m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02132
|
[1], [2] | |||
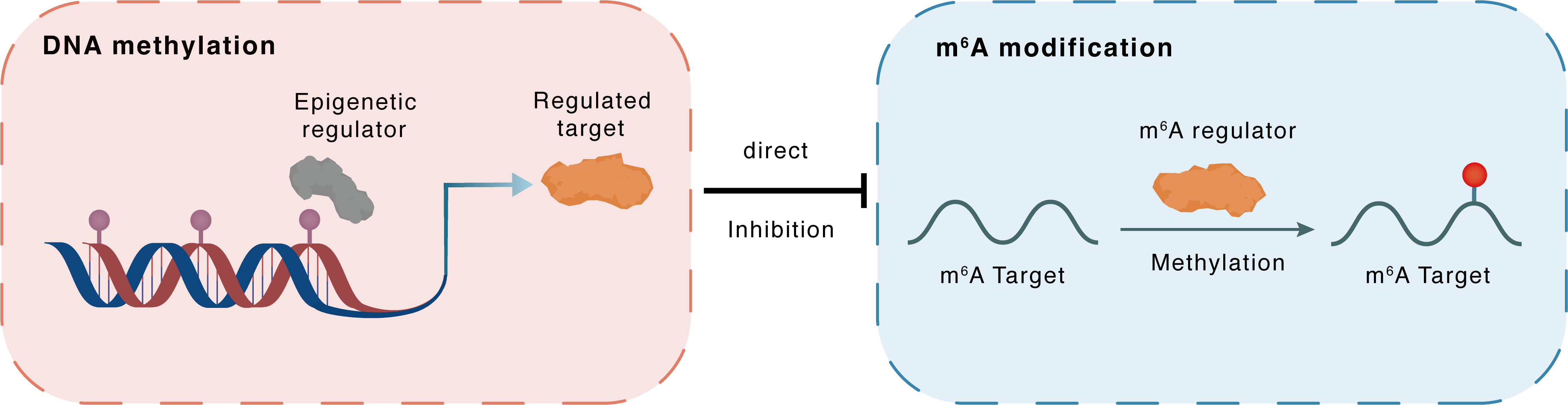 DNA methylation
DNMT3A
METTL3
Direct
Inhibition
m6A modification
BCL2
BCL2
METTL3
Methylation
DNA methylation
DNMT3A
METTL3
Direct
Inhibition
m6A modification
BCL2
BCL2
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Apoptosis regulator Bcl-2 (BCL2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | Cysteine methyltransferase DNMT3A (DNMT3A) | WRITER | View Details | ||
| Regulated Target | Methyltransferase-like protein 3 (METTL3) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | Quantitative chromatin immunoprecipitation (ChIP) assays showed that CSC substantially reduced the bindings of DNA methyltransferase 1 (DNMT1) and DNMT3A to the METTL3 promoter. celastrol treatment downregulated METTL3 and decreased m6A levels of Claspin and Apoptosis regulator Bcl-2 (BCL2) mRNA, leading to the degradation of Claspin and Bcl-2 mRNA in pancreatic cancer cells. Furthermore, we revealed that celastrol treatment downregulated Claspin and Bcl-2, at least in part, in an m6A-YTHDF3-mediated manner in pancreatic cancer cells. | ||||
| Responsed Disease | Pancreatic cancer | ICD-11: 2C10 | |||
| Responsed Drug | Celastrol | ||||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | Cell proliferation | ||||
In-vitro Model |
PANC-1 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| SW1990 | Pancreatic adenocarcinoma | Homo sapiens | CVCL_1723 | ||
| AsPC-1 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0152 | ||
| BxPC-3 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| In-vivo Model | Female BALB/c nude mice, aged 4-5 weeks, purchased from the Beijing Vital River Laboratory Animal Technology, were allowed to acclimate to local conditions for 1 week and maintained under a 12-h dark/12-h light cycle with food and water provided ad libitum. Mice (five in each group) were injected subcutaneously with 0.1 ml of cell suspension containing 2 × 106 cells in the back flank. When a tumor was palpable, it was measured every other day and the volume was calculated according to the formula volume = length × width2 × 0.5. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Apoptosis regulator Bcl-2 (BCL2) | 49 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| GDC-0199 | Approved | [3] | ||
| MOA | Modulator | |||
| Activity | Ki < 0.01 nM | |||
| External Link | ||||
| MCI-186 | Approved | [4] | ||
| Synonyms |
Edarabone; Edaravone; Methylphenylpyrazolone; Monopyrazolone; Norantipyrine; Norphenazone; Phenylmethylpyrazolone; Radicut; Developer Z; Edaravone [INN]; Phenyl methyl pyrazolone; CDS1_000986; CI Developer 1; IN1263; M0687; MCI 186; Edaravone(jan); Radicut (TN); AE-641/00371017; C.I. Developer 1; Edaravone (JAN/INN); (MCI-186); 1-Fenyl-3-methyl-2-pyrazolin-5-on; 1-Fenyl-3-methyl-2-pyrazolin-5-on [Czech]; 1-Phenyl-3-methyl-5-oxo-2-pyrazoline; 1-Phenyl-3-methyl-5-pyrazolone; 1-Phenyl-3-methylpyrazolone; 1-Phenyl-3-methylpyrazolone-5; 2,4-Dihydro-5-methyl-2-phenyl-3H-pyrazol-3-one; 3-METHYL-1-PHENYL-2-PYRAZOLIN-5-ONE; 3-Methyl-1-phenyl-2-pyrazoline-5-one; 3-Methyl-1-phenyl-5-pyrazolone; 3-Methyl-1-phenylpyrazol-5-one; 5-Methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one; 5-methyl-2-phenyl-4H-pyrazol-3-one
Click to Show/Hide
|
|||
| MOA | Regulator (upregulator) | |||
| External Link | ||||
| Taxol | Approved | [5] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Oblimersen | Phase 3 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| RG7601 | Phase 3 | [3] | ||
| MOA | Modulator | |||
| External Link | ||||
| Taxol/Paraplatin/Herceptin | Phase 3 | [7] | ||
| Synonyms |
Paclitaxel/carboplatin/trastuzumab
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| ABT-263 | Phase 3 | [8] | ||
| Synonyms |
Navitoclax; ABT 263; S1001_Selleck; ABT263, Navitoclax; 4-(4-{[2-(4-chlorophenyl)-5,5-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({4-({(1R)-3-morpholin-4-yl-1-[(phenylsulfanyl)methyl]propyl}amino)-3-[(trifluoromethyl)sulfonyl]phenyl}sulfonyl)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki < 0.01 nM | |||
| External Link | ||||
| Thymoquinone | Phase 2/3 | [9] | ||
| Synonyms |
490-91-5; Thymoquinon; p-Cymene-2,5-dione; 2-Isopropyl-5-methyl-1,4-benzoquinone; 2,5-CYCLOHEXADIENE-1,4-DIONE, 2-METHYL-5-(1-METHYLETHYL)-; 2-Isopropyl-5-methyl-p-benzoquinone; 2-Isopropyl-5-methylbenzoquinone; Polythymoquinone; 5-Isopropyl-2-methyl-1,4-benzoquinone; 2-Isopropyl-5-methylbenzo-1,4-quinone; p-Mentha-3,6-diene-2,5-dione; NSC 2228; 2-Isopropyl-5-methylcyclohexa-2,5-diene-1,4-dione; 2-Methyl-5-isopropyl-p-benzoquinone; 2-methyl-5-propan-2-ylcyclohexa-2,5-diene-1,4-dione; NSC2228; 2-methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione; UNII-O60IE26NUF; 2-Methyl-5-isopropyl-1,4-benzoquinone; O60IE26NUF; 2,5-Cyclohexadiene-1,4-dione, 5-isopropyl-2-methyl-; NSC-2228; 5-Isopropyl-2-methyl-p-benzoquinone; MFCD00001602; 2-Methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione; p-Mentha-3,6-diene-2,5-dione (8CI); 5-Isopropyl-2-methyl-2,5-Cyclohexadiene-1,4-dione; CCRIS 7152; EINECS 207-721-1; 2-methyl-5-(methylethyl)cyclohexa-2,5-diene-1,4-dione; BRN 1939047; thymolquinone; Thymoil; AI3-17758; 4hco; p-Mentha-3,5-dione; Spectrum_001237; SpecPlus_000457; Thymoquinone, >=98%; Spectrum2_000700; Spectrum3_001345; Spectrum4_001895; Spectrum5_000550; BSPBio_003129; KBioGR_002455; KBioSS_001717; DivK1c_006553; SCHEMBL542535; SPBio_000859; CHEMBL1672002; DTXSID9060079; KBio1_001497; KBio2_001717; KBio2_004285; KBio2_006853; KBio3_002349; Thymoquinone, analytical standard; CHEBI:113532; 2-Methyl-5-iso-propylbenzoquinone; BDBM166686; ZINC164367; BCP16946; HY-D0803; WLN: L6V DVJ B1 EY1&1; 2,4-dione, 5-isopropyl-2-methyl-; ANW-41600; CCG-40027; s4761; SBB008296; AKOS003368628; MCULE-9899033250; NCGC00178250-01; NCGC00178250-05; 73940-92-8; AK101679; AS-11327; 2-Isopropyl-5-methylbenzo-1,4-quinone #; 2,4-dione, 2-methyl-5-(1-methylethyl)-; CS-0012226; FT-0612708; ST45023960; K-9199; SR-05000002192; Q7799650; SR-05000002192-2; W-202869; BRD-K97566842-001-03-5; 2-methyl-5-(propan-2-yl)cyclohexa-2,5-diene-1,4-dione (F8); 2-Methyl-5-(1-methylethyl)-2,5-cyclohexadiene-1,4-dione, 9CI
