m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05638
|
[1] | |||
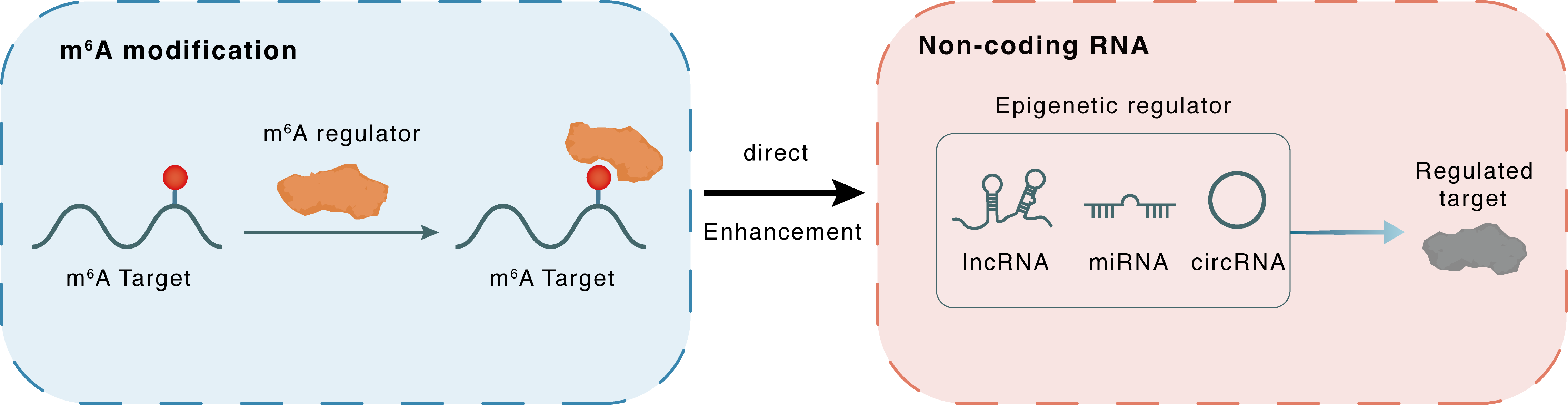 m6A modification
MALAT1
MALAT1
IGF2BP3
m6A modification
MALAT1
MALAT1
IGF2BP3
 : m6A sites
Direct
Enhancement
Non-coding RNA
MALAT1
S100A8
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
MALAT1
S100A8
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | READER | |||
| m6A Target | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | LncRNA | View Details | ||
| Regulated Target | Protein S100-A8 (S100A8) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | circRNA hsa_circ_0004287 was upregulated in peripheral blood mononuclear cells of both AD and psoriasis patients. hsa_circ_0004287 reduced the stability of its host gene Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) by competitively binding to IGF2BP3 with MALAT1 in an N6-methyladenosine (m6A)-dependent manner. Lower levels of MALAT1 promoted the ubiquitination degradation of Protein S100-A8 (S100A8)/S100A9, thereby impeding p38/mitogen-activated protein kinase phosphorylation and macrophage-mediated inflammation. | ||||
| Responsed Disease | Atopic eczema | ICD-11: EA80 | |||
| Cell Process | Ubiquitination degradation | ||||
| Inflammation. | |||||
In-vitro Model |
RAW 264.7 | Mouse leukemia | Mus musculus | CVCL_0493 | |
| In-vivo Model | IMQ-induced psoriatic model was constructed by applying 10 mg per ear 5% IMQ for 8 consecutive days, and 6 ug macrophage-specific control or hsa_circ_0004287 plasmid was topically applied every 2 days (5 mice per group per experiment). | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| EA80: Atopic eczema | 103 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Pimecrolimus | Approved | [2] | ||
| Synonyms |
Elidel; ASM 981; SDZ ASM 981; ASM-981; ASM-998; Elidel (TN); SDZ-ASM 981; Pimecrolimus [USAN:INN:BAN]; SDZ-ASM-981; Pimecrolimus (JAN/USAN/INN); 33-epi-Chloro-33-desoxyascomycin
Click to Show/Hide
|
|||
| External Link | ||||
| Roflumilast | Approved | [3] | ||
| Synonyms |
162401-32-3; DAXAS; Daliresp; 3-(CYCLOPROPYLMETHOXY)-N-(3,5-DICHLOROPYRIDIN-4-YL)-4-(DIFLUOROMETHOXY)BENZAMIDE; BY217; BYK20869; UNII-0P6C6ZOP5U; BY-217; Roflumilast (Daxas); B9302-107; 0P6C6ZOP5U; 3-(Cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)benzamide; Benzamide, 3-(cyclopropylmethoxy)-N-(3,5-dichloro-4-pyridinyl)-4-(difluoromethoxy)-; CHEMBL193240; CHEBI:47657; BYK-20869; ROF; Libertek; AK110425; 3-Cyclopropylmethoxy-N-(3,5-dichloropyridin-4-yl)-4-(difluoromethoxy)benzamide; Roflumilast [USAN]; APTA-2217; Roflumilast (JAN/USAN/INN); 3-cyclopropylmethoxy-4-difluoromethoxy-N-(3,5-di-chloropyrid-4-yl)benzamide; Alogliptin/roflumilast
Click to Show/Hide
|
|||
| External Link | ||||
| Secukinumab | Phase 2 | [4] | ||
| External Link | ||||
| cortisone | Approved | [5] | ||
| Synonyms |
11-dehydro-17-hydroxycorticosterone; 17-hydroxy-11-dehydrocorticosterone
Click to Show/Hide
|
|||
| External Link | ||||
| Prednisone | Approved | [6] | ||
