m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00444)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
ULK1
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Browse Drug
Fat mass and obesity-associated protein (FTO) [ERASER]
| Representative RNA-seq result indicating the expression of this target gene regulated by FTO | ||
| Cell Line | 253J cell line | Homo sapiens |
|
Treatment: siFTO 253J cells
Control: 253J cells
|
GSE150239 | |
| Regulation |
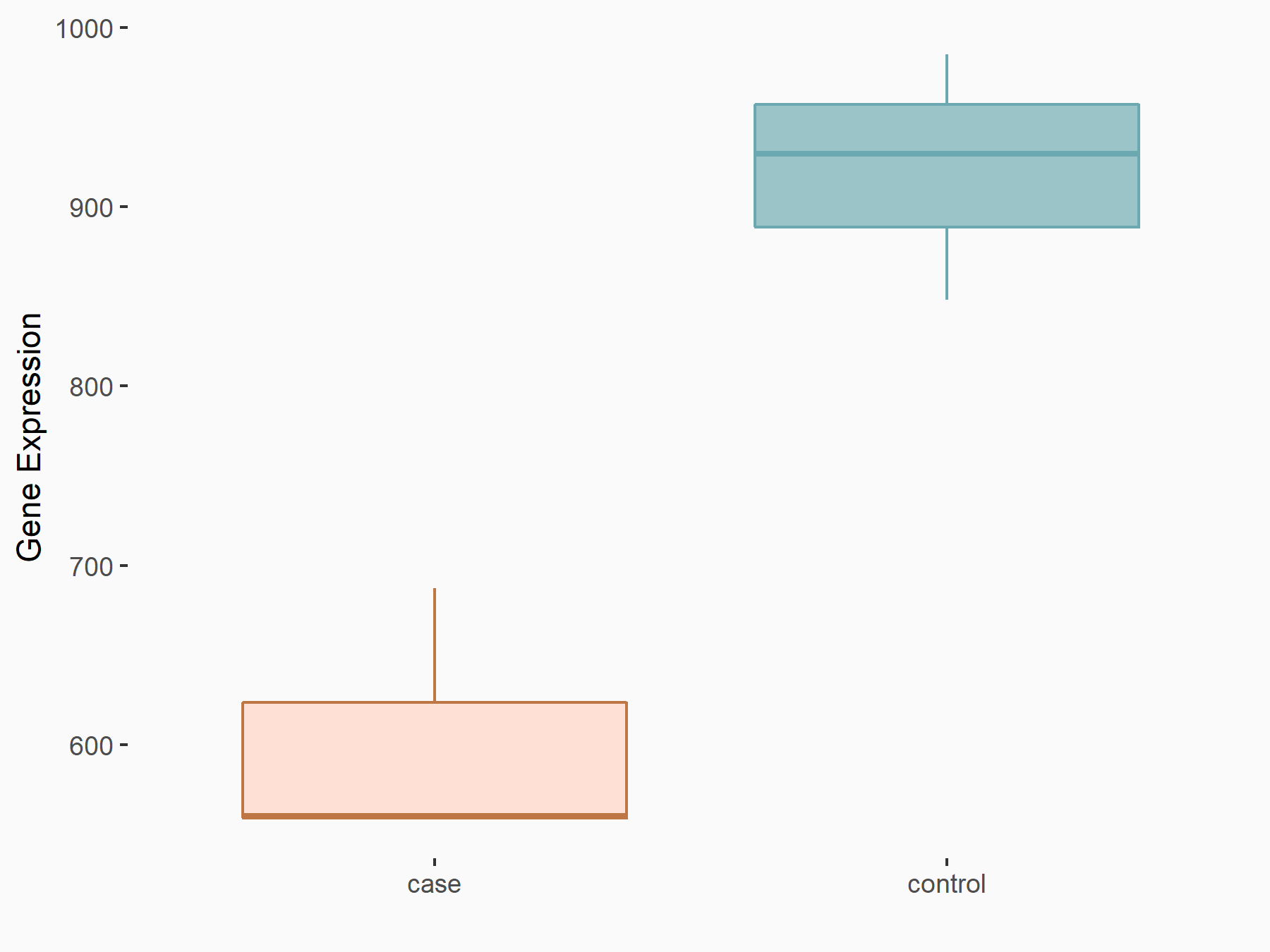  |
logFC: -6.13E-01 p-value: 1.25E-06 |
| More Results | Click to View More RNA-seq Results | |
| In total 2 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | The m6A changes caused by FTO influence the stability of ULK1 transcripts, likely through a YTHDF2-dependent manner.Under both basal and rapamycin-induced autophagy conditions, depletion of FTO significantly reduced the formation of GFP-LC3B puncta. The level of p62/SQSTM1 (an autophagy substrate) was higher in FTO-knockdown cells than that in control cells. FTO specifically upregulates the Serine/threonine-protein kinase ULK1 (ULK1) protein abundance. ULK1 mRNA undergoes m6A modification in the 3'-UTR and the m6A-marked ULK1 transcripts can further be targeted for degradation by YTHDF2. | |||
| Target Regulation | Up regulation | |||
| Responsed Drug | Rapamycin | Approved | ||
| Pathway Response | Autophagy | hsa04140 | ||
| Cell Process | RNA stability | |||
| Cell autophagy | ||||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| Experiment 2 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | Knockdown of FTO reversed cisplatin resistance of SGC-7901/DDP cells both in vitro and in vivo, which was attributed to the inhibition of Serine/threonine-protein kinase ULK1 (ULK1)-mediated autophagy. These findings indicate that the FTO/ULK1 axis exerts crucial roles in cisplatin resistance of gastric cancer. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | ||
| Responsed Drug | Cisplatin | Approved | ||
| In-vitro Model | GES-1 | Normal | Homo sapiens | CVCL_EQ22 |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| In-vivo Model | A total of 5 × 106 cells in 200 ul PBS were injected subcutaneously into the flanks of nude mice. After injection, cisplatin treatment was initiated on day 5. Mice were injected with 5 mg/kg cisplatin or PBS solution in the abdominal cavity once a week for 3 weeks. | |||
Methyltransferase-like 14 (METTL14) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL14 | ||
| Cell Line | BMDM | Mus musculus |
|
Treatment: METTL14 knockout mice BMDM
Control: Wild type mice BMDM
|
GSE153512 | |
| Regulation |
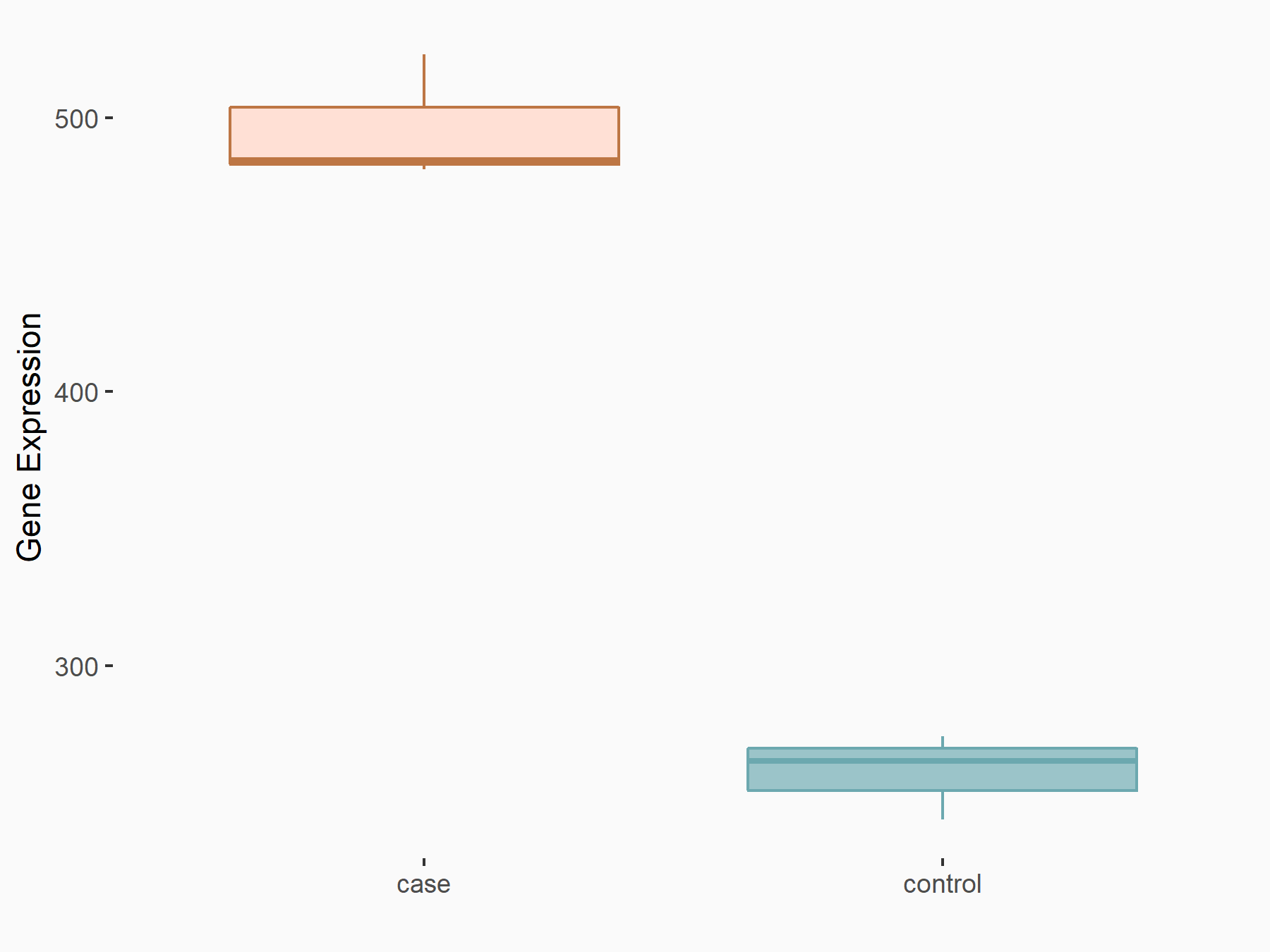  |
logFC: 9.25E-01 p-value: 4.64E-14 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | Linking mTORC1 signaling with m6A RNA methylation and demonstrates their roles in suppressing autophagy. Double-stranded RNA (dsRNA)-mediated depletion of either METTL3 or METTL14 increased the half-life of luciferase mRNA with either Serine/threonine-protein kinase ULK1 (ULK1/ATG1) or Atg8a 3' UTRs. WTAP stabilizes the interaction between the two METTL proteins, and RBM15/RBM15B have been proposed to recruit the MTC to its target transcripts. | |||
| Target Regulation | Down regulation | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | ||
| Autophagy | hsa04140 | |||
| Cell Process | Cell growth | |||
| Cell metabolism | ||||
| In-vitro Model | MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 |
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | LX2 cell line | Homo sapiens |
|
Treatment: shMETTL3 LX2 cells
Control: shLuc LX2 cells
|
GSE207909 | |
| Regulation |
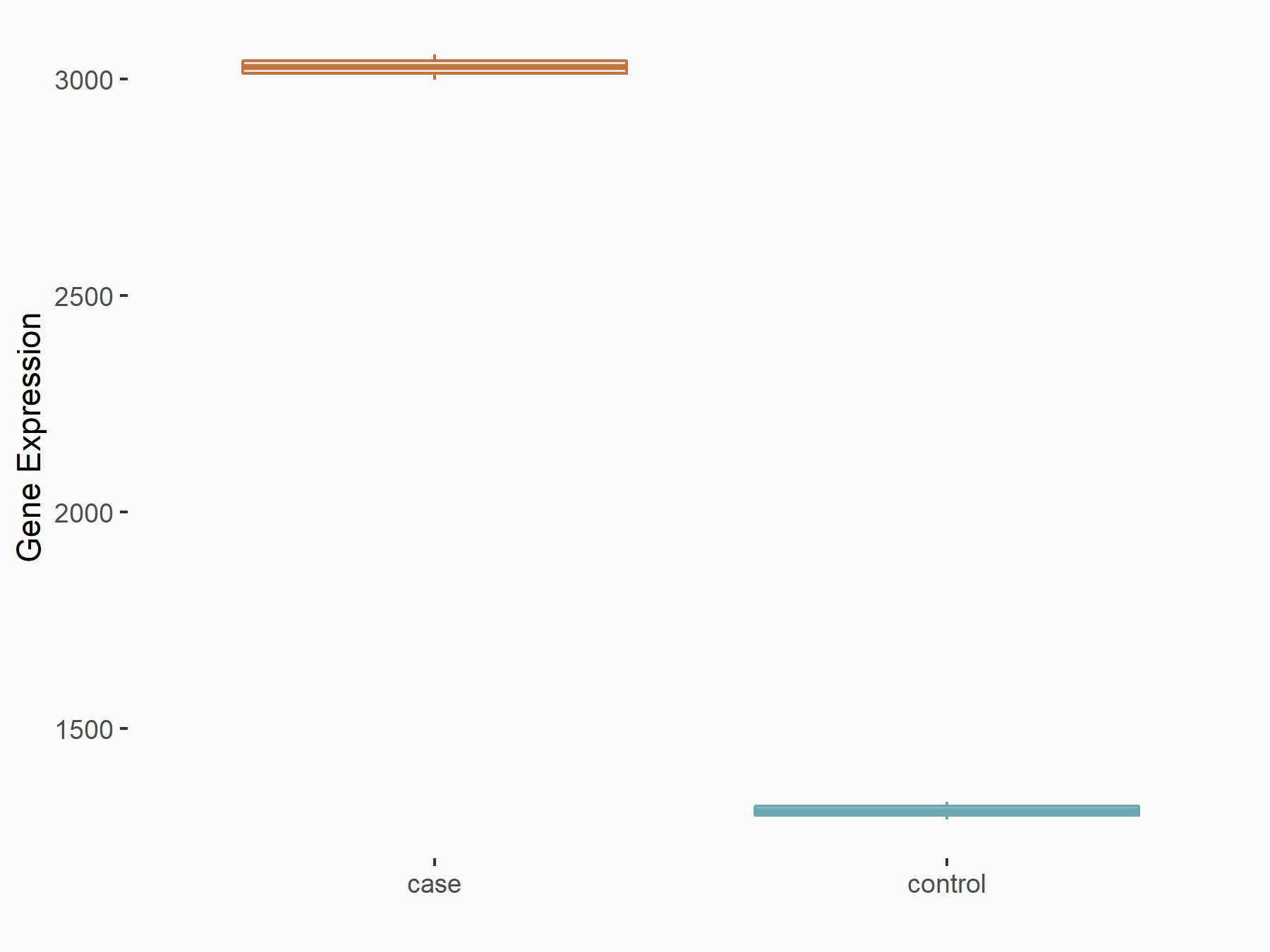  |
logFC: 1.21E+00 p-value: 7.24E-41 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | Linking mTORC1 signaling with m6A RNA methylation and demonstrates their roles in suppressing autophagy. Double-stranded RNA (dsRNA)-mediated depletion of either METTL3 or METTL14 increased the half-life of luciferase mRNA with either Serine/threonine-protein kinase ULK1 (ULK1/ATG1) or Atg8a 3' UTRs. WTAP stabilizes the interaction between the two METTL proteins, and RBM15/RBM15B have been proposed to recruit the MTC to its target transcripts. | |||
| Target Regulation | Down regulation | |||
| Pathway Response | mTOR signaling pathway | hsa04150 | ||
| Autophagy | hsa04140 | |||
| Cell Process | Cell growth | |||
| Cell metabolism | ||||
| In-vitro Model | MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 |
YTH domain-containing family protein 2 (YTHDF2) [READER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | The m6A changes caused by FTO influence the stability of ULK1 transcripts, likely through a YTHDF2-dependent manner.Under both basal and rapamycin-induced autophagy conditions, depletion of FTO significantly reduced the formation of GFP-LC3B puncta. The level of p62/SQSTM1 (an autophagy substrate) was higher in FTO-knockdown cells than that in control cells. FTO specifically upregulates the Serine/threonine-protein kinase ULK1 (ULK1) protein abundance. ULK1 mRNA undergoes m6A modification in the 3'-UTR and the m6A-marked ULK1 transcripts can further be targeted for degradation by YTHDF2. | |||
| Target Regulation | Up regulation | |||
| Responsed Drug | Rapamycin | Approved | ||
| Pathway Response | Autophagy | hsa04140 | ||
| Cell Process | RNA stability | |||
| Cell autophagy | ||||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
Gastric cancer [ICD-11: 2B72]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | Knockdown of FTO reversed cisplatin resistance of SGC-7901/DDP cells both in vitro and in vivo, which was attributed to the inhibition of Serine/threonine-protein kinase ULK1 (ULK1)-mediated autophagy. These findings indicate that the FTO/ULK1 axis exerts crucial roles in cisplatin resistance of gastric cancer. | |||