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Gossypol | Phase 2 | [10] | ||
| Synonyms |
303-45-7; (-)-Gossypol; 90141-22-3; (+)-Gossypol; Pogosin; Tash 1; racemic-Gossypol; (R)-(-)-Gossypol; (R)-Gossypol; (+-)-Gossypol; (+/-)-Gossypol; CCRIS 2689; NSC56817; NSC 56817; NSC 624336; BRN 1917878; CHEMBL51483; AI3-22957; 20300-26-9; 1,1',6,6',7,7'-Hexahydroxy-5,5'-diisopropyl-3,3'-dimethyl-[2,2'-binaphthalene]-8,8'-dicarbaldehyde; 2,2'-bi[8-Formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene]; 2,2'-Bis(1,6,7-trihydroxy-3-methyl-5-isopropyl-8-aldehydonaphthalene); NSC624336; Gossypol acetate
Click to Show/Hide
|
|||
| MOA | Regulator | |||
| Activity | Ki = 170 nM | |||
| External Link | ||||
| PI-88/Taxotere | Phase 2 | [11] | ||
| Synonyms |
PI-88/docetaxel
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Obatoclax | Phase 2 | [12] | ||
| Synonyms |
CHEMBL408194; UNII-QN4128B52A; QN4128B52A; SCHEMBL631676; SCHEMBL16340850; BDBM50376902; ZINC136873052; DB12191; NCGC00387855-01; BRD-K15600710-066-01-7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 300 nM | |||
| External Link | ||||
| APG-2575 | Phase 1/2 | [13] | ||
| Synonyms |
Bcl-2/Bcl-xl inhibitor 1; 2180923-05-9; OSL3FEZ1IF; UNII-OSL3FEZ1IF; SCHEMBL20580489; US10221174, Compound 6; BDBM361577; HY-129179; CS-0103865; (S)-N-((4-(((1,4-dioxan-2-yl)methyl)amino)-3- nitrophenyl)sulfonyl)-2-((1H-pyrrolo[2,3-b]pyridin- 5-yl)oxy)-4-(4-((6-(4-chlorophenyl)spiro[3.5]non-6- en-7-yl)methyl)piperazin-1-yl)benzamide; Benzamide, 4-(4-((6-(4-chlorophenyl)spiro(3.5)non-6-en-7-yl)methyl)-1-piperazinyl)-N-((4-(((2S)-1,4-dioxan-2-ylmethyl)amino)-3-nitrophenyl)sulfonyl)-2-(1H-pyrrolo(2,3-b)pyridin-5-yloxy)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| APG-1252 | Phase 1/2 | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| AZD0466 | Phase 1/2 | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| BCL201 | Phase 1 | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pc4 | Phase 1 | [17] | ||
| Synonyms |
Pc4 (topical formulation, cancer/psoriasis/dermatological disease) Fluence/CWRU/UHCMC
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| BGB-11417 | Phase 1 | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LP-108 | Phase 1 | [19] | ||
| External Link | ||||
| LOXO-338 | Phase 1 | [20] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| VOB560 | Phase 1 | [21] | ||
| Synonyms |
S 65487
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| AI-850 | Phase 1 | [22] | ||
| MOA | Modulator | |||
| External Link | ||||
| Irofulven/Taxotere | Phase 1 | [23] | ||
| Synonyms |
MGI 114/docetaxel
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Indole-based analog 2 | Patented | [24] | ||
| Synonyms |
PMID27744724-Compound-16
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 7100 nM | |||
| External Link | ||||
| PMID27744724-Compound-10 | Patented | [24] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 5800 nM | |||
| External Link | ||||
| PMID27744724-Compound-21 | Patented | [24] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 2000 nM | |||
| External Link | ||||
| Indole-based analog 3 | Patented | [24] | ||
| Synonyms |
PMID27744724-Compound-17
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1900 nM | |||
| External Link | ||||
| PMID27744724-Compound-18 | Patented | [24] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 33000 nM | |||
| External Link | ||||
| ABT-737 | Terminated | [25] | ||
| Synonyms |
852808-04-9; ABT 737; ABT737; UNII-Z5NFR173NV; Z5NFR173NV; 4-[4-[(4'-Chloro[1,1'-biphenyl]-2-yl)methyl]-1-piperazinyl]-N-[[4-[[(1R)-3-(dimethylamino)-1-[(phenylthio)methyl]propyl]amino]-3-nitrophenyl]sulfonyl]benzamide; CHEMBL376408; C42H45ClN6O5S2; 4-{4-[(4'-Chlorobiphenyl-2-Yl)methyl]piperazin-1-Yl}-N-{[4-({(1r)-3-(Dimethylamino)-1-[(Phenylthio)methyl]propyl}amino)-3-Nitrophenyl]sulfonyl}benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki < 0.6 nM | |||
| External Link | ||||
| WL-276 | Investigative | [26] | ||
| MOA | Modulator | |||
| External Link | ||||
| N-phenyl-2,3,4-trihydroxy-5-benzyl-benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL214965; SCHEMBL15313847
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5000 nM | |||
| External Link | ||||
| 5,10-Dioxy-2-phenyl-benzo[g]pteridin-4-ylamine | Investigative | [28] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1600 nM | |||
| External Link | ||||
| modified HA14-1 compounds (cancer) | Investigative | [29] | ||
| Synonyms |
Bcl-2 inhibitor (cancer), GL Pharmaceuticals; HA14-1, GL Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Apogossypol | Investigative | [30] | ||
| Synonyms |
BI-79D10; BI-97C1; CNDO-103; CNDO-133
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 640 nM | |||
| External Link | ||||
| 2,3,4-trihydroxy-5-isopropyl-N-phenyl-benzamide | Investigative | [27] | ||
| Synonyms |
CHEMBL214839; BDBM50196033
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7000 nM | |||
| External Link | ||||
| 4,5-dibenzylbenzene-1,2-diol | Investigative | [31] | ||
| Synonyms |
4,5-Dibenzyl-1,2-benzenediol; NSC664154; AC1L8EAW; CHEMBL481409; AC1Q79U2; SCHEMBL18604567; CTK7J8443; BDBM34138; 4,5-dibenzylbenzene-1,2-diol, 9; NSC-664154
