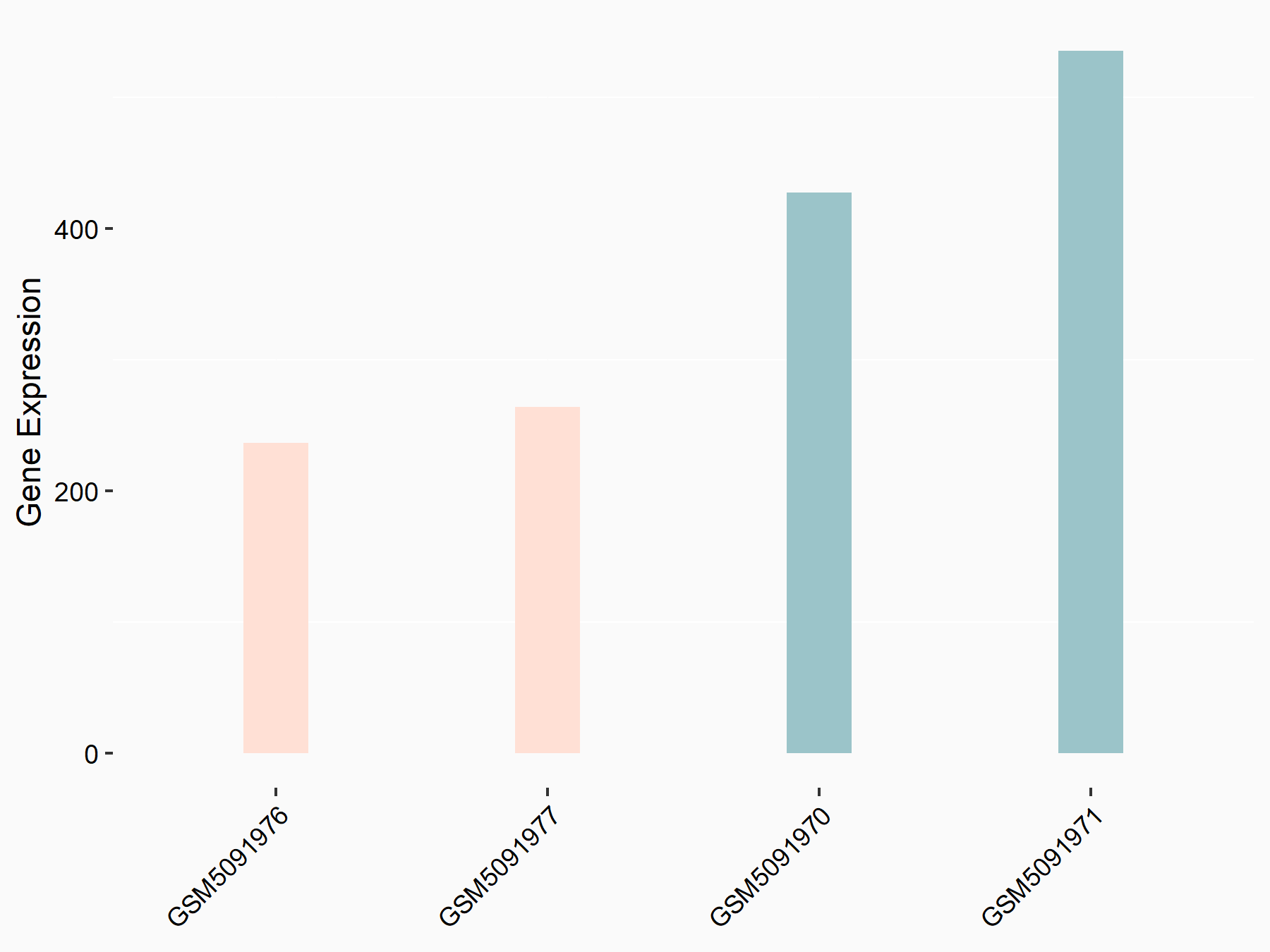 |
logFC: -9.34E-01 p-value: 1.41E-02 |
| More Results | Click to View More RNA-seq Results | |
Liver cancer [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [3] | |||
| Responsed Disease | Hepatocellular carcinoma [ICD-11: 2C12.02] | |||
| Responsed Drug | Cisplatin | Approved | ||
| Target Regulation | Down regulation | |||
| Pathway Response | Central carbon metabolism in cancer | hsa05230 | ||
| Glycolysis / Gluconeogenesis | hsa00010 | |||
| Cell Process | Glycolysis | |||
In-vitro Model |
SMMC-7721 | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 |
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hep 3B2.1-7 | Childhood hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| Response Summary | ZC3H13 overexpression sensitized to cisplatin and weakened metabolism reprogramming of HCC cells, ZC3H13-induced m6A modified patterns substantially abolished Pyruvate kinase PKM (PKM2/PKM) mRNA stability. | |||
Centromere protein K (CENPK)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [4] | |||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | |||
| Target Regulation | Up regulation | |||
| Pathway Response | Wnt signaling pathway | hsa04310 | ||
| p53 signaling pathway | hsa04115 | |||
| Cell Process | Epithelial-mesenchymal transition | |||
In-vitro Model |
SiHa | Cervical squamous cell carcinoma | Homo sapiens | CVCL_0032 |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| In-vivo Model | BALB/c-nu mice were grouped as follows to detect tumor growth: HeLa-sh-NC [mice inoculated with scramble shRNA-infected control HeLa cells (n = 5)] and HeLa-sh-CENPK [mice inoculated with CENPK-targeted shRNA-infected HeLa cells (n = 5)]. The mice were subcutaneously inoculated with 5 × 106 cells/100 uL in the flank. The longest and the shortest diameters of the growing tumors were measured every 3 d with a caliper, and the tumor volume (V) was counted by the following equation: V = (the longest diameter × the shortest diameter2)/2. The mice were grouped as follows to evaluate the tumor-initiating frequency and were inoculated with a series of 5 × 105, 2 × 105, and 5 × 104 cells subcutaneously: HeLa-sh-NC (n = 6); and HeLa-sh-CENPK (n = 6). The mice bearing subcutaneous xenograft tumors were grouped as follows to evaluate tumor chemoresistance: HeLa-sh-NC (n = 10); HeLa-sh-CENPK (n = 10); HeLa-sh-NC + cisplatin [mice inoculated with scramble shRNA-infected control HeLa cells and 3 mg/kg of cisplatin once a week intraperitoneally (ip) for 6 weeks (n = 10)]. | |||