| Responsed Disease | Gastric cancer [ICD-11: 2B72] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Cisplatin | Approved | ||
| In-vitro Model | GES-1 | Normal | Homo sapiens | CVCL_EQ22 |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| In-vivo Model | A total of 5 × 106 cells in 200 ul PBS were injected subcutaneously into the flanks of nude mice. After injection, cisplatin treatment was initiated on day 5. Mice were injected with 5 mg/kg cisplatin or PBS solution in the abdominal cavity once a week for 3 weeks. | |||
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [2] | |||
| Response Summary | Knockdown of FTO reversed cisplatin resistance of SGC-7901/DDP cells both in vitro and in vivo, which was attributed to the inhibition of Serine/threonine-protein kinase ULK1 (ULK1)-mediated autophagy. These findings indicate that the FTO/ULK1 axis exerts crucial roles in cisplatin resistance of gastric cancer. | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Gastric cancer | ICD-11: 2B72 | ||
| In-vitro Model | GES-1 | Normal | Homo sapiens | CVCL_EQ22 |
| SGC-7901 | Gastric carcinoma | Homo sapiens | CVCL_0520 | |
| In-vivo Model | A total of 5 × 106 cells in 200 ul PBS were injected subcutaneously into the flanks of nude mice. After injection, cisplatin treatment was initiated on day 5. Mice were injected with 5 mg/kg cisplatin or PBS solution in the abdominal cavity once a week for 3 weeks. | |||
Rapamycin
[Approved]
| In total 2 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [1] | |||
| Response Summary | The m6A changes caused by FTO influence the stability of ULK1 transcripts, likely through a YTHDF2-dependent manner.Under both basal and rapamycin-induced autophagy conditions, depletion of FTO significantly reduced the formation of GFP-LC3B puncta. The level of p62/SQSTM1 (an autophagy substrate) was higher in FTO-knockdown cells than that in control cells. FTO specifically upregulates the Serine/threonine-protein kinase ULK1 (ULK1) protein abundance. ULK1 mRNA undergoes m6A modification in the 3'-UTR and the m6A-marked ULK1 transcripts can further be targeted for degradation by YTHDF2. | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Autophagy | hsa04140 | ||
| Cell Process | RNA stability | |||
| Cell autophagy | ||||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
| Experiment 2 Reporting the m6A-centered Drug Response | [1] | |||
| Response Summary | The m6A changes caused by FTO influence the stability of ULK1 transcripts, likely through a YTHDF2-dependent manner.Under both basal and rapamycin-induced autophagy conditions, depletion of FTO significantly reduced the formation of GFP-LC3B puncta. The level of p62/SQSTM1 (an autophagy substrate) was higher in FTO-knockdown cells than that in control cells. FTO specifically upregulates the Serine/threonine-protein kinase ULK1 (ULK1) protein abundance. ULK1 mRNA undergoes m6A modification in the 3'-UTR and the m6A-marked ULK1 transcripts can further be targeted for degradation by YTHDF2. | |||
| Target Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Autophagy | hsa04140 | ||
| Cell Process | RNA stability | |||
| Cell autophagy | ||||
| In-vitro Model | HEK293T | Normal | Homo sapiens | CVCL_0063 |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00444)
| In total 4 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE005967 | Click to Show/Hide the Full List | ||
| mod site | chr12:131899907-131899908:+ | [5] | |
| Sequence | GGCGGGTGGATCATGAGGTCAGGAGATCGAGACTATCCTGG | ||
| Transcript ID List | ENST00000321867.6; rmsk_3952443 | ||
| External Link | RMBase: RNA-editing_site_33829 | ||
| mod ID: A2ISITE005968 | Click to Show/Hide the Full List | ||
| mod site | chr12:131906232-131906233:+ | [6] | |
| Sequence | CCTCCTAAGTAGCTGGGATTACAGGCACGCACCACCACGCC | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: RNA-editing_site_33830 | ||
| mod ID: A2ISITE005969 | Click to Show/Hide the Full List | ||
| mod site | chr12:131906287-131906288:+ | [6] | |
| Sequence | ATTTTAATAGAGACGGTTTCACCATGTTGGTCAGGCTGGTC | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: RNA-editing_site_33831 | ||
| mod ID: A2ISITE005970 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922591-131922592:+ | [5] | |
| Sequence | CCAGGCGGGCATGCCCTGCAAACCCCGCCTGGGCCTCCCTT | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: RNA-editing_site_33832 | ||
5-methylcytidine (m5C)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: M5CSITE000147 | Click to Show/Hide the Full List | ||
| mod site | chr12:131917877-131917878:+ | [7] | |
| Sequence | CCCAGCTGCCGTGGGCTGTGCTGTGTTGGTCAGGAGTTGCC | ||
| Cell/Tissue List | T24 | ||
| Seq Type List | Bisulfite-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000541761.2 | ||
| External Link | RMBase: m5C_site_11482 | ||
N6-methyladenosine (m6A)
| In total 125 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE016110 | Click to Show/Hide the Full List | ||
| mod site | chr12:131894804-131894805:+ | [8] | |
| Sequence | GGAGGGAGCGCGACCCTCGGACCCCGCCTGGCCCGCGGGGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; U2OS; GSC-11; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218274 | ||
| mod ID: M6ASITE016111 | Click to Show/Hide the Full List | ||
| mod site | chr12:131894829-131894830:+ | [8] | |
| Sequence | GCCTGGCCCGCGGGGCTGGGACCCGGCCCCGGCCTGCCCGA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; U2OS; GSC-11; iSLK | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218275 | ||
| mod ID: M6ASITE016112 | Click to Show/Hide the Full List | ||
| mod site | chr12:131895028-131895029:+ | [8] | |
| Sequence | CCGGCCGCGGCGGCACAGAGACCGTGGGCAAGTTCGAGTTC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; A549; Jurkat; GSC-11; HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218276 | ||
| mod ID: M6ASITE016113 | Click to Show/Hide the Full List | ||
| mod site | chr12:131895059-131895060:+ | [8] | |
