m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00348)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
NFE2L2
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Browse Drug
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | LX2 cell line | Homo sapiens |
|
Treatment: shMETTL3 LX2 cells
Control: shLuc LX2 cells
|
GSE207909 | |
| Regulation |
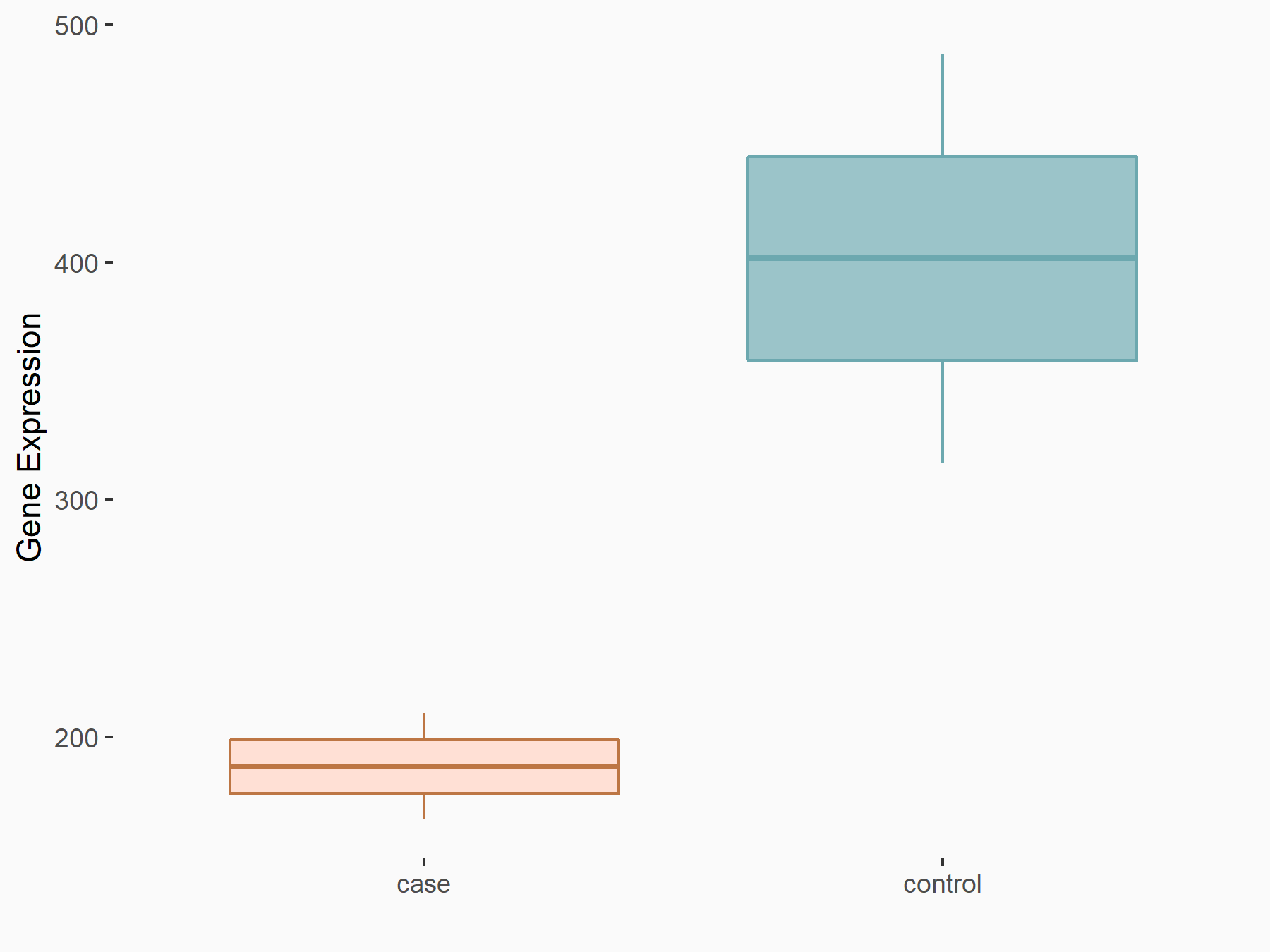  |
logFC: -1.10E+00 p-value: 4.72E-06 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | m6A methylation was involved in oxidative stress-mediated apoptosis in the mechanism of colistin nephrotoxicity. METTL3-mediated m6A methylation modification is involved in colistin-induced nephrotoxicity through apoptosis mediated by Keap1/Nuclear factor erythroid 2-related factor 2 (NFE2L2) signaling pathway. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Diseases of the urinary system | ICD-11: GC2Z | ||
| Responsed Drug | Colistin | Approved | ||
| Cell Process | Oxidative stress | |||
| Cell apoptosis | ||||
| In-vivo Model | The 60 female Kunming mice were divided into two groups (n = 30): control group (injection of physiological saline through the caudal vein) and colistin group (injection of 15 mg/kg colistin, twice a day, with an eight-hour interval). | |||
Fat mass and obesity-associated protein (FTO) [ERASER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [7] | |||
| Response Summary | DEHP worsened testicular histology, decreased testosterone concentrations, downregulated expression of spermatogenesis inducers, enhanced oxidative stress, inhibited the Nuclear factor erythroid 2-related factor 2 (NFE2L2)-mediated antioxidant pathway, and increased apoptosis in testes. DEHP is a common environmental endocrine disrupting chemical that induces male reproductive disorders. Additionally, DEHP increased global levels of m6A RNA modification and altered the expression of two important RNA methylation modulator genes, FTO and YTHDC2. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Male reproductive disorders | ICD-11: VV5Z | ||
| Cell Process | Oxidative stress | |||
| Cell apoptosis | ||||
| In-vivo Model | Exposed Sprague-Dawley rats to 0, 250, and 500 mg DEHP per kg body weight per day at the prepuberty stage from postnatal day 22 (PND 22) to PND 35 by oral gavage. | |||
YTH domain-containing family protein 1 (YTHDF1) [READER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [11] | |||
| Response Summary | YTHDF1 deficiency inhibits Non-small cell lung cancer cell proliferation and xenograft tumor formation through regulating the translational efficiency of CDK2, CDK4, p27, and cyclin D1, and that YTHDF1 depletion restrains de novo lung adenocarcinomas (ADC) progression. Mechanistic studies identified the Keap1-Nuclear factor erythroid 2-related factor 2 (NFE2L2)-AKR1C1 axis as the downstream mediator of YTHDF1. YTHDF1 high expression correlates with better clinical outcome, with its depletion rendering cancerous cells resistant to cisplatin (DDP) treatment. | |||
| Target Regulation | Down regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Chemical carcinogenesis - reactive oxygen species | hsa05208 | ||
| Cell cycle | hsa04110 | |||
| Cell Process | Biological regulation | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| A549-DDP (Human lung adenocarcinoma is resistant to cisplatin) | ||||
| GLC-82 | Endocervical adenocarcinoma | Homo sapiens | CVCL_3371 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| NCI-H1650 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1483 | |