| Response Summary | The study revealed firm links between m6A modification patterns and cervical cancer prognosis, especially through ZC3H13-mediated m6A modification of Centromere protein K (CENPK) mRNA. | |||
Homeodomain-interacting protein kinase 2 (HIPK2)
Keloid [ICD-11: EE60]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [5] | |||
| Responsed Disease | Keloid [ICD-11: EE60.0] | |||
| Target Regulation | Up regulation | |||
Putative C->U-editing enzyme APOBEC-4 (APOBEC4)
Ovarian cancer [ICD-11: 2C73]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [6] | |||
| Responsed Disease | Ovarian cancer [ICD-11: 2C73] | |||
In-vitro Model |
HEY | Ovarian serous adenocarcinoma | Homo sapiens | CVCL_0297 |
| THP-1 | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| Response Summary | Putative C->U-editing enzyme APOBEC-4 (APOBEC4) was found to be significantly correlated with m6A regulators such as WTAP, METTL14, ZC3H13, RBM15B, and FMR1. APOBEC3A was identified as a protective factor from comprehensive analyses based on the immune microenvironment and genomic instability of ovarian cancer. APOBEC3A had the potential to serve as a promising prognostic biomarker for foretelling the survival and immunotherapy response of ovarian cancer patients. | |||
Ras GTPase-activating-like protein IQGAP1 (IQGAP1)
Thyroid Cancer [ICD-11: 2D10]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [7] | |||
| Responsed Disease | Papillary thyroid cancer [ICD-11: 2D10.1] | |||
| Target Regulation | Down regulation | |||
In-vitro Model |
B-CPAP | Thyroid gland carcinoma | Homo sapiens | CVCL_0153 |
| KTC-1 | Thyroid carcinoma | Homo sapiens | CVCL_6300 | |
| K1 | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_2537 | |
| HTh83 | Thyroid gland anaplastic carcinoma | Homo sapiens | CVCL_0046 | |
| TPC-1 | Thyroid gland papillary carcinoma | Homo sapiens | CVCL_6298 | |
|
TEC
|
N.A. | Scophthalmus maximus | CVCL_J026 | |
| In-vivo Model | Female BALB/c nude mice were randomly divided into three groups (n = 5 of each group) and received neck subcutaneous injection of TPC-1 cell with stable expression of ZC3H13 and IQGAP1 genes. TPC-1 cells were prepared with a density of 1 × 107/mL cell suspension using a complete medium and 0.2 mL of cell suspension was used for injection. After injection, the volume of xenograft was measured every five days, and mice were sacrificed on day 30 to obtain the tumor. | |||