| Sequence | GTTCGAGTTCTCCCGCAAGGACCTGATCGGCCACGGCGCCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; Jurkat; HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218277 | ||
| mod ID: M6ASITE016114 | Click to Show/Hide the Full List | ||
| mod site | chr12:131895634-131895635:+ | [9] | |
| Sequence | GGTCGCCGTCAAGTGCATTAACAAGAAGAACCTCGCCAAGT | ||
| Motif Score | 2.168095238 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218278 | ||
| mod ID: M6ASITE016115 | Click to Show/Hide the Full List | ||
| mod site | chr12:131895643-131895644:+ | [8] | |
| Sequence | CAAGTGCATTAACAAGAAGAACCTCGCCAAGTCTCAGACGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218279 | ||
| mod ID: M6ASITE016116 | Click to Show/Hide the Full List | ||
| mod site | chr12:131895784-131895785:+ | [8] | |
| Sequence | CTTGGGTTTCTGTCCTAGGAACTGAAACATGAAAACATCGT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218280 | ||
| mod ID: M6ASITE016117 | Click to Show/Hide the Full List | ||
| mod site | chr12:131895790-131895791:+ | [8] | |
| Sequence | TTTCTGTCCTAGGAACTGAAACATGAAAACATCGTGGCCCT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218281 | ||
| mod ID: M6ASITE016118 | Click to Show/Hide the Full List | ||
| mod site | chr12:131895798-131895799:+ | [8] | |
| Sequence | CTAGGAACTGAAACATGAAAACATCGTGGCCCTGTACGACT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218282 | ||
| mod ID: M6ASITE016119 | Click to Show/Hide the Full List | ||
| mod site | chr12:131896616-131896617:+ | [8] | |
| Sequence | GCTTTGCTGTGGCCTTGGGGACCGAGGCTTGTCACAAGGTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000624048.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218283 | ||
| mod ID: M6ASITE016120 | Click to Show/Hide the Full List | ||
| mod site | chr12:131896675-131896676:+ | [8] | |
| Sequence | GGTCCAGCGAGTACCTGGAAACCCAGGCACACTGTGTCCTG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000624048.1 | ||
| External Link | RMBase: m6A_site_218284 | ||
| mod ID: M6ASITE016121 | Click to Show/Hide the Full List | ||
| mod site | chr12:131896816-131896817:+ | [8] | |
| Sequence | TTTGGAAGCCTCCTGCCTGGACCACAGCCTGCACATGCGTC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000624048.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218285 | ||
| mod ID: M6ASITE016122 | Click to Show/Hide the Full List | ||
| mod site | chr12:131896864-131896865:+ | [8] | |
| Sequence | TGGGAGCCAGCAAGGAGTGGACAGCTGTCCCTGGCCAGGCA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000624048.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218286 | ||
| mod ID: M6ASITE016123 | Click to Show/Hide the Full List | ||
| mod site | chr12:131896898-131896899:+ | [8] | |
| Sequence | CCAGGCAGCGACTCTGAGGGACTCCCCCTCCACCCTGGGCT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000624048.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218287 | ||
| mod ID: M6ASITE016124 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897010-131897011:+ | [8] | |
| Sequence | TGTCCGCACGACCCCCGCAGACCCGGGACCAGTATCCCCAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000624048.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218288 | ||
| mod ID: M6ASITE016125 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897017-131897018:+ | [8] | |
| Sequence | ACGACCCCCGCAGACCCGGGACCAGTATCCCCAGGCCCAGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000624048.1 | ||
| External Link | RMBase: m6A_site_218289 | ||
| mod ID: M6ASITE016126 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897268-131897269:+ | [8] | |
| Sequence | TGTTTGAGGCAGGCACCCAAACCCAGGAAAAAATAACTGGG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000624048.1 | ||
| External Link | RMBase: m6A_site_218290 | ||
| mod ID: M6ASITE016127 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897301-131897302:+ | [8] | |
| Sequence | TAACTGGGTTTTAAAAATGGACAATGAACGATTTGGGTGAT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000624048.1 | ||
| External Link | RMBase: m6A_site_218291 | ||
| mod ID: M6ASITE016128 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897338-131897339:+ | [8] | |
| Sequence | TGATAAAAGAAAATCCTCAAACATTACGGGAGGGAGTGCGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000624048.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218292 | ||
| mod ID: M6ASITE016129 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897443-131897444:+ | [8] | |
| Sequence | TTGTTTCCGGGCCACCGGGGACAGCCATCCTTACAAGGCGA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000624048.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218293 | ||
| mod ID: M6ASITE016130 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897694-131897695:+ | [8] | |
| Sequence | TAGCACTGTGGGAGGCCAAGACAGACCAGCCTGGGCAACAT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | rmsk_3952435; ENST00000624048.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218294 | ||
| mod ID: M6ASITE016131 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897721-131897722:+ | [8] | |
| Sequence | AGCCTGGGCAACATAGCAAGACTCGCTCTCTCTAAAAAAAA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000624048.1; rmsk_3952435 | ||
| External Link | RMBase: m6A_site_218295 | ||
| mod ID: M6ASITE016132 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897819-131897820:+ | [8] | |
| Sequence | GTTGTAGGAGGATTGATTAAACCCAGGAGTTTAAGGCTGCA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; rmsk_3952435; ENST00000624048.1 | ||
| External Link | RMBase: m6A_site_218296 | ||
| mod ID: M6ASITE016133 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897902-131897903:+ | [8] | |
| Sequence | AGTGTAACCCTGTCTCAAAAACACAAAACAAAACCCTCAAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | rmsk_3952435; ENST00000321867.6; ENST00000624048.1 | ||
| External Link | RMBase: m6A_site_218297 | ||
| mod ID: M6ASITE016134 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897909-131897910:+ | [8] | |