| NCI-H838 | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | |
| SPC-A1 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | |
| In-vivo Model | Mice were treated via nasal inhalation of adenovirus carrying Cre recombinase (5 × 106 p.f.u for Ad-Cre, Biowit Inc., Shenzhen, Guangdong), and were then killed at indicated times for gross inspection and histopathological examination. | |||
YTH domain-containing protein 2 (YTHDC2) [READER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [7] | |||
| Response Summary | DEHP worsened testicular histology, decreased testosterone concentrations, downregulated expression of spermatogenesis inducers, enhanced oxidative stress, inhibited the Nuclear factor erythroid 2-related factor 2 (NFE2L2)-mediated antioxidant pathway, and increased apoptosis in testes. DEHP is a common environmental endocrine disrupting chemical that induces male reproductive disorders. Additionally, DEHP increased global levels of m6A RNA modification and altered the expression of two important RNA methylation modulator genes, FTO and YTHDC2. | |||
| Responsed Disease | Male reproductive disorders | ICD-11: VV5Z | ||
| Cell Process | Oxidative stress | |||
| Cell apoptosis | ||||
| In-vivo Model | Exposed Sprague-Dawley rats to 0, 250, and 500 mg DEHP per kg body weight per day at the prepuberty stage from postnatal day 22 (PND 22) to PND 35 by oral gavage. | |||
Lung cancer [ICD-11: 2C25]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [11] | |||
| Response Summary | YTHDF1 deficiency inhibits Non-small cell lung cancer cell proliferation and xenograft tumor formation through regulating the translational efficiency of CDK2, CDK4, p27, and cyclin D1, and that YTHDF1 depletion restrains de novo lung adenocarcinomas (ADC) progression. Mechanistic studies identified the Keap1-Nuclear factor erythroid 2-related factor 2 (NFE2L2)-AKR1C1 axis as the downstream mediator of YTHDF1. YTHDF1 high expression correlates with better clinical outcome, with its depletion rendering cancerous cells resistant to cisplatin (DDP) treatment. | |||
| Responsed Disease | Non-small-cell lung carcinoma [ICD-11: 2C25.Y] | |||
| Target Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | ||
| Target Regulation | Down regulation | |||
| Responsed Drug | Cisplatin | Approved | ||
| Pathway Response | Chemical carcinogenesis - reactive oxygen species | hsa05208 | ||
| Cell cycle | hsa04110 | |||
| Cell Process | Biological regulation | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| A549-DDP (Human lung adenocarcinoma is resistant to cisplatin) | ||||
| GLC-82 | Endocervical adenocarcinoma | Homo sapiens | CVCL_3371 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| NCI-H1650 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1483 | |
| NCI-H838 | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | |
| SPC-A1 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | |
| In-vivo Model | Mice were treated via nasal inhalation of adenovirus carrying Cre recombinase (5 × 106 p.f.u for Ad-Cre, Biowit Inc., Shenzhen, Guangdong), and were then killed at indicated times for gross inspection and histopathological examination. | |||
Diseases of the urinary system [ICD-11: GC2Z]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | m6A methylation was involved in oxidative stress-mediated apoptosis in the mechanism of colistin nephrotoxicity. METTL3-mediated m6A methylation modification is involved in colistin-induced nephrotoxicity through apoptosis mediated by Keap1/Nuclear factor erythroid 2-related factor 2 (NFE2L2) signaling pathway. | |||