| Synonyms |
Adasone; Ancortone; Bicortone; Cartancyl; Colisone; Cortan; Cortidelt; Cotone; Dacorten; Dacortin; Decortancyl; Decortin; Decortisyl; Dehydrocortisone; Dekortin; Dellacort; Deltacortene; Deltacortisone; Deltacortone; Deltasone; Deltison; Deltisona; Deltisone; Deltra; Diadreson; Econosone; Encorton; Encortone; Enkortolon; Enkorton; Fiasone; Hostacortin; Incocortyl; Juvason; Kortancyl; Lisacort; Lodotra; Metacortandracin; Meticorten; Nisona; Nizon; Novoprednisone; Nurison; Orasone; Panafcort; Panasol; Paracort; Parmenison; Pehacort; Precort; Predeltin; Prednicorm; Prednicort; Prednicot; Prednidib; Prednilonga; Prednison; Prednisona; Prednisonum; Prednitone; Prednizon; Prednovister; Presone; Pronison; Pronisone; Rectodelt; Retrocortine; Servisone; Sone; Sterapred; Supercortil; Ultracorten; Ultracortene; Winpred; Wojtab; Zenadrid; Dellacort A; Delta E; Delta cortelan; Liquid Pred; Origen Prednisone; Prednisone Intensol; Zenadrid [veterinary]; P1276; U 6020; Apo-Prednisone; Delta E.; Delta-Cortelan; Delta-Cortisone; Delta-Cortone; Delta-Dome; Delta-E; Delta1-Cortisone; Delta1-Dehydrocortisone; Di-Adreson; In-Sone; Me-Korti; Meticortelone (TN); Meticorten (TN); Meticorten (Veterinary); Metrevet (Veterinary); Prednicen-M; Prednisona [INN-Spanish]; Prednisone [INN:BAN]; Prednisonum [INN-Latin]; SK-Prednisone; Zenadrid (veterinary); Delta(sup 1)-Cortisone; Delta(sup 1)-Dehydrocortisone; Delta(sup1)-Cortisone; Delta-1-Cortisone; Delta-1-Dehydrocortisone; Deltasone, Liquid Pred, Orasone, Adasone, Deltacortisone,Prednisone; (1S,2R,10S,11S,14R,15S)-14-hydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-diene-5,17-dione; (8S,9S,10R,13S,14S,17R)-17-hydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,12,14,15,16-octahydrocyclopenta[a]phenanthrene-3,11-dione; (8xi,9xi,14xi)-17,21-dihydroxypregna-1,4-diene-3,11,20-trione; 1,2-Dehydrocortisone; 1,4-Pregnadiene-17-alpha,21-diol-3,11,20-trione; 1,4-Pregnadiene-17.alpha.,21-diol-3,11,20-trione; 1,4-Pregnadiene-17alpha,21-diol-3,11,20-trione; 1-Cortisone; 1-Dehydrocortisone; 17,21-Dihydroxypregna-1,4-diene-3,11,20-trione; 17alpha,21-Dihydroxy-1,4-pregnadiene-3,11,20-trione
Click to Show/Hide
|
|||
| External Link | ||||
| Tralokinumab | Approved | [6] | ||
| Synonyms |
CAT-354; Anti-IL 13 monoclonal antibody, CAT/AstraZeneca; IL-13 antagonist, CAT/AstraZeneca; Interleukin-13 antagonist, CAT/AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| Cromolyn sodium | Approved | [7] | ||
| Synonyms |
Altoderm; Cromolyn sodium (topical, atopic dermatitis); Cromolyn sodium (topical, atopic dermatitis), Manhattan Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| crisaborole | Approved | [8] | ||
| Synonyms |
Crisaborole ointment 2%; PF-06930164
Click to Show/Hide
|
|||
| External Link | ||||
| Upadacitinib | Approved | [6] | ||
| Synonyms |
ABT-494
Click to Show/Hide
|
|||
| External Link | ||||
| Dupilumab | Approved | [9] | ||
| Synonyms |