| Sequence | CCCTGTCTCAAAAACACAAAACAAAACCCTCAAAAAGTTTA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | rmsk_3952435; ENST00000321867.6; ENST00000624048.1 | ||
| External Link | RMBase: m6A_site_218298 | ||
| mod ID: M6ASITE016135 | Click to Show/Hide the Full List | ||
| mod site | chr12:131897914-131897915:+ | [8] | |
| Sequence | TCTCAAAAACACAAAACAAAACCCTCAAAAAGTTTACCCCT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000624048.1; rmsk_3952435; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218299 | ||
| mod ID: M6ASITE016136 | Click to Show/Hide the Full List | ||
| mod site | chr12:131906663-131906664:+ | [8] | |
| Sequence | GCTGTTGCTCTTATGGGGGGACCAGGGGCCTTTGCCTTGAT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218300 | ||
| mod ID: M6ASITE016137 | Click to Show/Hide the Full List | ||
| mod site | chr12:131906693-131906694:+ | [8] | |
| Sequence | TTTGCCTTGATCAGATGGAGACCTGGCTGGCCACAAAACAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218301 | ||
| mod ID: M6ASITE016138 | Click to Show/Hide the Full List | ||
| mod site | chr12:131906710-131906711:+ | [8] | |
| Sequence | GAGACCTGGCTGGCCACAAAACAGAACACACCCACCTACAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5 | ||
| External Link | RMBase: m6A_site_218302 | ||
| mod ID: M6ASITE016139 | Click to Show/Hide the Full List | ||
| mod site | chr12:131906715-131906716:+ | [8] | |
| Sequence | CTGGCTGGCCACAAAACAGAACACACCCACCTACAAAGCAC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218303 | ||
| mod ID: M6ASITE016140 | Click to Show/Hide the Full List | ||
| mod site | chr12:131906830-131906831:+ | [8] | |
| Sequence | CCGAATTGGGGCTGAGCCAGACTCCCTGCAGTGGGGCCCGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218304 | ||
| mod ID: M6ASITE016141 | Click to Show/Hide the Full List | ||
| mod site | chr12:131906861-131906862:+ | [8] | |
| Sequence | TGGGGCCCGGTGGCACTGGGACCTCTCACAGCCTCTTTTCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218305 | ||
| mod ID: M6ASITE016142 | Click to Show/Hide the Full List | ||
| mod site | chr12:131907510-131907511:+ | [8] | |
| Sequence | GCAGTACTGCAACGGTGGGGACCTGGCCGACTACCTGCACG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5 | ||
| External Link | RMBase: m6A_site_218306 | ||
| mod ID: M6ASITE016143 | Click to Show/Hide the Full List | ||
| mod site | chr12:131908664-131908665:+ | [8] | |
| Sequence | CATGCGCACGCTGAGCGAGGACACCATCAGGCTCTTCCTGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5; ENST00000542313.2 | ||
| External Link | RMBase: m6A_site_218307 | ||
| mod ID: M6ASITE016144 | Click to Show/Hide the Full List | ||
| mod site | chr12:131908746-131908747:+ | [8] | |
| Sequence | ATCATCCACCGCGACCTGAAACCGCAGAACATCCTGCTGTC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000542313.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218308 | ||
| mod ID: M6ASITE016145 | Click to Show/Hide the Full List | ||
| mod site | chr12:131908754-131908755:+ | [8] | |
| Sequence | CCGCGACCTGAAACCGCAGAACATCCTGCTGTCCAACCCCG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000542313.2; ENST00000321867.6; ENST00000537421.5 | ||
| External Link | RMBase: m6A_site_218309 | ||
| mod ID: M6ASITE016146 | Click to Show/Hide the Full List | ||
| mod site | chr12:131908796-131908797:+ | [10] | |
| Sequence | CGGCCGCCGCGCCAACCCCAACAGCATCCGCGTCAAGATCG | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | brain; HEK293 | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000542313.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218310 | ||
| mod ID: M6ASITE016147 | Click to Show/Hide the Full List | ||
| mod site | chr12:131908930-131908931:+ | [9] | |
| Sequence | CGCGCGGTACCTCCAGAGCAACATGATGGCGGCCACACTCT | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5; ENST00000542313.2 | ||
| External Link | RMBase: m6A_site_218311 | ||
| mod ID: M6ASITE016148 | Click to Show/Hide the Full List | ||
| mod site | chr12:131909178-131909179:+ | [8] | |
| Sequence | GCACTACGACGGGAAGGCGGACCTGTGGAGCATCGGCACCA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218312 | ||
| mod ID: M6ASITE016149 | Click to Show/Hide the Full List | ||
| mod site | chr12:131909790-131909791:+ | [8] | |
| Sequence | GCAGGCCAGCAGCCCCCAGGACCTGCGCCTGTTCTACGAGA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5 | ||
| External Link | RMBase: m6A_site_218313 | ||
| mod ID: M6ASITE016150 | Click to Show/Hide the Full List | ||
| mod site | chr12:131909814-131909815:+ | [8] | |
| Sequence | GCGCCTGTTCTACGAGAAGAACAAGACGTTGGTCCCCACGT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5 | ||
| External Link | RMBase: m6A_site_218314 | ||
| mod ID: M6ASITE016151 | Click to Show/Hide the Full List | ||
| mod site | chr12:131909931-131909932:+ | [8] | |
| Sequence | GCCCCAGCATCCCCCGGGAGACCTCGGCCCCGCTGCGGCAG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000537421.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218315 | ||
| mod ID: M6ASITE016152 | Click to Show/Hide the Full List | ||
| mod site | chr12:131909980-131909981:+ | [9] | |
| Sequence | GGCCCTACTGCAACGCAACCACAAGGACCGCATGGACTTCG | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5 | ||
| External Link | RMBase: m6A_site_218316 | ||
| mod ID: M6ASITE016153 | Click to Show/Hide the Full List | ||
| mod site | chr12:131909986-131909987:+ | [8] | |
| Sequence | ACTGCAACGCAACCACAAGGACCGCATGGACTTCGGTGAGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5 | ||
| External Link | RMBase: m6A_site_218317 | ||
| mod ID: M6ASITE016154 | Click to Show/Hide the Full List | ||
| mod site | chr12:131909995-131909996:+ | [8] | |
| Sequence | CAACCACAAGGACCGCATGGACTTCGGTGAGCACCCACCAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000537421.5 | ||
| External Link | RMBase: m6A_site_218318 | ||
| mod ID: M6ASITE016155 | Click to Show/Hide the Full List | ||
| mod site | chr12:131911971-131911972:+ | [8] | |
| Sequence | AGATGCAGCAGCTGCAGAAGACCCTGGCCTCCCCGGCTGAC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218319 | ||