| Responsed Disease | Diseases of the urinary system [ICD-11: GC2Z] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Drug | Colistin | Approved | ||
| Cell Process | Oxidative stress | |||
| Cell apoptosis | ||||
| In-vivo Model | The 60 female Kunming mice were divided into two groups (n = 30): control group (injection of physiological saline through the caudal vein) and colistin group (injection of 15 mg/kg colistin, twice a day, with an eight-hour interval). | |||
Male reproductive disorders [ICD-11: VV5Z]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [7] | |||
| Response Summary | DEHP worsened testicular histology, decreased testosterone concentrations, downregulated expression of spermatogenesis inducers, enhanced oxidative stress, inhibited the Nuclear factor erythroid 2-related factor 2 (NFE2L2)-mediated antioxidant pathway, and increased apoptosis in testes. DEHP is a common environmental endocrine disrupting chemical that induces male reproductive disorders. Additionally, DEHP increased global levels of m6A RNA modification and altered the expression of two important RNA methylation modulator genes, FTO and YTHDC2. | |||
| Responsed Disease | Male reproductive disorders [ICD-11: VV5Z] | |||
| Target Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | ||
| Target Regulation | Up regulation | |||
| Cell Process | Oxidative stress | |||
| Cell apoptosis | ||||
| In-vivo Model | Exposed Sprague-Dawley rats to 0, 250, and 500 mg DEHP per kg body weight per day at the prepuberty stage from postnatal day 22 (PND 22) to PND 35 by oral gavage. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [7] | |||
| Response Summary | DEHP worsened testicular histology, decreased testosterone concentrations, downregulated expression of spermatogenesis inducers, enhanced oxidative stress, inhibited the Nuclear factor erythroid 2-related factor 2 (NFE2L2)-mediated antioxidant pathway, and increased apoptosis in testes. DEHP is a common environmental endocrine disrupting chemical that induces male reproductive disorders. Additionally, DEHP increased global levels of m6A RNA modification and altered the expression of two important RNA methylation modulator genes, FTO and YTHDC2. | |||
| Responsed Disease | Male reproductive disorders [ICD-11: VV5Z] | |||
| Target Regulator | YTH domain-containing protein 2 (YTHDC2) | READER | ||
| Cell Process | Oxidative stress | |||
| Cell apoptosis | ||||
| In-vivo Model | Exposed Sprague-Dawley rats to 0, 250, and 500 mg DEHP per kg body weight per day at the prepuberty stage from postnatal day 22 (PND 22) to PND 35 by oral gavage. | |||
Cisplatin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [11] | |||
| Response Summary | YTHDF1 deficiency inhibits Non-small cell lung cancer cell proliferation and xenograft tumor formation through regulating the translational efficiency of CDK2, CDK4, p27, and cyclin D1, and that YTHDF1 depletion restrains de novo lung adenocarcinomas (ADC) progression. Mechanistic studies identified the Keap1-Nuclear factor erythroid 2-related factor 2 (NFE2L2)-AKR1C1 axis as the downstream mediator of YTHDF1. YTHDF1 high expression correlates with better clinical outcome, with its depletion rendering cancerous cells resistant to cisplatin (DDP) treatment. | |||
| Target Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | ||
| Target Regulation | Down regulation | |||
| Responsed Disease | Non-small-cell lung carcinoma | ICD-11: 2C25.Y | ||