REGN-668; REGN-668); Anti-IL-4 monoclonal antibody (asthma/atopic dermatitis), sanofi-aventis; Anti-IL-4 receptor antibody (VelocImmune, allergy/immune disorder), Regeneron/sanofi-aventis
Click to Show/Hide
|
|||
| External Link | ||||
| Abrocitinib | Approved | [6] | ||
| External Link | ||||
| Ruxolitinib | Approved | [6] | ||
| Synonyms |
Ruxolitinib (JAK inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Tapinarof | Approved | [10] | ||
| Synonyms |
GSK-2894512
Click to Show/Hide
|
|||
| External Link | ||||
| Desonide | Approved | [11] | ||
| Synonyms |
Apolar; DesOwen; Desilux; Desonate; Desonida; Desonidum; Flusemidon; Hamiltoderm; Locapred; Prednacinolone; Prenacid; Reticus; Sterax; Steroderm; Topifug; Tridesilon; Tridesonit; Verdeso; Zotinar; Desfluorotriamcinolone acetonide; D 2083; D-2083; Desonida [INN-Spanish]; Desonidum [INN-Latin]; Desowen (TN); Verdeso (TN);Verdeso Foam (TN); Desonide (USAN/INN); Desonide [USAN:INN:BAN]; Locapred, Topifug, Tridesilon, Desonide; (4aR,4bS,5S,6aS,6bS,9aR,10aS,10bS)-5-hydroxy-6b-(hydroxyacetyl)-4a,6a,8,8-tetramethyl-4a,4b,5,6,6a,6b,9a,10,10a,10b,11,12-dodecahydro-2H-naphtho[2',1':4,5]indeno[1,2-d][1,3]dioxol-2-one; 11beta,16alpha,17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with acetone; 11beta,21-dihydroxy-16alpha,17-[(1-methylethylidene)bis(oxy)]pregna-1,4-diene-3,20-dione; 11beta,21-dihydroxy-16alpha,17-isopropylidenedioxypregna-1,4-diene-3,20-dione; 11beta,21-dihydroxy-16alpha,17alpha-isopropylidenedioxypregna-1,4-diene-3,20-dione; 16-alpha-Hydroxyprednisole-16,17-acetonide; 16alpha,17alpha-isopropylidenedioxyprednisolone; 16alpha-hydroxyprednisole-16,17-acetonide; 16alpha-hydroxyprednisolone-16alpha,17-acetonide
Click to Show/Hide
|
|||
| External Link | ||||
| Anapsos | Approved | [12] | ||
| Synonyms |
Psoriacen; EQA-00; Polypodium leucotomos extract, ASAC
Click to Show/Hide
|
|||
| External Link | ||||
| ARQ-151 | Phase 3 | [13] | ||
| External Link | ||||
| CBP-201 | Phase 3 | [14] | ||
| External Link | ||||
| IDP-124 | Phase 3 | [6] | ||
| External Link | ||||
| Lebrikizumab | Phase 3 | [6] | ||
| Synonyms |
RG3637
Click to Show/Hide
|
|||
| External Link | ||||
| SAR231893 | Phase 3 | [15] | ||
| External Link | ||||
| LY-686017 | Phase 3 | [6] | ||
| Synonyms |
NK1 antagonist (anxiety/alcoholism), Eli Lilly
Click to Show/Hide
|
|||
| External Link | ||||
| Omiganan | Phase 2 | [6] | ||
| Synonyms |
CLS-001; MBI-594AN; MX-594AN; Omiganan (topical, acne/rosacea); Omiganan (topical, acne/rosacea), Biowest Therapeutics/Cutanea; Omiganan (topical, acne/rosacea), MIGENIX/Cutanea
Click to Show/Hide
|
|||
| External Link | ||||
| Nemolizumab | Phase 3 | [16] | ||
| External Link | ||||
| MABp1 | Phase 2 | [6] | ||
| External Link | ||||
| Dupixent | Phase 3 | [6] | ||
| External Link | ||||
| Rocatinlimab | Phase 3 | [17] | ||
| Synonyms |
AMG 451 / KHK4083
Click to Show/Hide
|
|||
| External Link | ||||
| Bimosiamose | Phase 2a | [18] | ||
| Synonyms |
TBC 1269; TBC-1269
Click to Show/Hide
|
|||
| External Link | ||||
| SAR 444727 | Phase 2 | [19] | ||