Dual oxidase 1 (DUOX1)
Laryngeal cancer [ICD-11: 2C23]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [8] | |||
| Responsed Disease | Laryngeal squamous cell carcinoma [ICD-11: 2C23.Z] | |||
| Target Regulation | Down regulation | |||
In-vitro Model |
AMC-HN-8 | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_5966 |
| Tu 177 | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_4913 | |
| Tu 686 | Laryngeal squamous cell carcinoma | Homo sapiens | CVCL_4916 | |
Dual specificity protein phosphatase 9 (DUSP9)
Brain cancer [ICD-11: 2A00]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [9] | |||
| Responsed Disease | Brain cancer [ICD-11: 2A00] | |||
| Responsed Drug | Levetiracetam | Approved | ||
| Target Regulation | Up regulation | |||
In-vitro Model |
HMC3
|
N.A. | Homo sapiens | CVCL_II76 |
| BV-2 | Normal | Mus musculus | CVCL_0182 | |
| LN-229 | Glioblastoma | Homo sapiens | CVCL_0393 | |
| A-172 | Glioblastoma | Homo sapiens | CVCL_0131 | |
| In-vivo Model | One microliter of AAV [pAAV-hSyn-HA-hM3D(Gq)-IRES-mCitrine, AAV9-hSyn-EGFP-miR-NC-knockdown (KD) or AAV9-hSyn-EGFP-miR-200c-3p-KD; 5 × 1012 v.g/mL] was injected into the mouse motor cortex (M1; 1 mm anterior, 1.5 mm lateral to the bregma and at a depth of 1 mm). | |||
hsa_circ_0081723 (Circ_POLR2J2)
Cervical cancer [ICD-11: 2C77]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [10] | |||
| Responsed Disease | Cervical cancer [ICD-11: 2C77] | |||
In-vitro Model |
HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 |
| Ca Ski | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | |
| In-vivo Model | A total of six female BALB/c nude mice (4-week-old) were evenly divided to sh-NC group and sh-circ group. Then, 2 × 107 HeLa cells, transfected with either sh-NC or sh-circ, were subcutaneously injected into the mice. The tumor volume was observed and recorded weekly using a vernier caliper. After 4 weeks, the mice were sacrificed, and the tumors were dissected. The tumor from each mouse was imaged, and their weights were measured. This experiment was approved by the Ethics Committee of Wuhan Third Hospital. | |||
Unspecific Target Gene
Liver cancer [ICD-11: 2C12]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [11] | |||
| Responsed Disease | Liver cancer [ICD-11: 2C12] | |||