| Pathway Response | Chemical carcinogenesis - reactive oxygen species | hsa05208 | ||
| Cell cycle | hsa04110 | |||
| Cell Process | Biological regulation | |||
| In-vitro Model | A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 |
| A549-DDP (Human lung adenocarcinoma is resistant to cisplatin) | ||||
| GLC-82 | Endocervical adenocarcinoma | Homo sapiens | CVCL_3371 | |
| NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| HEK293T | Normal | Homo sapiens | CVCL_0063 | |
| NCI-H1650 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1483 | |
| NCI-H838 | Lung adenocarcinoma | Homo sapiens | CVCL_1594 | |
| SPC-A1 | Endocervical adenocarcinoma | Homo sapiens | CVCL_6955 | |
| In-vivo Model | Mice were treated via nasal inhalation of adenovirus carrying Cre recombinase (5 × 106 p.f.u for Ad-Cre, Biowit Inc., Shenzhen, Guangdong), and were then killed at indicated times for gross inspection and histopathological examination. | |||
Colistin
[Approved]
| In total 1 item(s) under this drug | ||||
| Experiment 1 Reporting the m6A-centered Drug Response | [1] | |||
| Response Summary | m6A methylation was involved in oxidative stress-mediated apoptosis in the mechanism of colistin nephrotoxicity. METTL3-mediated m6A methylation modification is involved in colistin-induced nephrotoxicity through apoptosis mediated by Keap1/Nuclear factor erythroid 2-related factor 2 (NFE2L2) signaling pathway. | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Responsed Disease | Diseases of the urinary system | ICD-11: GC2Z | ||
| Cell Process | Oxidative stress | |||
| Cell apoptosis | ||||
| In-vivo Model | The 60 female Kunming mice were divided into two groups (n = 30): control group (injection of physiological saline through the caudal vein) and colistin group (injection of 15 mg/kg colistin, twice a day, with an eight-hour interval). | |||
Full List of Crosstalk(s) between m6A Modification and Epigenetic Regulation Related to This Regulator
Non-coding RNA
m6A Regulator: Fat mass and obesity-associated protein (FTO)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05072 | ||
| Epigenetic Regulator | Urothelial cancer associated 1 (UCA1) | |
| Regulated Target | FTO alpha-ketoglutarate dependent dioxygenase (FTO) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | DOX-induced cardiotoxicity | |
m6A Regulator: Staphylococcal nuclease domain-containing protein 1 (SND1)
| In total 1 item(s) under this m6A regulator | ||
| Crosstalk ID: M6ACROT05108 | ||
| Epigenetic Regulator | SNAI3 antisense RNA 1 (SNAI3-AS1) | |
| Regulated Target | Staphylococcal nuclease domain-containing protein 1 (SND1) | |
| Crosstalk relationship | ncRNA → m6A | |
| Disease | Brain cancer | |
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00348)
| In total 52 m6A sequence/site(s) in this target gene | |||
| mod ID: A2ISITE010119 | Click to Show/Hide the Full List | ||
| mod site | chr2:177235658-177235659:- | [12] | |
| Sequence | AATTTGTTTTGATTTTGGGTAGAAACAGGTGTCACTATGGT | ||
| Transcript ID List | ENST00000397063.8; ENST00000586532.5; ENST00000464747.5; ENST00000588123.1; ENST00000423513.5; ENST00000477534.1; ENST00000446151.6; ENST00000448782.5; ENST00000397062.8; ENST00000462023.1; ENST00000421929.5; ENST00000449627.1; ENST00000430047.1 | ||
| External Link | RMBase: RNA-editing_site_81811 | ||
| mod ID: A2ISITE010120 | Click to Show/Hide the Full List | ||
| mod site | chr2:177235714-177235715:- | [12] | |
| Sequence | CTCACTTCAGTCTCCCTAATAGTTGGGACTACAGGCTCGTA | ||