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4700 nM | |||
| External Link | ||||
| TW-37 | Investigative | [27] | ||
| Synonyms |
877877-35-5; N-[4-(2-tert-Butylphenylsulfonyl)phenyl]-2,3,4-trihydroxy-5-(2-isopropylbenzyl)benzamide; tw 37; CHEMBL217354; N-(4-((2-(tert-butyl)phenyl)sulfonyl)phenyl)-2,3,4-trihydroxy-5-(2-isopropylbenzyl)benzamide; N-(4-(2-tert-butylphenylsulfonyl)phenyl)-2,3,4-trihydroxy-5-(2-isopropylbenzyl)benzamide; N-[4-[(2-tert-Butylphenyl)sulfonyl]phenyl]-2,3,4-trihydroxy-5-[(2-isopropylphenyl)methyl]benzamide; N-(4-((2-Tert-Butylphenyl)sulfonyl)phenyl)-2,3,4-trihydroxy-5-((2-isopropylphenyl)methyl)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| QEDIIRNIARHLAQVGDSMDR | Investigative | [27] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PNT-2258 | Phase 2 | [32] | ||
| External Link | ||||
| Beclanorsen | Phase 1/2 | [32] | ||
| Synonyms |
SPC-2004; SPC-2993; SPC-2996; Anti-Bcl-2 (LNA antisense therapy), Santaris
Click to Show/Hide
|
|||
| External Link | ||||
| BP1002 | Phase 1 | [32] | ||
| External Link | ||||
| G3139 + Thalidomide | Investigative | [32] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [32] | ||
| External Link | ||||
| G3139 + G-CSF | Investigative | [32] | ||
| External Link | ||||
| G3139 + Fludarabine | Investigative | [32] | ||
| External Link | ||||
| BP-100-1.02 | Investigative | [32] | ||
| Synonyms |
Liposomal Bcl-2 (lymphoma/solid tumor), Bio-Path
Click to Show/Hide
|
|||
| External Link | ||||
| G3139 + Paclitaxel | Investigative | [32] | ||
| External Link | ||||
| G3139 + Dexamethasone | Investigative | [32] | ||
| External Link | ||||
| G3139 + Dacarbazine | Investigative | [32] | ||
| External Link | ||||
| G3139 + cytarabine (ARA-C) | Investigative | [32] | ||
| External Link | ||||
| Cysteine methyltransferase DNMT3A (DNMT3A) | 8 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PMID27376512-Compound-Figure3CN | Patented | [33] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 1100 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure3CG | Patented | [33] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 2400 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure3CM | Patented | [33] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 1100 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure2aExample1 | Patented | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3000 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [33] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| 2C10: Pancreatic cancer | 182 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Atezolizumab | Approved | [16] | ||
| External Link | ||||
| Trimethadione | Approved | [34] | ||
| Synonyms |
Absentol; Absetil; Convenixa; Convexina; Edion; Epidione; Epidone; Epixal; Etydion; Minoaleuiatin; Minoaleviatin; Petidion; Petidon; Petilep; Petimalin; Pitmal; Ptimal; Tioxanona; Tredione; Tricione; Tridilona; Tridion; Tridione; Tridone; Trilidona; Trimedal; Trimedone; Trimetadiona; Trimetadione; Trimethadion; Trimethadionum; Trimethdione; Trimethin; Trimethinum; Trimetin; Trioksal; Trioxanona; Triozanona; Tromedone; Troxidone; Abbott Brand of Trimethadione; Trimetadione [DCIT]; A 2297; Mino-Aleviatin; Neo-Absentol; Tridione (TN); Trimetadiona [INN-Spanish]; Trimethadione [INN:JAN]; Trimethadionum [INN-Latin]; Trimethadione (JP15/INN); 3,3,5-Trimethyl-2,4-diketooxazolidine; 3,5,5,-Trimethyloxazolidine-2,4-dione; 3,5,5-TRIMETHYL-OXAZOLIDINE-2,4-DIONE; 3,5,5-Trimethyl-1,3-oxazolidine-2,4-dione; 3,5,5-Trimethyl-2,4-oxazolidinedione; 3,5,5-Trojmetylooksazolidyno-2,4-dion; 3,5,5-Trojmetylooksazolidyno-2,4-dion [Polish]
Click to Show/Hide
|
|||
| External Link | ||||
| Motixafortide | Approved | [16] | ||
| External Link | ||||
| Uridine triacetate | Approved | [35] | ||
| Synonyms |
PN401
Click to Show/Hide
|
|||
| External Link | ||||
| Bentiromide | Approved | [36] | ||
| Synonyms |
Bentiromide sodium; 41748-47-4; N-Benzoyl-L-tyrosyl-4-aminobenzoic acid sodium salt; NCGC00164607-01; EINECS 255-530-7; DSSTox_CID_26476; DSSTox_RID_81647; DSSTox_GSID_46476; DTXSID6046476; CHEMBL3188891; Tox21_112229; AKOS024373587; ACM41748474; Sodium (S)-4-((2-(benzoylamino)-3-(4-hydroxyphenyl)-1-oxopropyl)amino)benzoate; CAS-41748-47-4; FT-0771579; ST51012404; N-Benzoyl-L-tyrosine p-amidobenzoic acid sodium salt; sodium (S)-4-(2-benzamido-3-(4-hydroxyphenyl)propanamido)benzoate; N-Benzoyl-L-tyrosine p-amidobenzoic acid so
Click to Show/Hide
|
|||
| External Link | ||||
| Olaparib | Approved | [16] | ||
| Synonyms |
AZD 2281; AZD2281; AZD-2281; Acylpiperazine analogue, 47; KU-0059436; KU-59436; Olaparib, KU-0059436, AZD2281,KU0059436, AZD2281; 4-[(3-{[4-Cyclopropylcarbonyl)piperazin-4-yl]carbonyl}-4-fluorophenyl)methyl]phtalazin-1(2H)-one; 4-[3-(4-Cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one
Click to Show/Hide
|
|||
| External Link | ||||
| Streptozocin | Approved | [37] | ||
| Synonyms |
Estreptozocina; STREPTOZOTOCIN; STRZ; Streptozocine; Streptozocinium; Streptozocinum; Streptozosin; Zanosar; Alkylating agent; Binds to DNA; Streptozocinium [Latin]; Streptozocine [INN-French]; Streptozocinum [INN-Latin]; Zanosar (TN); Streptozocin (USAN/INN); Streptozocin, Zanosar, STZ,Streptozotocin;N-(Methylnitrosocarbamoyl)-alpha-D-glucosamine; N-D-Glucosyl-(2)-N'-nitrosomethylharnstoff; N-D-Glucosyl-(2)-N'-nitrosomethylurea; D-Glucose, 2-deoxy-2-(((methylnitrosoamino)carbonyl)amino)-(9CI); 1-methyl-1-nitroso-3-[(2S,3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]urea; 2-Deoxy-2-(((methylnitrosoamino)carbonyl)amino)-D-glucopyranose; 2-Deoxy-2-(3-methyl-3-nitrosoureido)-D-glucopyranose; 2-Deoxy-2[[(methylnitrosoamino)-carbonyl]amino]-D-glucopyranose; 2-deoxy-2-{[methyl(nitroso)carbamoyl]amino}-alpha-D-glucopyranose