| mod ID: M6ASITE016156 | Click to Show/Hide the Full List | ||
| mod site | chr12:131912020-131912021:+ | [8] | |
| Sequence | CTTCCTGCACAGCTCCCGGGACTCTGGTGGCAGCAAGGACT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218320 | ||
| mod ID: M6ASITE016157 | Click to Show/Hide the Full List | ||
| mod site | chr12:131912038-131912039:+ | [8] | |
| Sequence | GGACTCTGGTGGCAGCAAGGACTCTTCCTGTGACACAGACG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218321 | ||
| mod ID: M6ASITE016158 | Click to Show/Hide the Full List | ||
| mod site | chr12:131913228-131913229:+ | [8] | |
| Sequence | GCTGAGGCGCCCAGTGCCAAACCCCCGCCAGACAGCCTGAT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218322 | ||
| mod ID: M6ASITE016159 | Click to Show/Hide the Full List | ||
| mod site | chr12:131913239-131913240:+ | [8] | |
| Sequence | CAGTGCCAAACCCCCGCCAGACAGCCTGATGTGCAGTGGGT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218323 | ||
| mod ID: M6ASITE016160 | Click to Show/Hide the Full List | ||
| mod site | chr12:131913789-131913790:+ | [8] | |
| Sequence | GCTTGGAGAGCCACGGCCGGACCCCATCTCCATCCCCACCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218324 | ||
| mod ID: M6ASITE016161 | Click to Show/Hide the Full List | ||
| mod site | chr12:131914422-131914423:+ | [8] | |
| Sequence | AGTCCCCACGCAGGTGCAGAACTACCAGCGCATTGAGCGAA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218325 | ||
| mod ID: M6ASITE016162 | Click to Show/Hide the Full List | ||
| mod site | chr12:131914443-131914444:+ | [8] | |
| Sequence | CTACCAGCGCATTGAGCGAAACCTGCAGTCACCCACCCAGT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218326 | ||
| mod ID: M6ASITE016163 | Click to Show/Hide the Full List | ||
| mod site | chr12:131914469-131914470:+ | [8] | |
| Sequence | AGTCACCCACCCAGTTCCAAACACCTCGGTGAGTGTGGAGC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218327 | ||
| mod ID: M6ASITE016164 | Click to Show/Hide the Full List | ||
| mod site | chr12:131915213-131915214:+ | [9] | |
| Sequence | TCTGGGTGGAGGCCGGCCCTACACGCCATCTCCTCAAGGTG | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218328 | ||
| mod ID: M6ASITE016165 | Click to Show/Hide the Full List | ||
| mod site | chr12:131915339-131915340:+ | [8] | |
| Sequence | TCTCTTTTCCCCAAGTTGGAACCATCCCTGAGCGGCCAGGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218329 | ||
| mod ID: M6ASITE016166 | Click to Show/Hide the Full List | ||
| mod site | chr12:131915996-131915997:+ | [8] | |
| Sequence | GTCCGCCCCAAGCTGCCCAAACCCCCCACGGACCCCCTGGG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; MT4; MM6; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218330 | ||
| mod ID: M6ASITE016167 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916007-131916008:+ | [8] | |
| Sequence | GCTGCCCAAACCCCCCACGGACCCCCTGGGAGCTGTGTTCA | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; MT4; MM6; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000541761.2 | ||
| External Link | RMBase: m6A_site_218331 | ||
| mod ID: M6ASITE016168 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916082-131916083:+ | [8] | |
| Sequence | CGGCCTGCAGTCCTGCCGGAACCTGCGGGGCTCACCCAAGC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2; MT4; MM6; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218332 | ||
| mod ID: M6ASITE016169 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916124-131916125:+ | [8] | |
| Sequence | GCCCGACTTCCTGCAGCGAAACCCCCTGCCCCCCATCCTGG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; MT4; MM6; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218333 | ||
| mod ID: M6ASITE016170 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916229-131916230:+ | [8] | |
| Sequence | GTGGGAATGGCCAGTCCTGAACAAAGACCGAGGAAGGCAGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000541761.2 | ||
| External Link | RMBase: m6A_site_218334 | ||
| mod ID: M6ASITE016171 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916235-131916236:+ | [8] | |
| Sequence | ATGGCCAGTCCTGAACAAAGACCGAGGAAGGCAGCTCTGTA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000541761.2 | ||
| External Link | RMBase: m6A_site_218335 | ||
| mod ID: M6ASITE016172 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916318-131916319:+ | [8] | |
| Sequence | ATGCCTGCCAGTTCCTGCGGACTCGGCCCCTTCCCCAGGCA | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218336 | ||
| mod ID: M6ASITE016173 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916358-131916359:+ | [8] | |
| Sequence | ACCGGGCCCCAGGGCTGCAGACCTGCGGGGCAGTCTTCACC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218337 | ||
| mod ID: M6ASITE016174 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916424-131916425:+ | [8] | |
| Sequence | CCTCCTTTGACTTCCCGAAGACCCCCAGCTCCCAGAACCTG | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218338 | ||
| mod ID: M6ASITE016175 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916440-131916441:+ | [8] | |
| Sequence | GAAGACCCCCAGCTCCCAGAACCTGCTGGCCCTCCTAGCCC | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218339 | ||
| mod ID: M6ASITE016176 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916491-131916492:+ | [8] | |
| Sequence | GGTGATGACGCCCCCTCGAAACCGGACGCTGCCCGACCTCT | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218340 | ||
| mod ID: M6ASITE016177 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916522-131916523:+ | [8] | |
| Sequence | CCCGACCTCTCGGAGGTGGGACCCTTCCATGGTCAGCCGTT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218341 | ||
| mod ID: M6ASITE016178 | Click to Show/Hide the Full List | ||
| mod site | chr12:131916569-131916570:+ | [8] | |
| Sequence | TGGCCTGCGGCCAGGCGAGGACCCCAAGGGCCCCTTTGGCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218342 | ||
| mod ID: M6ASITE016179 | Click to Show/Hide the Full List | ||
| mod site | chr12:131917004-131917005:+ | [8] | |
| Sequence | TCCTTAAGGCGGCGTTTGGGACACAAGCCCCGGACCCGGGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218343 | ||
