m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05484
|
[1] | |||
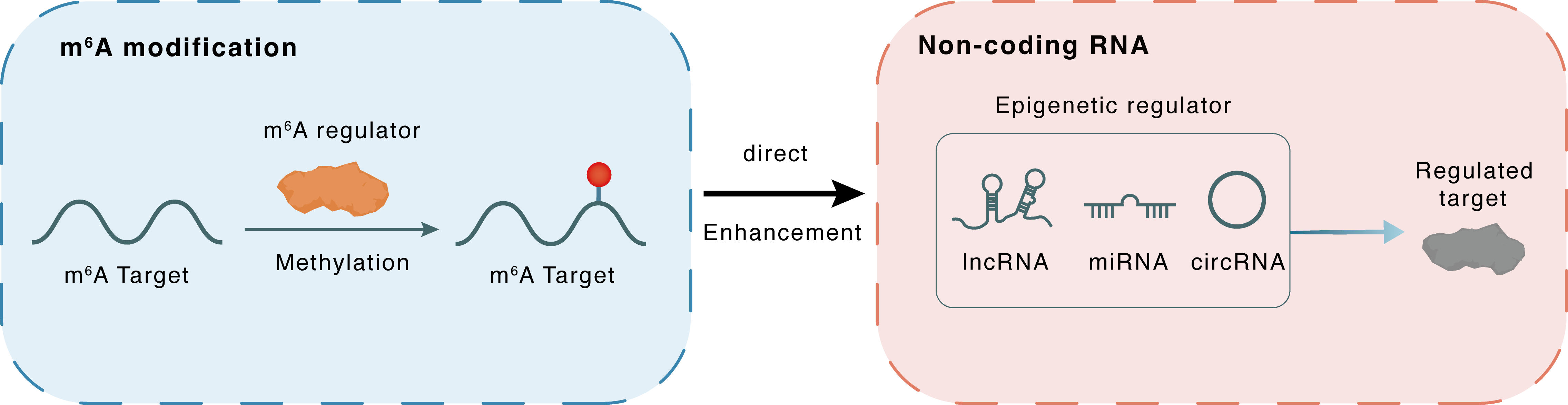 m6A modification
Circ_CUX1
Circ_CUX1
METTL3
Methylation
m6A modification
Circ_CUX1
Circ_CUX1
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
Circ_CUX1
CASP1
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
Circ_CUX1
CASP1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | hsa_circ_0081609 (circ_CUX1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa_circ_0081609 (Circ_CUX1) | circRNA | View Details | ||
| Regulated Target | Caspase-1 (CASP1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | METTL3-mediated m6A modification plays a key role in stabilizing the expression of hsa_circ_0081609 (circ_CUX1), thereby inhibiting the expression of Caspase-1 (CASP1) and conferring the radiotherapy resistance of hypopharyngeal squamous cell carcinoma. | ||||
| Responsed Disease | Hypopharyngeal cancer | ICD-11: 2B6D | |||
In-vitro Model |
SCC-4 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1684 | |
| SCC-9 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1685 | ||
| SCC-15 | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1681 | ||
| CLA-27 cell line (The head and neck tumor cell lines) | |||||
| SAS | Tongue squamous cell carcinoma | Homo sapiens | CVCL_1675 | ||
| Tca8113 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6851 | ||
| HOK | Normal | Hexagrammos otakii | CVCL_YE19 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Caspase-1 (CASP1) | 14 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Belnacasan | Phase 2 | [2] | ||
| Synonyms |
Second generation ICE inhibitors, Vertex; VRT-043198; VRT-43198; VX-765; VX-765)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PRALNACASAN | Phase 2 | [3] | ||
| Synonyms |
VX-740; UNII-N986NI319S; 192755-52-5; N986NI319S; HMR3480/VX-740; Pralnacasan [USAN:INN]; HMR 3480; VX 470; Pralnacasan (USAN/INN); AC1L4A1A; SCHEMBL142187; GTPL6467; CHEMBL437526; DTXSID60172873; HMR3480; HMR-3480; BDBM50189360; AKOS030230853; DB04875; D08978; (4S,7S)-N-[(2R,3S)-2-ethoxy-5-oxooxolan-3-yl]-7-(isoquinoline-1-carbonylamino)-6,10-dioxo-2,3,4,7,8,9-hexahydro-1H-pyridazino[1,2-a]diazepine-4-carboxamide
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 3.6 nM | |||
| External Link | ||||
| Nivocasan | Phase 2 | [4] | ||
| Synonyms |
GS-9450; LB-84451
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| AC-201 | Phase 2 | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Ac-YVAD-FMK | Patented | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Ac-YVAD-cmk | Patented | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| VE-16084 | Terminated | [7] | ||
| Synonyms |
ICE inhibitors, Sanofi Winthrop; WIN-67694
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-709049 | Terminated | [8] | ||
| Synonyms |
Ac-Yvad-cho; 143313-51-3; Acetyl-tyrosyl-valyl-alanyl-aspartal; Caspase-1 Inhibitor I; CHEMBL37630; Ac-Tyr-Val-Ala-Asp-H; IL-1beta Converting Enzyme (ICE) Inhibitor I; acetyl-Tyr-Val-Ala-Asp-aldehyde; N-acetyl-Tyr-Val-Ala-Asp-aldehyde; (3S)-3-[(2S)-2-[(2S)-2-[(2S)-2-acetamido-3-(4-hydroxyphenyl)propanamido]-3-methylbutanamido]propanamido]-4-oxobutanoic acid; Ac-Tyr-Val-Ala-Asp-Aldehyde; AC1NSK2K; SCHEMBL4349143; BDBM10355; MolPort-016-580-695; N-acetyl-Tyr-Val-Ala-Asp aldehyde; ZINC3915255; 1600AH; NCGC00167338-01
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.7 nM | |||
| External Link | ||||
| SDZ-224-015 | Terminated | [9] | ||
| Synonyms |
VE-13045
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| YVAD | Investigative | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Z-YVAD-CHO | Investigative | [11] | ||
| Synonyms |
CHEMBL159822
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 180 nM | |||
| External Link | ||||
| Z-VAD-CHO | Investigative | [11] | ||
| Synonyms |
CHEMBL320954; ZVAD-CHO; BDBM50176519; (S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methyl-butyrylamino)-propionylamino]-4-oxo-butyric acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 8 nM | |||
| External Link | ||||
| Z-YVAD-FMK | Investigative | [12] | ||
| Synonyms |
benzyloxycarbonyl-Tyr-Val-Ala-Asp(OMe)-fluoromethylketone; caspase-1 Inhibitor VI
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| M826 | Investigative | [13] | ||
| Synonyms |
compound 4sx
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
References