Click to Show/Hide
|
|||
| External Link | ||||
| Plazomicin | Phase 3 | [38] | ||
| Synonyms |
ACHN-490; UNII-LYO9XZ250J; 1154757-24-0; LYO9XZ250J; Plazomicin [USAN:INN]; Plazomicin (USAN); ZINC68150640; DB12615; D10151; D-Streptamine,
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [16] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Ibrutinib | Phase 3 | [16] | ||
| Synonyms |
PCI-32765; Ibrutinib (BTK inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Erlotinib | Approved | [39] | ||
| Synonyms |
Erlotinin; Tarceva; Erlotinib Base; OSI 744; R 1415; CP 358,774; CP-358774; Erlotinib(Tarceva); Tarceva (TN); CP-358,774; Erlotinib, OS-774; N-(3-ethynylphenyl)[6,7-bis(2-methoxyethoxy)quinazolin-4-yl]amine; N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine; N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine; [6,7-BIS(2-METHOXY-ETHOXY)QUINAZOLINE-4-YL]-(3-ETHYNYLPHENYL)AMINE; [6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-(3-ethynyl-phenyl)-amine; 4-[(3-Ethynylphenyl)amino]-6,7-bis(2-methoxyethoxy)quinazoline
Click to Show/Hide
|
|||
| External Link | ||||
| Ruxolitinib | Approved | [40] | ||
| Synonyms |
Ruxolitinib (JAK inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Nivolumab | Approved | [16] | ||
| External Link | ||||
| Coenzyme Q10 | Phase 2 | [16] | ||
| Synonyms |
CoQ10; Coenzyme Q10 (oral formulation); CoQ10 platform technology, Ryan (Receptagen); Coenzyme Q10 (oral formulation), Receptagen
Click to Show/Hide
|
|||
| External Link | ||||
| Aglatimagene besadenovec | Phase 1/2 | [16] | ||
| External Link | ||||
| Zolbetuximab | Phase 3 | [41] | ||
| Synonyms |
IMAB362
Click to Show/Hide
|
|||
| External Link | ||||
| AC-1204 | Phase 3 | [42] | ||
| Synonyms |
isoindoline hydrochloride; 32372-82-0; 2,3-Dihydroisoindole hydrochloride; 2,3-dihydro-1H-isoindole hydrochloride; 2,3-Dihydro-1H-isoindole HCl; Isoindoline HCl salt; 1H-Isoindole, 2,3-dihydro-, hydrochloride; Isoindoline hydrochloride, 97%; Isoindolinehydrochloride; Isoindoline, HCl; ISOINDOLINE HCL; AC1Q38WR; dihydroisoindole hydrochloride; KSC491I3F; AMBZ0192; SCHEMBL4702076; CTK3J1432; DTXSID50487241; MolPort-003-986-749; NOVIRODZMIZUPA-UHFFFAOYSA-N; BH168; CS-D1516; ACT08858; ACN-S003258; KS-000001RA
Click to Show/Hide
|
|||
| External Link | ||||
| Radiosensitizer gene therapy | Phase 3 | [43] | ||
| Synonyms |
Radiosensitizer gene therapy (prostate cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| Glufosfomide | Phase 3 | [44] | ||
| External Link | ||||
| Yttrium (90Y) clivatuzumab tetraxetan | Phase 3 | [45] | ||
| Synonyms |
PAM4 mAb; Yttrium Y 90 clivatuzumab tetraxetan; Anti-MUC1 PAM4 monoclonal antibody; Clivatuzumab tetraxetan-[90Y]; HPAM4-Cide; IMMU-107; PAM-4; PAM4-Y-90; Yttrium-90-hPAM4; 90Y-clivatuzumab tetraxetan; 90Y-hPAM4
Click to Show/Hide
|
|||
| External Link | ||||
| Y-90 Clivatuzumab | Phase 3 | [46] | ||
| External Link | ||||
| Civacir | Phase 3 | [47] | ||
| External Link | ||||
| GV1001 | Phase 3 | [48] | ||
| External Link | ||||
| Masitinib | Phase 3 | [16] | ||
| Synonyms |
790299-79-5; AB1010; Masatinib; Masitinib (AB1010); Masivet; AB-1010; AB 1010; UNII-M59NC4E26P; Masitinib [INN]; M59NC4E26P; 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-thiazolyl]amino]phenyl]benzamide; CHEMBL1908391; CHEBI:63450; Masitinib (INN); N-(4-Methyl-3-((4-(pyridin-3-yl)thiazol-2-yl)amino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide; Q-201339; C28H30N6OS; N-(4-methyl-3-(4-(pyridin-3-yl)thiazol-2-ylamino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| Glufosfamide | Phase 3 | [16] | ||
| Synonyms |
Glucosylifostamide mustard; D 19575; D-19575; Glc-IPM; Glucosyl-ifosfamide mustard; Beta-D-Glucopyranose 1-(N,N'-bis(2-chloroethyl)phosphorodiamidate; Beta-D-Glucopyranose, 1-(N,N'-bis(2-chloroethyl)phosphorodiamidate); (2S,3R,4S,5S,6R)-2-bis(2-chloroethylamino)phosphoryloxy-6-(hydroxymethyl)oxane-3,4,5-triol
Click to Show/Hide
|
|||
| External Link | ||||
| Sapacitabine | Phase 3 | [16] | ||
| Synonyms |
CYC682
Click to Show/Hide
|
|||
| External Link | ||||
| Pelareorep | Phase 2 | [49] | ||
| External Link | ||||
| Pamrevlumab | Phase 3 | [50] | ||
| External Link | ||||
| GRASPA | Phase 1 | [51] | ||
| Synonyms |
L-asparaginase (erythrocyte-encapsulated, acute lymphoblastic leukemia/solid tumor), ERYtech
Click to Show/Hide
|
|||
| External Link | ||||
| Pancreas algenpantucel-L | Phase 3 | [52] | ||
| Synonyms |
HyperAcute (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| CPI-613 | Phase 3 | [53] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Zarnestra | Phase 3 | [6] | ||
| Synonyms |
JAN; Tipifarnib; Tipifarnib [USAN]; R 115777; R115777; R-11577; R-115777; Tipifarnib (USAN/INN); Zarnestra, IND 58359, R115777, Tipifarnib; (R)-6-(Amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl)-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone; (R)-R115777; 2 (1H))-Quinolinone,6-(amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl)-4-(3-chlorophenyl)-1-methyl-, 2(1H)-quinolinone; 6-[(R)-amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2(1H)-one; 6-[(R)-amino-(4-chlorophenyl)-(3-methylimidazol-4-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2-one; 6-[(S)-AMINO(4-CHLOROPHENYL)(1-METHYL-1H-IMIDAZOL-5-YL)METHYL]-4-(3-CHLOROPHENYL)-1-METHYLQUINOLIN-2(1H)-ONE
Click to Show/Hide
|
|||
| External Link | ||||
| MM-398 | Phase 3 | [54] | ||
| External Link | ||||
| Marimastat | Phase 3 | [55] | ||
| Synonyms |
Marimastat [USAN]; BB 2516; BB-2516; Marimastat (USAN/INN); (2R,3S)-N-[(2S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl]-N',3-dihydroxy-2-(2-methylpropyl)butanediamide; (2S,3R)-3-(((1S)-2,2-Dimethyl-1-(methylcarbamoxy)propyl)carboyl)-2-hydroxy-5-methylhexanohydroxamic acid; (2S,3R)-3-(((1S)-2,2-Dimethyl-1-(methylcarbamoyl)propyl)carbamoyl)-2-hydroxy-5-methylhexanohydroxamic acid; (2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl]-N(1),2-dihydroxy-3-(2-methylpropyl)butanediamide