| mod ID: M6ASITE016180 | Click to Show/Hide the Full List | ||
| mod site | chr12:131917017-131917018:+ | [8] | |
| Sequence | GTTTGGGACACAAGCCCCGGACCCGGGCAGCACGGAGAGCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218344 | ||
| mod ID: M6ASITE016181 | Click to Show/Hide the Full List | ||
| mod site | chr12:131918386-131918387:+ | [8] | |
| Sequence | TTGGGATATAACAGGTGTAAACCGAGGCAGAGGCACATTGG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000540647.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218345 | ||
| mod ID: M6ASITE016182 | Click to Show/Hide the Full List | ||
| mod site | chr12:131918587-131918588:+ | [8] | |
| Sequence | CCTGAGCTCCCTGCTCCAGGACACGGCTGCAGCTTTGCCGA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000540647.5; ENST00000541761.2; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218346 | ||
| mod ID: M6ASITE016183 | Click to Show/Hide the Full List | ||
| mod site | chr12:131918622-131918623:+ | [8] | |
| Sequence | TGCCGACCCCATTACTGCGAACCTGGAGGGGGCTGTGACCT | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000540647.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218347 | ||
| mod ID: M6ASITE016184 | Click to Show/Hide the Full List | ||
| mod site | chr12:131918669-131918670:+ | [8] | |
| Sequence | CCCCCGACCTCCCTGAGGAGACCCTCATGGAGGTGAGGGCT | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540647.5; ENST00000541761.2 | ||
| External Link | RMBase: m6A_site_218348 | ||
| mod ID: M6ASITE016185 | Click to Show/Hide the Full List | ||
| mod site | chr12:131919218-131919219:+ | [9] | |
| Sequence | CCGCTTCCTGCAGCAAGAGCACACGGAGATCCTGCGTGGCC | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000541761.2; ENST00000540647.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218349 | ||
| mod ID: M6ASITE016186 | Click to Show/Hide the Full List | ||
| mod site | chr12:131919434-131919435:+ | [8] | |
| Sequence | GTGGTGGCCTGGGGGCCAGGACCAACCGGCCTCCTCTGATC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000540647.5; ENST00000321867.6; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218350 | ||
| mod ID: M6ASITE016187 | Click to Show/Hide the Full List | ||
| mod site | chr12:131919480-131919481:+ | [8] | |
| Sequence | GCCGCCCCCAGCTTCGCGGAACAGCTGGTGCTGTACCTGAA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540647.5; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218351 | ||
| mod ID: M6ASITE016188 | Click to Show/Hide the Full List | ||
| mod site | chr12:131919758-131919759:+ | [11] | |
| Sequence | CACAGAGGCCATTGGGTCGGACAGCACCCTGGAGCCCAGTA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540647.5; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218352 | ||
| mod ID: M6ASITE016189 | Click to Show/Hide the Full List | ||
| mod site | chr12:131919818-131919819:+ | [8] | |
| Sequence | GGGGTCGGATGGCACCCGGGACAAGGTGCGGAGCCTGGCCA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544718.1; ENST00000540647.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218353 | ||
| mod ID: M6ASITE016190 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920002-131920003:+ | [9] | |
| Sequence | GCGCAGGCTGAATGAGCTGTACAAGGCCAGCGTGGTGTCCT | ||
| Motif Score | 2.856142857 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000540647.5; ENST00000544718.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218354 | ||
| mod ID: M6ASITE016191 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920062-131920063:+ | [8] | |
| Sequence | GCTGCAGCGCTTCTTCCTGGACAAGCAGCGGCTCCTGGACC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; hESC-HEK293T; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000544718.1; ENST00000540647.5 | ||
| External Link | RMBase: m6A_site_218355 | ||
| mod ID: M6ASITE016192 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920080-131920081:+ | [8] | |
| Sequence | GGACAAGCAGCGGCTCCTGGACCGCATTCACAGCATCACTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544718.1; ENST00000540568.1; ENST00000321867.6; ENST00000540647.5 | ||
| External Link | RMBase: m6A_site_218356 | ||
| mod ID: M6ASITE016193 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920089-131920090:+ | [10] | |
| Sequence | GCGGCTCCTGGACCGCATTCACAGCATCACTGCCGAGAGGC | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1; ENST00000544718.1; ENST00000540647.5 | ||
| External Link | RMBase: m6A_site_218357 | ||
| mod ID: M6ASITE016194 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920143-131920144:+ | [8] | |
| Sequence | CGCTGTGCAGATGGTACGGGACAGACAGACACCAGTGGGGC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000540647.5; ENST00000540568.1; ENST00000544718.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218358 | ||
| mod ID: M6ASITE016195 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920151-131920152:+ | [8] | |
| Sequence | AGATGGTACGGGACAGACAGACACCAGTGGGGCAGGGGCCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000544718.1; ENST00000540647.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218359 | ||
| mod ID: M6ASITE016196 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920175-131920176:+ | [8] | |
| Sequence | CAGTGGGGCAGGGGCCAGGAACTTCCAGGCCCGCCGTCTCC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; peripheral-blood; HEK293T; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000321867.6; ENST00000544718.1; ENST00000540647.5 | ||
| External Link | RMBase: m6A_site_218360 | ||
| mod ID: M6ASITE016197 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920207-131920208:+ | [8] | |
| Sequence | GCCGTCTCCACTGGGACGGGACCTTGATGCCCACAGCGTGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; peripheral-blood; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1; ENST00000540647.5; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218361 | ||
| mod ID: M6ASITE016198 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920232-131920233:+ | [8] | |