| Synonyms |
(2E)-2-[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl-1H-pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4,4-dimethylpent-2-enenitrile; (alphaE,3R)-3-(4-amino-3-(2-fluoro-4-phenoxyphenyl)-1hpyrazolo(3,4-d)pyrimidin-1-yl)-alpha-(2,2-dimethylpropylidene)-beta-oxo-1-piperidinepropanenitrile; (alphaE,3R)-3-[4-Amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-alpha-(2,2-dimethylpropylidene)-beta-oxo-1-piperidinepropanenitrile; (E)-2-[(3R)-3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)pyrazolo[3,4-d]pyrimidin-1-yl]piperidine-1-carbonyl]-4,4-dimethylpent-2-enenitrile; 1581714-49-9; 1-Piperidinepropanenitrile, 3-(4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo(3,4-d)pyrimidin-1-yl)-alpha-(2,2-dimethylpropylidene)-beta-oxo-, (alphaE,3R)-; 1-Piperidinepropanenitrile, 3-[4-amino-3-(2-fluoro-4-phenoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-1-yl]-alpha-(2,2-dimethylpropylidene)-beta-oxo-, (alphaE,3R)-; AKOS040756908; atuzabrutinib; Atuzabrutinib [INN]; ATUZABRUTINIB [USAN]; BDBM197260; BDBM50589191; CHEMBL4114766; compound 11 [PMID: 35302767]; CS-0204055; GTPL11666; HY-132808; PRN473; PRN-473; SAR444727; SAR-444727; SCHEMBL15515897; SCHEMBL15516108; UNII-YZ68ZB8LWA; US9090621, 125A; YZ68ZB8LWA
Click to Show/Hide
|
|||
| External Link | ||||
| UCB1381 | Phase 2 | [20] | ||
| External Link | ||||
| Amlitelimab | Phase 2 | [21] | ||
| External Link | ||||
| Q301 | Phase 2 | [22] | ||
| Synonyms |
Boc-N-Me-Val-OH; 45170-31-8; Boc-N-methyl-L-valine; N-Boc-N-methyl-L-valine; Boc-MeVal-OH; (S)-2-((tert-Butoxycarbonyl)(methyl)amino)-3-methylbutanoic acid; Boc-N-a-methyl-L-valine; L-Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-; MFCD00038760; n-(tert-butoxycarbonyl)-n-methyl-l-valine; N-Boc-N-methylvaline; (2S)-3-methyl-2-[methyl-[(2-methylpropan-2-yl)oxycarbonyl]amino]butanoic acid; PubChem12254; Boc-N-Me-L-Val-OH; Boc-Nalpha-methyl-L-valine; Boc-N-; A-Methyl-L-valine; N-Boc-N-Methyl-L-Val-OH; SCHEMBL59435; DTXSID10426676; ZINC2391126; ANW-41482; AKOS015836686; AKOS015905243; AC-8571; AM82390; AT-5684; CS-W008976; AS-15695; BP-21374; AB0017502; Boc-N-Me-Val-OH, >=99.0% (TLC); DB-038153; 170B318; J-300310; N-alpha-t-Butyloxycarbonyl-N-alpha-methyl-L-valine; Valine, N-[(1,1-dimethylethoxy)carbonyl]-N-methyl-; (2S)-(tert-Butoxycarbonyl-methyl-amino)-3-methyl-butyric acid; (s)-2-(t-butoxycarbonyl(methyl)amino)-3-methylbutanoic acid; (s)-2-(tert-butoxycarbonyl(methyl)amino)-3-methylbutanoic acid; (S)-2-(tert-butoxycarbonyl-methyl-amino)-3-methyl-butyric acid; (2S)-2-[[(tert-butoxy)carbonyl](methyl)amino]-3-methyl butanoic acid; (2S)-2-[[(tert-Butoxy)carbonyl](methyl)amino]-3-methylbutanoic acid; (2S)-3-methyl-2-[methyl-[(2-methylpropan-2-yl)oxycarbonyl]amino]butanoicacid
Click to Show/Hide
|
|||
| External Link | ||||
| DMT210 | Phase 2 | [6] | ||
| External Link | ||||
| PF-07038124 | Phase 2 | [23] | ||
| Synonyms |