Click to Show/Hide
|
|||
| External Link | ||||
| AM0010 | Phase 3 | [56] | ||
| External Link | ||||
| ANX-510 | Phase 3 | [57] | ||
| External Link | ||||
| Napabucasin | Phase 3 | [16] | ||
| Synonyms |
83280-65-3; UNII-Z1HHM49K7O; 2-acetylnaphtho[2,3-b]furan-4,9-dione; Z1HHM49K7O; 2-Acetylnaphtho(2,3-b)furan-4,9-dione; 2-Acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione; Napabucasin [USAN:INN]; Napabucasin (BBI608); 2-Acetylfuranonaphthoquinone; CHEMBL64130; Napabucasin (JAN/USAN/INN); SCHEMBL1883845; Napabucasin - BBI 608/ FNQ; 2-Acetylfuro-1,4-naphthoquinone; DPHUWDIXHNQOSY-UHFFFAOYSA-N; MolPort-039-101-321; EX-A1314; ZINC13306865; s7977; AKOS027470201; DB12155; CS-1747; ACN-053294; HY-13919
Click to Show/Hide
|
|||
| External Link | ||||
| Algenpantucel-L | Phase 3 | [58] | ||
| Synonyms |
HyperAcute pancreas (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| OT-101 | Phase 2/3 | [16] | ||
| External Link | ||||
| RP101 | Phase 2/3 | [59] | ||
| Synonyms |
SCHEMBL15589316; CHEMBL3703295; BDBM149820; US8975415,
Click to Show/Hide
|
|||
| External Link | ||||
| NLG8189 | Phase 2/3 | [16] | ||
| Synonyms |
1-Methyl-D-tryptophan; Indoximod; 110117-83-4; D-Tryptophan, 1-methyl-; D-1MT; Indoximod (NLG-8189); D-1-methyltryptophan; UNII-TX5CYN1KMZ; D-(+)-1-Methyltryptophan; TX5CYN1KMZ; (R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methylindol-3-yl)propanoic acid; NSC-721782; (2R)-2-amino-3-(1-methyl-3-indolyl)propanoic acid; 1-MT; (2R)-2-azanyl-3-(1-methylindol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; D-l-Methyltryptophan; Indoximod [USAN:INN]; NLG-8189; NLG 8189
Click to Show/Hide
|
|||
| External Link | ||||
| LY2157299 | Phase 2/3 | [16] | ||
| Synonyms |
Galunisertib; 700874-72-2; LY 2157299; LY-2157299; UNII-3OKH1W5LZE; 4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide; 3OKH1W5LZE; Galunisertib (LY2157299); AK-79916; 4-[5,6-Dihydro-2-(6-methyl-2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-6-quinolinecarboxamide; 4-(2-(6-Methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo-[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide; 4-[2-(6-methylpyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline-6-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| SM-88 | Phase 2/3 | [60] | ||
| External Link | ||||
| SiG12D-LODER | Phase 2 | [16] | ||
| External Link | ||||
| BNT141 | Phase 2 | [61] | ||
| External Link | ||||
| BNT321 | Phase 2 | [62] | ||
| Synonyms |
MVT-5873
Click to Show/Hide
|
|||
| External Link | ||||
| BPM 31510 | Phase 2 | [63] | ||
| External Link | ||||
| GC4711 | Phase 2 | [64] | ||
| Synonyms |
UNII-FW5T90VM32; FW5T90VM32; GC-4419 dipropionate; Bis-propionato(gc4419); Avasopasem manganese dipropionate; GC-4711; 2248030-85-3; Manganese(II), bis-propionato((4aS,13aS,17aS,21aS)-1,2,3,4,4a,5,6,12,13,13a,14,15,16,17,17a,18,19,20,21,21a-eicosahydro-11,7-nitrilo-7H-dibenzo(b,H)(1,4,7,10)tetraaza-cycloheptadecine-kn5,kn13,kn18,kn21,kn22)-,
Click to Show/Hide
|
|||
| External Link | ||||
| CYTO-401 | Phase 2 | [65] | ||
| External Link | ||||
| Zenocutuzumab | Phase 2 | [66] | ||
| External Link | ||||
| Cabiralizumab | Phase 2 | [16] | ||
| External Link | ||||
| VS-6063 | Phase 2 | [16] | ||
| Synonyms |
Defactinib hydrochloride; 1073160-26-5; Defactinib (hydrochloride); UNII-L2S469LM49; Defactinib hydrochloride [USAN]; L2S469LM49; Defactinib hydrochloride (USAN); Benzamide, N-methyl-4-[[4-[[[3-[methyl(methylsulfonyl)amino]-2-pyrazinyl]methyl]amino]-5-(trifluoromethyl)-2-pyrimidinyl]amino]-, hydrochloride; Defactinib HCl; Benzamide, N-methyl-4-((4-(((3-(methyl(methylsulfonyl)amino)-2-pyrazinyl)methyl)amino)-5-(trifluoromethyl)-2-pyrimidinyl)amino)-, hydrochloride (1:1); Benzamide, N-methyl-4-[[4-[[[3-[methyl(methylsu
Click to Show/Hide
|
|||
| External Link | ||||
| MENK | Phase 2 | [44] | ||
| External Link | ||||
| CO-101 | Phase 2 | [67] | ||
| Synonyms |
methyl 2-(dimethylcarbamoyl)benzoate; 26593-43-1; Phthalamic acid, N,N-dimethyl-, methyl ester; CO 101; BRN 2504723; N,N-Dimethylphthalamic acid methyl ester; N,N-Dimethylphthalamic acid, methyl ester; AC1Q5ZAU; 2-09-00-00601 (Beilstein Handbook Reference); AC1L4V19; CTK4F8203; DTXSID50181156; 2-methoxycarbonyl-N,N-dimethylbenzamide; LS-109082; Benzoic acid,2-[(dimethylamino)carbonyl]-, methyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| MENK | Phase 2 | [16] | ||
| Synonyms |
IRT-101
Click to Show/Hide
|
|||
| External Link | ||||
| TL-118 | Phase 2 | [68] | ||
| Synonyms |
Hamsa 1; TL-111; TL-112; Combination anti-angiogenic therapy (oral suspension, solid tumors), Tiltan Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| Antroquinonol | Phase 2 | [16] | ||
| Synonyms |
Hocena; Fungal extract (cancer), Golden Biotechnology
Click to Show/Hide
|
|||
| External Link | ||||
| NPC-1C | Phase 2 | [16] | ||
| Synonyms |
Ensituximab
Click to Show/Hide
|
|||
| External Link | ||||
| Necuparanib | Phase 2 | [69] | ||
| External Link | ||||
| CCX872 | Phase 2 | [16] | ||
| External Link | ||||
| GC4419 | Phase 1/2 | [16] | ||
| Synonyms |
Avasopasem manganese; UNII-EY1WA413UL; EY1WA413UL; Avasopasem manganese [USAN]; SC-72325A; M-40419; 435327-40-5; Manganese, dichloro((4aS,13aS,17aS,21aS)-1,2,3,4,4a,5,6,12,13,13a,14,15,16,17,17a,18,19,20,21,21a-eicosahydro-7,11-nitrilo-7H-dibenzo(b,H)-5,13,18,21-tetraazacycloheptadecine-kappaN5,kappaN13,kappaN18,kappaN21,kappaN22)-, (pb-7-11-2344'3')-
Click to Show/Hide
|
|||
| External Link | ||||
| Istiratumab | Phase 2 | [49] | ||