In-vitro Model |
Hep 3B2.1-7 | Childhood hepatocellular carcinoma | Homo sapiens | CVCL_0326 |
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
Cardiomyopathy [ICD-11: BC43]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response of This Target Gene | [12] | |||
| Responsed Disease | Cardiomyopathy [ICD-11: BC43] | |||
In-vitro Model |
BHK HL-1
|
N.A. | Mesocricetus auratus | CVCL_XY23 |
Pyruvate kinase PKM (PKM2/PKM)
| Representative RNA-seq result indicating the expression of this target gene regulated by ZC3H13 | ||
| Cell Line | Human umbilical vein endothelial cells | Homo sapiens |
|
Treatment: siKIAA0853 HUVECs
Control: siControl HUVECs
|
GSE167067 | |
| Regulation |
 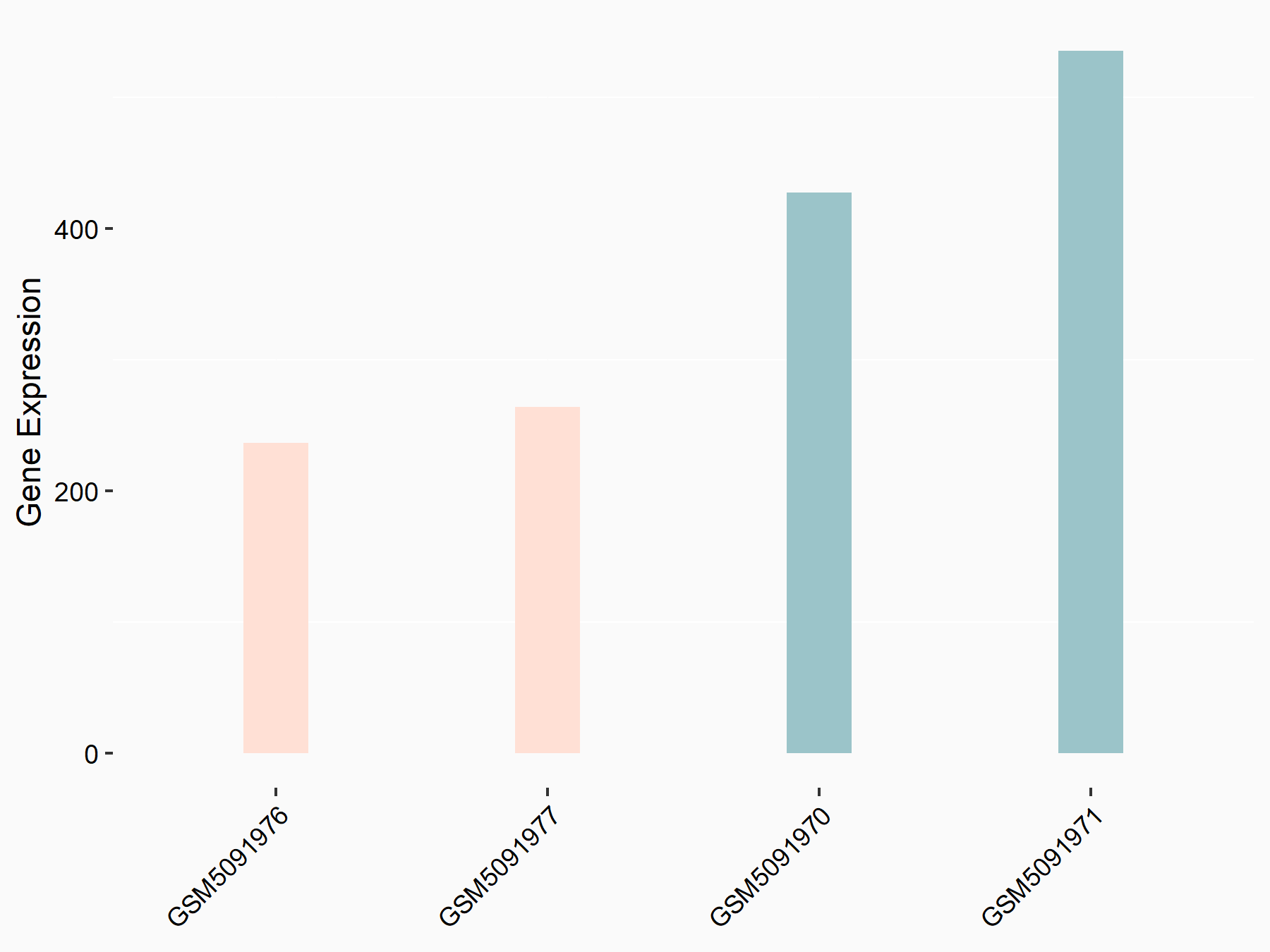 |
logFC: -9.34E-01 p-value: 1.41E-02 |
| More Results | Click to View More RNA-seq Results | |
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [3] | |||
| Responsed Disease | Hepatocellular carcinoma | ICD-11: 2C12.02 | ||
| Target Regulation | Down regulation | |||
| Pathway Response | Central carbon metabolism in cancer | hsa05230 | ||
| Glycolysis / Gluconeogenesis | hsa00010 | |||
| Cell Process | Glycolysis | |||
| In-vitro Model | SMMC-7721 | Endocervical adenocarcinoma | Homo sapiens | CVCL_0534 |
| Huh-7 | Adult hepatocellular carcinoma | Homo sapiens | CVCL_0336 | |
| Hep-G2 | Hepatoblastoma | Homo sapiens | CVCL_0027 | |
| Hep 3B2.1-7 | Childhood hepatocellular carcinoma | Homo sapiens | CVCL_0326 | |
| Response Summary | ZC3H13 overexpression sensitized to cisplatin and weakened metabolism reprogramming of HCC cells, ZC3H13-induced m6A modified patterns substantially abolished Pyruvate kinase PKM (PKM2/PKM) mRNA stability. | |||
Dual specificity protein phosphatase 9 (DUSP9)
Levetiracetam
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response of This Target Gene | [9] | |||
| Responsed Disease | Brain cancer | ICD-11: 2A00 | ||
| Target Regulation | Up regulation | |||
| In-vitro Model |
HMC3
|
N.A. | Homo sapiens | CVCL_II76 |
| BV-2 | Normal | Mus musculus | CVCL_0182 | |
| LN-229 | Glioblastoma | Homo sapiens | CVCL_0393 | |
| A-172 | Glioblastoma | Homo sapiens | CVCL_0131 | |
| In-vivo Model | One microliter of AAV [pAAV-hSyn-HA-hM3D(Gq)-IRES-mCitrine, AAV9-hSyn-EGFP-miR-NC-knockdown (KD) or AAV9-hSyn-EGFP-miR-200c-3p-KD; 5 × 1012 v.g/mL] was injected into the mouse motor cortex (M1; 1 mm anterior, 1.5 mm lateral to the bregma and at a depth of 1 mm). | |||