(R)-4-(5-(4-Methoxy-3-propoxyphenyl)pyridin-3-yl)-1,2-oxaborolan-2-ol; 2415085-44-6; 3-[(4R)-2-hydroxyoxaborolan-4-yl]-5-(4-methoxy-3-propoxyphenyl)pyridine; BDBM589740; CS-0433935; Example 4 [US2020108083A1]; GLXC-25702; GTPL11950; HY-144683; M6ZU548FWD; PF 07038124 [WHO-DD]; PF07038124; PF-07038124; Pyridine, 3-((4R)-2-hydroxy-1,2-oxaborolan-4-yl)-5-(4-methoxy-3-propoxyphenyl)-; UNII-M6ZU548FWD; US11559538, Example 4
Click to Show/Hide
|
|||
| External Link | ||||
| ATI-502 | Phase 2 | [24] | ||
| Synonyms |
ifidancitinib; UNII-R105E71J13; R105E71J13; A-301; 5-[[2-(4-fluoro-3-methoxy-5-methylanilino)-5-methylpyrimidin-4-yl]amino]-3H-1,3-benzoxazol-2-one; Ifidancitinib [INN]; SCHEMBL342002; CHEMBL4594441; GTPL10638; 2(3H)-Benzoxazolone, 5-((2-((4-fluoro-3-methoxy-5-methylphenyl)amino)-5-methyl-4-pyrimidinyl)amino)-; 5-((2-((4-Fluoro-3-methoxy-5-methylphenyl)amino)-5-methyl-4-pyrimidinyl)amino)-2(3H)-benzoxazolone; 5-((2-(4-Fluoro-3-methoxy-5-methylphenylamino)-5-methylpyrimidin-4-yl)amino)benzo(d)oxazol-2(3H)-one; 5-(2-(4-fluoro-3-methoxy-5-methylphenylamino)-5-methylpyrimidin-4-ylamino)benzo[d]oxazol-2(3h)-one
Click to Show/Hide
|
|||
| External Link | ||||
| Zabedosertib | Phase 2 | [25] | ||
| Synonyms |
1931994-81-8; 2-Pyridinecarboxamide, N-(6-(1-hydroxy-1-methylethyl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)-; AKOS040755617; BAY 1834845; BAY1834845; BAY-1834845; BDBM395297; CS-0198831; D96922; EX-A5143; GTPL11415; HY-139374; MFCD32900903; MS-28697; N-(6-(1-Hydroxy-1-methylethyl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)-2-pyridinecarboxamide; N-(6-(2-Hydroxypropan-2-yl)-2-(2-(methanesulfonyl)ethyl)- 2H-indazol-5-yl)-6-(trifluoromethyl)pyridine-2- carboxamide; N-(6-(2-Hydroxypropan-2-yl)-2-(2-(methylsulfonyl)ethyl)-2H-indazol-5-yl)-6-(trifluoromethyl)picolinamide; N-[6-(1-hydroxy-1-methyl-ethyl)-2-(2-methylsulfonylethyl)indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; N-[6-(2-Hydroxy-2-propyl)-2-[2-(methylsulfonyl)ethyl]-2H-indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; N-[6-(2-hydroxypropan-2-yl)-2-(2-methylsulfonylethyl)indazol-5-yl]-6-(trifluoromethyl)pyridine-2-carboxamide; N-{6-(2-Hydroxypropan-2-yl)-2-[2-(methylsulphonyl)ethyl]-2H-indazol-5-yl}-6-(trifluoromethyl)pyridine-2-carboxamide; N1GRK350ZM; OQAMEEFUUFJZRS-UHFFFAOYSA-N; SB74225; SCHEMBL17785221; SY323200; UNII-N1GRK350ZM; US10308634, Example 12; Zabedosertib; Zabedosertib [INN]
Click to Show/Hide
|
|||
| External Link | ||||
| E6005 | Phase 2 | [26] | ||
| Synonyms |
Lotamilast; 947620-48-6; E-6005; UNII-TO043KKB9C; TO043KKB9C; Methyl 4-((3-(6,7-dimethoxy-2-(methylamino)quinazolin-4-yl)phenyl)carbamoyl)benzoate; RVT-501; Methyl 4-({3-[6,7-dimethoxy-2-(methylamino)quinazolin-4-yl]phenyl}carbamoyl)benzoate; Lotamilast [USAN]; SCHEMBL369445; CHEMBL3989967; MolPort-046-033-604; BCP25249; EX-A1382; ZINC113676839; AKOS027337123; SB16962; DB12776; CS-7517; (Methyl 4-(((3-(6,7-dimethoxy-2-(methylamino)quinazolin-4-yl)phenyl(amino)carbonyl)benzoate; HY-12740; AS-52366; Benzoic acid, 4-(((3-(6,7
Click to Show/Hide
|