| External Link | ||||
| GI-4000 | Phase 2 | [70] | ||
| External Link | ||||
| OCV-101 | Phase 2 | [71] | ||
| Synonyms |
OTS-11101
Click to Show/Hide
|
|||
| External Link | ||||
| RX-3117 | Phase 2 | [16] | ||
| Synonyms |
Antimetabolite (cancer), Rexahn; Antimetabolite (cancer), Rexahn/ Teva
Click to Show/Hide
|
|||
| External Link | ||||
| Ensitiximab | Phase 2 | [44] | ||
| External Link | ||||
| GI-4000 + gemcitabine | Phase 2 | [72] | ||
| External Link | ||||
| BC-819 | Phase 2 | [73] | ||
| External Link | ||||
| IRT-102 | Phase 2 | [74] | ||
| External Link | ||||
| LE-DT | Phase 2 | [75] | ||
| Synonyms |
Liposomal docetaxel
Click to Show/Hide
|
|||
| External Link | ||||
| TH-302 | Phase 2 | [16] | ||
| Synonyms |
evofosfamide; 918633-87-1; TH 302; TH302; UNII-8A9RZ3HN8W; Evofosfamide(TH 302); n,n'-bis(2-bromoethyl)phosphorodiamidic acid (1-methyl-2-nitro-1h-imidazol-5-yl)methyl ester; 8A9RZ3HN8W; compound 3b; Evofosfamide;HAP-302; Phosphorodiamidic acid, N,N'-bis(2-bromoethyl)-, (1-methyl-2-nitro-1H-imidazol-5-yl)methyl ester; 2-bromo-N-[(2-bromoethylamino)-[(3-methyl-2-nitroimidazol-4-yl)methoxy]phosphoryl]ethanamine; Evofosfamide [USAN:INN]; Evofosfamide(TH-302); C9H16Br2N5O4P; CHEMBL260046; SCHEMBL2357174
Click to Show/Hide
|
|||
| External Link | ||||
| Demcizumab | Phase 2 | [44] | ||
| External Link | ||||
| Anti-PSCA mab | Phase 2 | [76] | ||
| External Link | ||||
| ALT-803 | Phase 2 | [49] | ||
| Synonyms |
IL-15 agonist/ IL-15R alpha-Fc fusion complex (cancer), Altor BioScience
Click to Show/Hide
|
|||
| External Link | ||||
| ARQ 761 | Phase 2 | [16] | ||
| External Link | ||||
| Reolysinpelareorep | Phase 2 | [16] | ||
| External Link | ||||
| PBI-05204 | Phase 2 | [16] | ||
| External Link | ||||
| PCI-27483 | Phase 2 | [77] | ||
| External Link | ||||
| RX-0201 | Phase 2 | [44] | ||
| External Link | ||||
| CP-613 | Phase 2 | [78] | ||
| External Link | ||||
| CART 19 | Preclinical | [79] | ||
| External Link | ||||
| VT-122 | Phase 1 | [44] | ||
| External Link | ||||
| PEGPH20 | Phase 2 | [80] | ||
| External Link | ||||
| Ficlatuzumab | Phase 2 | [16] | ||
| Synonyms |
AV-299
Click to Show/Hide
|
|||
| External Link | ||||
| Tigatuzumab | Phase 2 | [81] | ||
| External Link | ||||
| CRS-207 | Phase 2 | [82] | ||
| External Link | ||||
| CAP1-6D | Phase 2 | [83] | ||
| Synonyms |
Modified CEA peptide (pancreatic cancer), University of Chicago
Click to Show/Hide
|
|||
| External Link | ||||
| SGT-53 | Phase 2 | [16] | ||
| Synonyms |
P53 gene stimulator (solid tumor), Synergene Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Tarextumab | Phase 2 | [84] | ||
| External Link | ||||
| BVD-523 | Phase 2 | [16] | ||
| External Link | ||||
| Ocaperidone | Phase 2 | [49] | ||
| Synonyms |
Ocaperidona; 129029-23-8; UNII-26HUS7139V; 3-(2-(4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino)ethyl)-2,9-dimethyl-4H-pyrido(1,2-a)pyrimidin-4-one; 26HUS7139V; Ocaperidonum; Ocaperidonum [INN-Latin]; Ocaperidona [INN-Spanish]; 4H-Pyrido[1,2-a]pyrimidin-4-one,3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-2,9-dimethyl-; Ocaperidone (USAN); Ocaperidone [USAN:INN:BAN]; 3-[2-[4-(6-Fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl]ethyl]-2,9-dimethyl-4H-pyrido[1,2-a]pyrimidin-4-one; 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2,9-dimethylpyrido[1,2-a]pyrimidin-4-one; 8-[2-[4-(6-fluorobenzo[d]isoxazol-3-yl)-1-piperidyl]ethyl]-2,9-dimethyl-6,10-diazabicyclo[440]deca-2,4,8,10-tetraen-7-one; FG-3019
Click to Show/Hide
|
|||
| External Link | ||||
| Encapsulated live cells converting ifosfamide | Phase 2 | [16] | ||
| External Link | ||||
| LY2603618 | Phase 2 | [85] | ||
| Synonyms |
Rabusertib; 911222-45-2; LY 2603618; LY-2603618; UNII-3S9L1NU6U7; 3S9L1NU6U7; IC-83; ly2603618 IC-83; (S)-1-(5-bromo-4-methyl-2-(morpholin-2-ylmethoxy)phenyl)-3-(5-methylpyrazin-2-yl)urea; n-(5-bromo-4-methyl-2-((2s)-2-morpholinylmethoxy)phenyl)-n'-(5-methyl-2-pyrazinyl)urea; LY2603618 (IC-83); Rabusertib [USAN:INN]; 3-(5-Bromo-4-methyl-2-[(2s)-morpholin-2-ylmethoxy]phenyl)-1-(5-methylpyrazin-2-yl)urea; N-[5-Bromo-4-methyl-2-[(2S)-2-morpholinylmethoxy)phenyl]-N'-(5-methyl-2-pyrazinyl)urea
Click to Show/Hide
|
|||
| External Link | ||||
| Salirasib | Discontinued in Phase 1/2 | [86] | ||
| Synonyms |
162520-00-5; Farnesylthiosalicylic acid; S-Farnesylthiosalicylic acid; UNII-MZH0OM550M; MZH0OM550M; CHEMBL23293; AK186909; Farnesyl Thiosalicylic Acid; 2-[[(2E,6E)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-yl]thio]benzoic Acid; 2-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]sulfanylbenzoic acid; 2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienylthio)benzoic acid; 2-(((2E,6E)-3,7,11-Trimethyl-2,6,10-dodecatrienyl)sulfanyl)benzoic acid; Benzoic acid, 2-(((2E,6E)-3,7,11-trimethyl-2,6,10-dodecatrienyl)thio)-; FTS; Farnesylthiosalicyclic acid; FTS, Thyreos; Ras antagonists, Thyreos; S-trans; Th-101; Trans-farnesylthiosalicylicacid; FTS (oral, cancer), Concordia; Farnesylthiosalicyclic acid (oral, cancer), Concordia; Ras-inhibitors (cancer), Concordia; FTS (oral, cancer), Concordia/Ono; KD032
Click to Show/Hide
|
|||
| External Link | ||||
| MM-141 | Phase 2 | [16] | ||
| External Link | ||||
| GB1275 | Phase 1/2 | [87] | ||
| External Link | ||||
| ABTL0812 | Phase 1/2 | [88] | ||
| Synonyms |
(9Z,12Z)-2-Hydroxy-9,12-octadecadienoic acid; (9Z,12Z)-2-hydroxyoctadeca-9,12-dienoic acid; (9Z,12Z)-2-hydroxyoctadecadienoic acid; (alpha)-Hydroxylinoleic acid; .ALPHA.-HYDROXYLINOLEIC ACID; 0DE74TJ7EZ; 2-hydroxy-9Z,12Z-Octadecadienoic acid; 2-hydroxylinoleic acid; 57818-44-7; 9,12-Octadecadienoic acid, 2-hydroxy-, (9Z,12Z)-; 9,12-Octadecadienoic acid, 2-hydroxy-, (Z,Z)-; ABTL0812; ABTL-0812; a-Hydroxylinoleic acid; AKOS040740632; alpha-Hydroxylinoleic acid; CHEBI:136927; CS-7178; DTXSID301258077; hydroxylinoleic acid; HY-U00141; LMFA02000290; MS-24253; s9611; SCHEMBL320069; UNII-0DE74TJ7EZ