| Sequence | GATGCCCACAGCGTGGTGAGACTTTGGGGGGTGTCACTTCT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; A549; HEK293A-TOA; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544718.1; ENST00000540568.1; ENST00000540647.5; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218362 | ||
| mod ID: M6ASITE016199 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920400-131920401:+ | [11] | |
| Sequence | CCCCAATTGTAAAATGAGAGACTTGGTCTTCAGGCCTCGTA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540647.5; ENST00000544718.1; rmsk_3952464; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218363 | ||
| mod ID: M6ASITE016200 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920459-131920460:+ | [11] | |
| Sequence | GAAGCAGCACCTGGTCCCGGACAACCTGCCTGGAAGTGTGG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544718.1; ENST00000540647.5; ENST00000321867.6; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218364 | ||
| mod ID: M6ASITE016201 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920507-131920508:+ | [11] | |
| Sequence | AGGTGGTACCACGGGCAAAGACTCCCTGCTTGGGCTCTGAA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000544718.1; ENST00000540568.1; ENST00000540647.5 | ||
| External Link | RMBase: m6A_site_218365 | ||
| mod ID: M6ASITE016202 | Click to Show/Hide the Full List | ||
| mod site | chr12:131920765-131920766:+ | [11] | |
| Sequence | GTAGTAAAAATCCAGTCTGAACAAACATGGGAAAAGTCAGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HEK293A-TOA | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000544718.1; ENST00000321867.6; ENST00000540647.5; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218366 | ||
| mod ID: M6ASITE016203 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921160-131921161:+ | [9] | |
| Sequence | GGGCTGCGTCCCACGCTACCACAAGGCCCTGCTGCTCCTGG | ||
| Motif Score | 2.053113095 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000540647.5; ENST00000544718.1; ENST00000540568.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218367 | ||
| mod ID: M6ASITE016204 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921205-131921206:+ | [8] | |
| Sequence | GCTGCAGCACATGCTCTCGGACCAGGCCGACATCGAGAACG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000321867.6; ENST00000540647.5; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218368 | ||
| mod ID: M6ASITE016205 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921214-131921215:+ | [10] | |
| Sequence | CATGCTCTCGGACCAGGCCGACATCGAGAACGTCACCAAGT | ||
| Motif Score | 2.865571429 | ||
| Cell/Tissue List | kidney; hESC-HEK293T | ||
| Seq Type List | m6A-REF-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000544718.1; ENST00000321867.6; ENST00000540647.5; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218369 | ||
| mod ID: M6ASITE016206 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921327-131921328:+ | [8] | |
| Sequence | AAGCTGTGCATTGAGCGGAGACTCTCGGCGCTGCTGACTGG | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000321867.6; ENST00000540647.5; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218370 | ||
| mod ID: M6ASITE016207 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921428-131921429:+ | [8] | |
| Sequence | GGGCTCTGTGTGCTGGCTGGACTCCTCGGGACAAGCCCATG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; H1A; H1B; hESCs; GM12878; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000544718.1; ENST00000321867.6; ENST00000540647.5; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218371 | ||
| mod ID: M6ASITE016208 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921438-131921439:+ | [8] | |
| Sequence | TGCTGGCTGGACTCCTCGGGACAAGCCCATGGCGCTGATCG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; hESC-HEK293T; H1A; H1B; hESCs; GM12878; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000540647.5; ENST00000544718.1; ENST00000540568.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218372 | ||
| mod ID: M6ASITE016209 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921490-131921491:+ | [8] | |
| Sequence | CCCTGCCCTGGGCCCCACGGACAGTCAGCCTGCCGGCCTCC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; brain; A549; hESC-HEK293T; H1A; H1B; hESCs; fibroblasts; GM12878; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-REF-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000540647.5; ENST00000321867.6; ENST00000544718.1; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218373 | ||
| mod ID: M6ASITE016210 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921530-131921531:+ | [8] | |
| Sequence | CCTGCAGCTCACGGGGCAGAACCAGCACATCTGGAGCCACA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; H1A; H1B; hESCs; fibroblasts; GM12878; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000540647.5; ENST00000321867.6; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218374 | ||
| mod ID: M6ASITE016211 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921671-131921672:+ | [8] | |
| Sequence | CCCCAGGGCAGCCCCGGAGGACAGGCAAGGGCCTGAGACCA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1A; H1B; GM12878; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000544718.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218375 | ||
| mod ID: M6ASITE016212 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921688-131921689:+ | [8] | |
| Sequence | AGGACAGGCAAGGGCCTGAGACCACTGCCGACTCAAAGCCA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; GM12878; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218376 | ||
| mod ID: M6ASITE016213 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921854-131921855:+ | [8] | |
| Sequence | AGCACTTTATGCATATAGAGACAGAACCTGGACCTCACCAG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; hESC-HEK293T; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218377 | ||
| mod ID: M6ASITE016214 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921859-131921860:+ | [8] | |