|||
| External Link | ||||
| K-201 | Phase 2 | [27] | ||
| Synonyms |
DKKLXCRMAXNIJF-UHFFFAOYSA-N; UNII-0I621Y6R4Q; K201; 0I621Y6R4Q; 1038410-88-6; K 201; SCHEMBL194018; CHEMBL2440857; DTXSID90146108; K-201, Moberg; Dermatological drug combination (kaprolac, atopic dermatitis), Moberg; Urea + PEG/propylene glycol formulation (atopic dermatitis), Moberg; JTV 519
Click to Show/Hide
|
|||
| External Link | ||||
| JNJ-26113100 | Phase 2 | [28] | ||
| Synonyms |
Dermatitis agent (oral), Alza
Click to Show/Hide
|
|||
| External Link | ||||
| SRD-441 | Phase 2 | [29] | ||
| Synonyms |
Aureostat; ST-441; STP-01a; Protease inhibitor (atopic dermatitis), Surface Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| S-777469 | Phase 2 | [30] | ||
| External Link | ||||
| PMX-53 | Phase 2 | [31] | ||
| Synonyms |
YOKBGCTZYPOSQM-HPSWDUTRSA-N; PMX53; CHEMBL41547; (2S)-2-acetamido-N-[(3S,9S,12S,15R,18S)-15-(cyclohexylmethyl)-9-[3-(diaminomethylideneamino)propyl]-12-(1H-indol-3-ylmethyl)-2,8,11,14,17-pentaoxo-1,7,10,13,16-pentazabicyclo[1630]henicosan-3-yl]-3-phenylpropanamide; PMX 53; AcF-[OP(D-Cha)WR]; Ac-Phe-[Orn-Pro-cha-Trp-Arg]; C5aR-AP; AC1OCFH0; GTPL579; SCHEMBL16492460; SCHEMBL12971688; 219639-75-5; BDBM50111445; N-Acetyl-L-phenylalanyl-L-ornithyl-L-prolyl-3-cyclohexyl-D-alanyl-L-tryptophyl-D-arginine N-52-C-16-lactam
Click to Show/Hide
|
|||
| External Link | ||||
| NCX 1022 | Phase 2 | [32] | ||
| External Link | ||||
| ALX-101 | Phase 2 | [6] | ||
| Synonyms |
Rovazolac; UNII-W51K389XIL; W51K389XIL; Rovazolac [INN]; GTPL9625; SCHEMBL15242823; ZUMNJDGBYXHASJ-UHFFFAOYSA-N; example 36 [WO2013130892]; HY-109073; CS-0033536; A-110; ethyl 2-[5-[4-(3-methylsulfonylphenyl)phenyl]-3-(trifluoromethyl)pyrazol-1-yl]acetate; ethyl 2-(5-(3'-(methylsulfonyl)biphenyl-4-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)acetate; ethyl 2-(5-(3'-(methylsulfonyl)-[1,1'-biphenyl]-4-yl)-3-(trifluoromethyl)-1H-pyrazol-1-yl)acetate; Ethyl 2-(5-(3'-(methylsulfonyl)-(1,1'-biphenyl)-4-yl)-3-(trifluoromethy
Click to Show/Hide
|
|||
| External Link | ||||
| ATI-502 | Phase 2 | [6] | ||
| External Link | ||||
| Cerdulatinib | Phase 2 | [6] | ||
| Synonyms |
PRT-062070; Syk + JAK multikinase inhibitor (NHL/CLL/RA), Portola Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| ASB17061 | Phase 2 | [33] | ||
| External Link | ||||
| RVT-505 | Phase 2 | [6] | ||
| External Link | ||||
| Q-301 | Phase 2 | [6] | ||
| External Link | ||||
| PAC-14028 | Phase 2 | [34] | ||
| External Link | ||||
| MM36 | Phase 2 | [6] | ||
| Synonyms |
CHEMBL225555
Click to Show/Hide
|
|||
| External Link | ||||
| BRT-FC-83C | Phase 2 | [35] | ||
| Synonyms |
Dermatological agent (topical, atopic dermatitis), Biomed Research & Technologies
Click to Show/Hide
|
|||
| External Link | ||||
| ADX-914 | Phase 2 | [36] | ||
| External Link | ||||
| HL-009 | Phase 2 | [37] | ||
| Synonyms |
Adenosylcobamide; Adenosylcobalamin (vitamin B12) (nanoliposome, allergy), HanAll
Click to Show/Hide
|
|||
| External Link | ||||