Click to Show/Hide
|
|||
| External Link | ||||
| GP-2250 | Phase 1/2 | [89] | ||
| External Link | ||||
| Delolimogene mupadenorepvec | Phase 1/2 | [90] | ||
| Synonyms |
LOAd703
Click to Show/Hide
|
|||
| External Link | ||||
| GSK3145095 | Phase 1/2 | [91] | ||
| Synonyms |
1622849-43-7; CHEMBL4452233; (S)-5-benzyl-N-(7,9-difluoro-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-yl)-4H-1,2,4-triazole-3-carboxamide; UNII-B4D3WPS7JY; B4D3WPS7JY; SCHEMBL17312826; BCP31015; EX-A3069; BDBM50502339; s8845; GSK-3145095; HY-111946; CS-0094287; GSK 3145095; FC1=CC2=C(NC(=O)[C@H](CC2)NC(=O)C2=NN=C(CC3=CC=CC=C3)N2)C(F)=C1; (S)-5-Benzyl-N-(7,9-difluoro-2-oxo-2,3,4,5-tetrahydro-1hbenzo(b)azepin-3-yl)-1H-1,2,4-triazole-3-carboxamide (7,7-dimethyl-2- oxobicyclo(2.2.1)heptan-1-yl)
Click to Show/Hide
|
|||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [92] | ||
| External Link | ||||
| ETBX-011 cancer vaccine | Phase 1/2 | [70] | ||
| External Link | ||||
| BPX-601 | Phase 1/2 | [93] | ||
| External Link | ||||
| NANT | Phase 1/2 | [16] | ||
| External Link | ||||
| BrevaRex | Phase 1/2 | [94] | ||
| Synonyms |
BrevaRex MAb; monoclonal antibody
Click to Show/Hide
|
|||
| External Link | ||||
| DCVax-Pancreas | Phase 1/2 | [95] | ||
| Synonyms |
Dendritic cell-based immunotherapy (pancreatic cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| PEGylated hyaluronidase (human recombinant) | Phase 1/2 | [96] | ||
| Synonyms |
PEGylated hyaluronidase; PEGylated hyaluronidase (human recombinant) (intravenous, stroke/cancer), Halozyme
Click to Show/Hide
|
|||
| External Link | ||||
| CAR-T cells targeting mesothelin | Phase 1/2 | [97] | ||
| External Link | ||||
| MALP-2S | Phase 1/2 | [98] | ||
| External Link | ||||
| Anti-Mesothelin CAR-T cells | Phase 1/2 | [99] | ||
| External Link | ||||
| Anti-MUC1 CAR T Cells | Phase 1/2 | [100] | ||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [101] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [102] | ||
| External Link | ||||
| Anti-MUC1 AR20.5 | Phase 1/2 | [49] | ||
| External Link | ||||
| AR20.5 | Phase 1/2 | [16] | ||
| External Link | ||||
| G0-203-2c | Phase 1/2 | [103] | ||
| External Link | ||||
| LOAd703 | Phase 1/2 | [16] | ||
| External Link | ||||
| Anti-mesothelin CAR transduced PBL | Phase 1/2 | [104] | ||
| External Link | ||||
| M9241 | Phase 1 | [105] | ||
| Synonyms |
NHS-IL12
Click to Show/Hide
|
|||
| External Link | ||||
| NBF-006 | Phase 1 | [106] | ||
| External Link | ||||
| AB680 | Phase 1 | [107] | ||
| Synonyms |
AB-680; UNII-J6K8WSV73A; J6K8WSV73A; CHEMBL4471306; 2105904-82-1; (((((2R,3S,4R,5R)-5-(6-chloro-4-(((S)-1-(2-fluorophenyl)ethyl)amino)-1H-pyrazolo[3,4-b]pyridin-1-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)methyl)phosphonic acid; [[(2~{R},3~{S},4~{R},5~{R})-5-[6-chloranyl-4-[[(1~{S})-1-(2-fluorophenyl)ethyl]amino]pyrazolo[3,4-b]pyridin-1-yl]-3,4-bis(oxidanyl)oxolan-2-yl]methoxy-oxidanyl-phosphoryl]methylphosphonic acid; SCHEMBL19100484; GTPL10707; BDBM50527134; HY-125286; CS-0090231; [[(2R,3S,4R,5R)-5-[6-chloro-4-[[(1S)-1-(2-fluorophenyl)ethyl]amino]pyrazolo[3,4-b]pyridin-1-yl]-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]methylphosphonic acid; QDH
Click to Show/Hide
|
|||
| External Link | ||||
| STAT400 | Phase 1 | [108] | ||
| External Link | ||||
| CEND-1 | Phase 1 | [109] | ||
| Synonyms |
iRGD; UNII-Z8MXU5GH4Q; Z8MXU5GH4Q; iRGD-peptide; 1392278-76-0; Internalized-arginylglycylaspartic acid cyclic peptide; Q48988348; L-Cysteine, L-cysteinyl-L-arginylglycyl-L-alpha-aspartyl-L-lysylglycyl-L-prolyl-L-alpha-aspartyl-, cyclic (1->9)-disulfide
Click to Show/Hide
|
|||
| External Link | ||||
| CAR-T Cells targeting EGFRvIII | Phase 1 | [110] | ||
| External Link | ||||
| HuCART-meso cells | Phase 1 | [111] | ||
| External Link | ||||
| OCV-105 | Phase 1 | [112] | ||
| Synonyms |
Cancer vaccine (pancreas), Otsuka/OncoTherapy
Click to Show/Hide
|
|||
| External Link | ||||
| SBP-101 | Phase 1 | [44] | ||
| Synonyms |
diethyl dihydroxyhomospermine
Click to Show/Hide
|
|||
| External Link | ||||
| RG7882 | Phase 1 | [49] | ||
| External Link | ||||
| CART-meso-19 T cells | Phase 1 | [113] | ||
| External Link | ||||
| MOv19-BBz CAR T cells | Phase 1 | [114] | ||
| External Link | ||||
| Anti-MUC1 AR20.5 mab | Phase 1 | [115] | ||
| External Link | ||||
| Anti-CEA-CAR T | Phase 1 | [116] | ||
| External Link | ||||
| MVT-5873 | Phase 1 | [49] | ||
| External Link | ||||
| CART-meso cells | Phase 1 | [117] | ||
| External Link | ||||
| CAR-20/19-T Cells | Phase 1 | [118] | ||
| External Link | ||||
| CARTmeso/19 | Phase 1 | [119] | ||
| External Link | ||||
| CAR-T Cells targeting Mesothelin | Phase 1 | [110] | ||
| External Link | ||||
| CAR-T Cells targeting CEA | Phase 1 | [110] | ||
| External Link | ||||
| HLA-A*2402-restricted KIF20A and VEGFR-1 epitope peptide vaccine | Phase 1 | [120] | ||
| Synonyms |
HLA-A*2402-restricted KIF20A and VEGFR-1 epitope peptide vaccine (pancreatic cancer, subcutaneous)
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-hCD70 CAR transduced PBL | Phase 1 | [121] | ||
| External Link | ||||
| MORAb-066 | Phase 1 | [122] | ||
| Synonyms |
Anti-tissue factor monoclonal antibody (pancreatic tumor), Morphotek
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-MUC1 mab | Phase 1 | [123] | ||
| External Link | ||||
| Anti-meso-CAR vector transduced T cells | Phase 1 | [124] | ||
| External Link | ||||
| CRS-207 + GVAX | Phase 2 | [44] | ||
| External Link | ||||