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
RNA modification
m6A Target: Zinc finger protein SNAI1 (SNAI1)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT00484 | ||
| Epigenetic Regulator | Fat mass and obesity-associated protein (FTO) | |
| Regulated Target | hsa-mir-22 | |
| Crosstalk relationship | m6Am → m6A | |
Non-coding RNA
m6A Target: Dual specificity protein phosphatase 9 (DUSP9)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05165 | ||
| Epigenetic Regulator | hsa-miR-200c-3p | |
| Regulated Target | Zinc finger CCCH domain-containing protein 13 (ZC3H13) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Brain cancer | |
| Drug | Levetiracetam | |
m6A Target: hsa_circ_0081723 (Circ_POLR2J2)
| In total 1 item(s) under this m6A target | ||
| Crosstalk ID: M6ACROT05827 | ||
| Epigenetic Regulator | hsa_circ_0081723 (Circ_POLR2J2) | |
| Crosstalk relationship | m6A → ncRNA | |
| Disease | Cervical cancer | |
Xenobiotics Compound(s) Regulating the m6A Methylation Regulator
| Compound Name | Ginsenoside Rh2 | Investigative |
|---|---|---|
| Synonyms |
Ginsenoside Rh2; 78214-33-2; 20(S)-Ginsenoside Rh2; Ginsenoside-Rh2; (20S)-ginsenoside Rh2; 20(S)-Ginsenoside; CHEBI:77147; 20S-Ginsenoside Rh2; UNII-0JU44A5KWG; 0JU44A5KWG; (2R,3R,4S,5S,6R)-2-[[(3S,5R,8R,9R,10R,12R,13R,14R,17S)-12-hydroxy-17-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl]oxy]-6-(hydroxymethyl)oxane-3,4,5-triol; (20R)-Ginsenoside Rh2; (3beta,12beta)-12,20-dihydroxydammar-24-en-3-yl beta-D-glucopyranoside; C36H62O8; (20R)Ginsenoside Rh2; 20(S)-Ginsenoside-RH2; (S)Ginsenoside-Rh2; CHEMBL1783834; DTXSID70999457; 20(S)-Rh2; 112246-15-8; 67400-17-3; HY-N0605; BDBM50023457; Ginsenoside Rh2, analytical standard; MFCD00800712; s9023; s9036; ZINC72129809; AKOS037514675; CCG-270259; CCG-270261; CS-3835; AC-33940; Ginsenoside Rh2; 20(s)-Ginsenoside Rh2; X1143; C22128; 12,20-Dihydroxydammar-24-en-3-yl hexopyranoside; 214G332; Q-100827; Q27146703; 3beta-(beta-D-glucopyranosyloxy)dammar-24-ene-3beta,20beta-diol; beta-D-Glucopyranoside, (3beta,12beta)-12,20-dihydroxydammar-24-en-3-yl
Click to Show/Hide
|
|
| External link | ||
| Description |
Ginsenoside Rh2 reduces m6A RNA methylation via downregulating KIF26B expression in some cancer cells. KIF26B elevates m6A RNA methylation via enhancing ZC3H13/CBLL1 nuclear localization.
|
[1] |
References