| GW-870086-X | Phase 2 | [38] | ||
| Synonyms |
GW-870086-X (topical, atopic dermatitis), GlaxoSmithKline
Click to Show/Hide
|
|||
| External Link | ||||
| DPK-060 | Phase 2 | [39] | ||
| Synonyms |
DP-9011; DP-9012; DP-9013; DP-9011-DP-9013 series (atopic dermatitis/impetigo), DermaGen
Click to Show/Hide
|
|||
| External Link | ||||
| Santalum | Phase 2 | [6] | ||
| External Link | ||||
| TS-022 | Phase 2 | [40] | ||
| Synonyms |
Prostaglandin derivative (topical, dermatitis), Taisho
Click to Show/Hide
|
|||
| External Link | ||||
| BMX-010 | Phase 2 | [6] | ||
| Synonyms |
UNII-BN93L2NT5I; BN93L2NT5I; Manganese(III)meso-tetrakis(N-ethylpyridinium-2-yl) porphyrin chloride; Manganese(4+), chloro((2,2',2'',2'''-(21H,23H-porphine-5,10,15,20-tetrayl-kappaN21,kappaN22,kappaN23,kappaN24)tetrakis(1-ethylpyridiniumato))(2-))-, tetrachloride, (sp-5-12)-; 219818-60-7
Click to Show/Hide
|
|||
| External Link | ||||
| ANB-020 | Phase 2 | [6] | ||
| Synonyms |
etokimab
Click to Show/Hide
|
|||
| External Link | ||||
| PRO22 | Phase 2 | [6] | ||
| External Link | ||||
| LEO-29102 | Phase 2 | [41] | ||
| External Link | ||||
| LAS-41004 | Phase 2 | [42] | ||
| Synonyms |
Anti-inflammatory (psoriasis/atopic dermatitis), Almirall
Click to Show/Hide
|
|||
| External Link | ||||
| Cis-urocanic acid | Phase 2 | [43] | ||
| Synonyms |
ProtoCure; ProtoCure (emulsion, atopic dermatitis), Biocis Pharma; Anti-inflammatory (topical emulsion, dermatological disease), BioCis; ProtoCure (emulsion, atopic dermatitis/psoriasis), Biocis Pharma; ProtoCure (emulsion, atopic dermatitis/psoriasis), Laurantis; Cis-UCA (topical emulsion, atopic dermatitis/psoriasis), BioCis Pharma; Cis-UCA (topical emulsion, atopic dermatitis/psoriasis), Laurantis; Cis-urocanic acid (topical emulsion, atopic dermatitis/psoriasis), BioCis Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| AN0128 | Phase 2 | [44] | ||
| Synonyms |
Bis(3-chloro-4-methyl-phenyl)boranyl 3-hydroxypyridine-2-carboxylate; 2-Pyridinecarboxylic acid, 3-hydroxy-, anhydride with bis(3-chloro-4-methylphenyl)borinic acid; 3-Hydroxypyridine-2-carbonyloxy-bis(3-chloro-4-methylphenyl)borane
Click to Show/Hide
|
|||
| External Link | ||||
| GBR 830 | Phase 2 | [6] | ||
| External Link | ||||
| TA-7906 | Phase 2 | [45] | ||
| Synonyms |
T-2585; PDE4 inhibitor (skin disease), Maruho; PDE4 inhibitors (inflammation), Tanabe Seiyaku; T-2585.HCl
Click to Show/Hide
|
|||
| External Link | ||||
| PF-3893787 | Phase 2 | [6] | ||
| Synonyms |
PF-03893787; PF-3826719
Click to Show/Hide
|
|||
| External Link | ||||
| VTB-38543 | Phase 2 | [6] | ||
| External Link | ||||
| AN-2898 | Phase 2 | [46] | ||
| Synonyms |
PDE4 inhibitor (topical, psoriasis/atopic dermatitis), Anacor
Click to Show/Hide
|
|||
| External Link | ||||
| KP-413 | Phase 1/2 | [47] | ||
| External Link | ||||
| REGN-846 | Phase 1/2 | [48] | ||
| Synonyms |
RENG-846; SAR-302352; SAR-302532
Click to Show/Hide
|
|||
| External Link | ||||
| ZPL521 | Phase 1/2 | [6] | ||