| Autologous T cells transfected with chimeric anti-mesothelin immunoreceptor SS1 | Phase 1 | [125] | ||
| External Link | ||||
| Meso-CART | Phase 1 | [126] | ||
| External Link | ||||
| ASG-5ME | Phase 1 | [127] | ||
| External Link | ||||
| Anti-CD133-CAR vector-transduced T cells | Phase 1 | [128] | ||
| External Link | ||||
| IRX4204 | Phase 1 | [16] | ||
| Synonyms |
220619-73-8; CHEMBL75133; UNII-877M97Z38Y; VTP-194204; 877M97Z38Y; KB-145960; SCHEMBL3437269; MolPort-042-665-869; ZINC1550770; IRX-4204; 3-Methyl-5-[2-methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-cyclopropyl]-penta-2,4-dienoic acid; BDBM50101445; DB11806; VTP 194204; (+)-VTP-194204; AGN 4204; (2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-cyclopropyl]-penta-2,4-dienoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| AbGn-107 | Phase 1 | [16] | ||
| External Link | ||||
| LMB-100 | Phase 1/2 | [16] | ||
| External Link | ||||
| PLX7486 | Phase 1 | [129] | ||
| External Link | ||||
| CAR-T Cells targeting HER2 | Phase 1 | [110] | ||
| External Link | ||||
| CAR-T Cells targeting MUCI | Phase 1 | [110] | ||
| External Link | ||||
| SEL-403 | Phase 1 | [16] | ||
| External Link | ||||
| CAR-T Cells targeting PSCA | Phase 1 | [110] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [130] | ||
| External Link | ||||
| MVT-1075 | Phase 1 | [16] | ||
| External Link | ||||
| CAR-CLD18 T cell | Clinical trial | [131] | ||
| External Link | ||||
| CART-meso cells | Clinical trial | [132] | ||
| External Link | ||||
| PMID28460551-Compound-1 | Patented | [133] | ||
| External Link | ||||
| Tanomastat | Discontinued in Phase 3 | [134] | ||
| Synonyms |
Tanomastat (USAN/INN); (2S)-4-[4-(4-chlorophenyl)phenyl]-4-oxo-2-(phenylsulfanylmethyl)butanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Larotaxel | Discontinued in Phase 3 | [135] | ||
| Synonyms |
Benzenepropanoic acid; PNU 100940; XRP 9881; XRP9881
Click to Show/Hide
|
|||
| External Link | ||||
| Apricoxib | Discontinued in Phase 2 | [136] | ||
| Synonyms |
TG01
Click to Show/Hide
|
|||
| External Link | ||||
| Lintitript | Discontinued in Phase 2 | [137] | ||
| Synonyms |
SR 27897; SR 27897B; SR27897; SR-27897; SR-27897B; 1-((2-(4-(2-Chlorophenyl)thiazol-2-yl)aminocarbonyl)indolyl)acetic acid; 2-((4-(o-Chlorophenyl)-2-thiazolyl)carbamoyl)indole-1-acetic acid; 2-[2-[[4-(2-chlorophenyl)-1,3-thiazol-2-yl]carbamoyl]indol-1-yl]acetic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Merbarone | Discontinued in Phase 2 | [138] | ||
| Synonyms |
NSC-336628
Click to Show/Hide
|
|||
| External Link | ||||
| LY293111 | Discontinued in Phase 2 | [139] | ||
| Synonyms |
Etalocib; 161172-51-6; UNII-THY6RIW44R; LY 293111; THY6RIW44R; CHEMBL329123; LY-193111; 2-[3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxyphenoxy]propoxy]-2-propylphenoxy]benzoic acid; VML295; Etalocib [USAN:INN]; Etalocib (USAN); GTPL2948; SCHEMBL1649516; CTK8E7596; C33H33FO6; VML 295; DTXSID70167073; YFIZRWPXUYFCSN-UHFFFAOYSA-N; MolPort-009-019-411; ZINC3930629; AC1L4328; PDSP2_001221; BDBM50029450; PDSP1_001237; 1758AH; DB12850; RT-013626; D04074; L001468; J-009797; Benzoic acid, 2-(3-(3-((5-ethyl-4'-fluoro-2-hydroxy(1,1'-bipheny
Click to Show/Hide
|
|||
| External Link | ||||
| HMN-214 | Discontinued in Phase 1 | [140] | ||
| Synonyms |
N-(4-methoxyphenyl)sulfonyl-N-[2-[(E)-2-(1-oxidopyridin-4-yl)ethenyl]phenyl]acetamide; (E)-4-(2-(2-(N-Acetyl-N-((p-methoxyphenyl)sulfonyl)amino)phenyl)ethenyl)pyridine 1-oxide
Click to Show/Hide
|
|||
| External Link | ||||
| RG7600 | Discontinued in Phase 1 | [141] | ||
| External Link | ||||
| IC261 | Preclinical | [142] | ||
| Synonyms |
IC-261; IC 261
Click to Show/Hide
|
|||
| External Link | ||||
| ANAVEX 1007 | Preclinical | [143] | ||
| External Link | ||||
| IPH-4201 | Terminated | [144] | ||
| Synonyms |
MAb-16D10; MAb-J28; FAPP-targeting mAb (pancreatic cancer), Innate Pharma; FAPP-targeting mAb (pancreatic cancer), Universite de la Mediterranee/ INSERM; Feto-acinar pancreatic protein-targeting monoclonal antibodies (pancreatic cancer), Innate Pharma; Feto-acinar pancreatic protein-targeting monoclonal antibodies (pancreatic cancer), Universite de la Mediterranee/ INSERM
Click to Show/Hide
|
|||
| External Link | ||||
| MesoTarg | Investigative | [145] | ||
| External Link | ||||
| PAT-PM-1 | Investigative | [145] | ||
| Synonyms |
PM-1; Human monoclonal antibody (pancreatic cancer), Patrys; Human MAb (pancreas cancer), OncoMab/ Acceptys; Human monoclonal antibody (pancreatic cancer), OncoMab/ Acceptys; PM-1 antibody, OncoMab/ Acceptys
Click to Show/Hide
|
|||
| External Link | ||||
| OP-04 | Investigative | [145] | ||
| Synonyms |
OP-04 program (prodrug, pancreatic cancer); OP-04 program (prodrug, pancreatic cancer), Onco-Pharmakon
Click to Show/Hide
|
|||
| External Link | ||||
| VLI-27 | Investigative | [146] | ||
| Synonyms |
AKT inhibitor (pancreatic cancer), NovaLead Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| GS-326 | Investigative | [145] | ||
| Synonyms |
GS-326C
Click to Show/Hide
|
|||
| External Link | ||||
| PX-12 | Phase 2 | [147] | ||
| External Link | ||||
| Prodigiosin | Investigative | [145] | ||
| External Link | ||||
| Pbi-shPDX-1 LP | Investigative | [145] | ||
| External Link | ||||
| Gastrin 17C diphtheria toxoid conjugate | Investigative | [145] | ||
| Synonyms |
Gastrin 17C diphtheria toxoid conjugate (pancreatic cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| HS-P1 | Investigative | [145] | ||
| Synonyms |
HS-310; Endoplasmin modulator (pancreas tumor, HeatShock/fusion protein/antigen), Heat Biologics; Gp-96-Ig + unspecified tumor antigen secreting live cell vaccine (pancreas tumor, HeatShock), Heat Biologics
Click to Show/Hide
|
|||
| External Link | ||||
References