| Sequence | TTTATGCATATAGAGACAGAACCTGGACCTCACCAGGGACT | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000544718.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218378 | ||
| mod ID: M6ASITE016215 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921865-131921866:+ | [8] | |
| Sequence | CATATAGAGACAGAACCTGGACCTCACCAGGGACTGCTGGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000544718.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218379 | ||
| mod ID: M6ASITE016216 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921877-131921878:+ | [8] | |
| Sequence | GAACCTGGACCTCACCAGGGACTGCTGGGCAGCGATTCCTG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1; ENST00000544718.1 | ||
| External Link | RMBase: m6A_site_218380 | ||
| mod ID: M6ASITE016217 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921933-131921934:+ | [8] | |
| Sequence | TTGTACATACACATATGCAGACACATGCCAGGGCCCCCCAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000544718.1; ENST00000321867.6; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218381 | ||
| mod ID: M6ASITE016218 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921967-131921968:+ | [8] | |
| Sequence | CCCCCAAGCCCGAGCACCGGACCACGTTGCTGCCCAGGTCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218382 | ||
| mod ID: M6ASITE016219 | Click to Show/Hide the Full List | ||
| mod site | chr12:131921990-131921991:+ | [8] | |
| Sequence | ACGTTGCTGCCCAGGTCTGGACCTCAGCGGGAGAACTGGCT | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218383 | ||
| mod ID: M6ASITE016220 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922004-131922005:+ | [8] | |
| Sequence | GTCTGGACCTCAGCGGGAGAACTGGCTCCGGGGGGAGTGGG | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; MM6; Huh7; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; GSCs; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218384 | ||
| mod ID: M6ASITE016221 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922075-131922076:+ | [8] | |
| Sequence | GTTCAAGCGTTCCTCTGGGGACCGGCAGCAGAGGCACCGTG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; GM12878; LCLs; H1299; MM6; Jurkat; CD4T; peripheral-blood; GSC-11; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; NB4 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218385 | ||
| mod ID: M6ASITE016222 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922146-131922147:+ | [12] | |
| Sequence | TTTCACACTTTATTCCTAAAACGTGTCTTATTTTTATGCAG | ||
| Motif Score | 2.179660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218386 | ||
| mod ID: M6ASITE016223 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922191-131922192:+ | [8] | |
| Sequence | TTTTTTCTTTAAAGGAGAAAACTTGTAGGTGTTTAAGAATT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; iSLK; MSC | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000540568.1; ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218387 | ||
| mod ID: M6ASITE016224 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922230-131922231:+ | [8] | |
| Sequence | TTGGTTTTGGGAGGGCGAGGACTGGGCCAGGTTAGAGGCAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6; ENST00000540568.1 | ||
| External Link | RMBase: m6A_site_218388 | ||
| mod ID: M6ASITE016225 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922344-131922345:+ | [8] | |
| Sequence | CTCCCTGTGGCAGCAGCAGGACAGGTGTGTGCCCAGCACCC | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218389 | ||
| mod ID: M6ASITE016226 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922592-131922593:+ | [8] | |
| Sequence | CAGGCGGGCATGCCCTGCAAACCCCGCCTGGGCCTCCCTTG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218390 | ||
| mod ID: M6ASITE016227 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922700-131922701:+ | [13] | |
| Sequence | AGGCAGTGGTGGGGCCATGGACCCATGCGGGGGGTTCCAGG | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HEK293T; HeLa | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218391 | ||
| mod ID: M6ASITE016228 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922740-131922741:+ | [13] | |
| Sequence | GGTCACACGCCACATAACAGACAAAAATACACACACGTGTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HEK293T; HeLa; A549; iSLK; TIME | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218392 | ||
| mod ID: M6ASITE016229 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922752-131922753:+ | [9] | |
| Sequence | CATAACAGACAAAAATACACACACGTGTGTTTTTCTTTGCA | ||
| Motif Score | 2.084928571 | ||
| Cell/Tissue List | hESC-HEK293T | ||
| Seq Type List | MAZTER-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218393 | ||
| mod ID: M6ASITE016230 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922754-131922755:+ | [12] | |
| Sequence | TAACAGACAAAAATACACACACGTGTGTTTTTCTTTGCAAT | ||
| Motif Score | 2.058863095 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218394 | ||
| mod ID: M6ASITE016231 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922790-131922791:+ | [12] | |
| Sequence | GCAATACTTGAAATATTGCCACTGTGCTTGGACTTAGAAGA | ||
| Motif Score | 2.475107143 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218395 | ||
| mod ID: M6ASITE016232 | Click to Show/Hide the Full List | ||
| mod site | chr12:131922801-131922802:+ | [13] | |
| Sequence | AATATTGCCACTGTGCTTGGACTTAGAAGAAGAAAATCCCC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HEK293T; HeLa; A549; iSLK | ||
| Seq Type List | MeRIP-seq; m6A-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218396 | ||
| mod ID: M6ASITE016233 | Click to Show/Hide the Full List | ||
| mod site | chr12:131923053-131923054:+ | [8] | |
| Sequence | TGTTCTCTTGATAGTGCTGGACCCTTTGTCTATTTTAAAGC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218397 | ||
| mod ID: M6ASITE016234 | Click to Show/Hide the Full List | ||
| mod site | chr12:131923135-131923136:+ | [8] | |
| Sequence | ATACCAACGTAAGGAAATAAACCTTTGGAATTGTTGGGCTG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000321867.6 | ||
| External Link | RMBase: m6A_site_218398 | ||
References