| External Link | ||||
| PF-07295324 | Phase 1 | [49] | ||
| External Link | ||||
| PF-07242813 | Phase 1 | [50] | ||
| External Link | ||||
| PF-07275315 | Phase 1 | [51] | ||
| External Link | ||||
| STMC-103H | Phase 1 | [52] | ||
| External Link | ||||
| UCB9741 | Phase 1 | [53] | ||
| External Link | ||||
| PF-07264660 | Phase 1 | [54] | ||
| External Link | ||||
| PF-06817024 | Phase 1 | [6] | ||
| External Link | ||||
| EDP1066 | Phase 1 | [6] | ||
| External Link | ||||
| SNA-125 | Phase 1 | [6] | ||
| External Link | ||||
| SAR444656 | Phase 1 | [55] | ||
| External Link | ||||
| KPL-716 | Phase 1 | [6] | ||
| External Link | ||||
| SB414 | Phase 1 | [6] | ||
| External Link | ||||
| Quinazoline derivative 13 | Patented | [56] | ||
| Synonyms |
PMID26936077-Compound-24
Click to Show/Hide
|
|||
| External Link | ||||
| Piperazine derivative 5 | Patented | [56] | ||
| Synonyms |
PMID26936077-Compound-16
Click to Show/Hide
|
|||
| External Link | ||||
| P-1 | Discontinued in Phase 3 | [57] | ||
| Synonyms |
Zemaphyte; Zemaphyte (P1)
Click to Show/Hide
|
|||
| External Link | ||||
| AVAC | Discontinued in Phase 2 | [58] | ||
| External Link | ||||
| GW842470X | Discontinued in Phase 2 | [30] | ||
| External Link | ||||
| Dermolastin | Discontinued in Phase 2 | [59] | ||
| External Link | ||||
| CD-581 | Discontinued in Phase 1 | [60] | ||
| Synonyms |
1-(Eicosa-5,8,11-triynoyl)-4-(2-hydroxyethyl)piperazine; 4-(1-Oxo-5,8,11-eicosatriynyl)-1-piprazineethanol
Click to Show/Hide
|
|||
| External Link | ||||
| RESP-6000 | Investigative | [61] | ||
| External Link | ||||
| J-555Y | Investigative | [61] | ||
| External Link | ||||
| SWT-01113 | Investigative | [61] | ||
| External Link | ||||
| PBI-1308 | Investigative | [61] | ||
| Synonyms |
PBI-1308 (dermatological, dermatological diseases); PBI-1308 (dermatological, dermatological diseases), Darier/ProMetic
Click to Show/Hide
|
|||
| External Link | ||||
| DPS-201 | Investigative | [61] | ||
| External Link | ||||
| RTU-1096 | Investigative | [62] | ||
| External Link | ||||
| KF-66490 | Investigative | [63] | ||
| Synonyms |
K-34; PDE 4 inhibitors (dermatitis/rheumatoid arthritis); PDE 4 inhibitors (dermatitis/rheumatoid arthritis), Kyowa Hakko Kirin
Click to Show/Hide
|
|||
| External Link | ||||
| MLR-1130 | Investigative | [61] | ||
| Synonyms |
Non-steroidal antiinflammatory (topical, atopic dermatitis), Melior
Click to Show/Hide
|
|||
| External Link | ||||
| AP-1189 | Investigative | [64] | ||
| Synonyms |
AP-1089; Melanocortin receptor-1 modulator (oral, inflammation), Action Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| DM-107 | Investigative | [65] | ||
| Synonyms |
MDPK-67; MDPK-67b; Kallikrein 2 inhibitor (anticancer), Med Discovery; Kallikrein 2 inhibitor (dermatological disorders), Dermadis
Click to Show/Hide
|
|||
| External Link | ||||
| SWT-05141 | Investigative | [61] | ||
| External Link | ||||
| C-0333158 | Investigative | [61] | ||
| External Link | ||||
References