| Transcript ID List | ENST00000446151.6; ENST00000423513.5; ENST00000586532.5; ENST00000477534.1; ENST00000397063.8; ENST00000464747.5; ENST00000462023.1; ENST00000397062.8; ENST00000588123.1; ENST00000449627.1; ENST00000430047.1; ENST00000448782.5; ENST00000421929.5 | ||
| External Link | RMBase: RNA-editing_site_81812 | ||
| mod ID: A2ISITE010121 | Click to Show/Hide the Full List | ||
| mod site | chr2:177235774-177235775:- | [12] | |
| Sequence | GAGTGCAGTGGTACAATCACAGCTCACCATGGCCTTGACTT | ||
| Transcript ID List | ENST00000449627.1; ENST00000462023.1; ENST00000477534.1; ENST00000397062.8; ENST00000423513.5; ENST00000446151.6; ENST00000464747.5; ENST00000448782.5; ENST00000586532.5; ENST00000430047.1; ENST00000397063.8; ENST00000421929.5; ENST00000588123.1 | ||
| External Link | RMBase: RNA-editing_site_81813 | ||
| mod ID: A2ISITE010122 | Click to Show/Hide the Full List | ||
| mod site | chr2:177237114-177237115:- | [13] | |
| Sequence | GTAATCCTAGCACTTTGGGAAGCTGAGGCAGGAAAATCAAT | ||
| Transcript ID List | ENST00000423513.5; ENST00000464747.5; ENST00000448782.5; ENST00000588123.1; ENST00000397062.8; ENST00000462023.1; ENST00000397063.8; ENST00000586532.5; ENST00000446151.6; rmsk_751799; ENST00000477534.1; ENST00000430047.1; ENST00000421929.5; ENST00000449627.1 | ||
| External Link | RMBase: RNA-editing_site_81814 | ||
| mod ID: A2ISITE010123 | Click to Show/Hide the Full List | ||
| mod site | chr2:177239654-177239655:- | [14] | |
| Sequence | AGACAAGGTCTCACTTTGTTACCCAGGCTGGAGTGCAGTGG | ||
| Transcript ID List | ENST00000464747.5; ENST00000449627.1; ENST00000588123.1; ENST00000397063.8; ENST00000421929.5; ENST00000430047.1; ENST00000477534.1; ENST00000446151.6; ENST00000423513.5; ENST00000462023.1; ENST00000397062.8; ENST00000586532.5; ENST00000448782.5 | ||
| External Link | RMBase: RNA-editing_site_81815 | ||
| mod ID: A2ISITE010124 | Click to Show/Hide the Full List | ||
| mod site | chr2:177243602-177243603:- | [15] | |
| Sequence | ACTTGAGCCTGGGAATTAGAAGCTGCAGTGAGCTAGGAAGG | ||
| Transcript ID List | ENST00000446151.6; ENST00000421929.5; ENST00000397063.8; ENST00000397062.8; ENST00000464747.5; ENST00000423513.5; ENST00000588123.1; ENST00000448782.5; ENST00000449627.1; ENST00000462023.1; rmsk_751808; ENST00000477534.1; ENST00000586532.5; ENST00000430047.1 | ||
| External Link | RMBase: RNA-editing_site_81816 | ||
| mod ID: A2ISITE010125 | Click to Show/Hide the Full List | ||
| mod site | chr2:177243617-177243618:- | [15] | |
| Sequence | CAAGGTGGGAGGATCACTTGAGCCTGGGAATTAGAAGCTGC | ||
| Transcript ID List | rmsk_751808; ENST00000588123.1; ENST00000421929.5; ENST00000464747.5; ENST00000397063.8; ENST00000430047.1; ENST00000462023.1; ENST00000423513.5; ENST00000448782.5; ENST00000449627.1; ENST00000586532.5; ENST00000477534.1; ENST00000446151.6; ENST00000397062.8 | ||
| External Link | RMBase: RNA-editing_site_81817 | ||
| mod ID: A2ISITE010126 | Click to Show/Hide the Full List | ||
| mod site | chr2:177243622-177243623:- | [15] | |
| Sequence | GAGGCCAAGGTGGGAGGATCACTTGAGCCTGGGAATTAGAA | ||
| Transcript ID List | ENST00000464747.5; ENST00000446151.6; rmsk_751808; ENST00000449627.1; ENST00000462023.1; ENST00000588123.1; ENST00000477534.1; ENST00000397063.8; ENST00000448782.5; ENST00000430047.1; ENST00000586532.5; ENST00000423513.5; ENST00000421929.5; ENST00000397062.8 | ||
| External Link | RMBase: RNA-editing_site_81818 | ||
| mod ID: A2ISITE010127 | Click to Show/Hide the Full List | ||
| mod site | chr2:177243636-177243637:- | [15] | |
| Sequence | TGCATGCTACTTGGGAGGCCAAGGTGGGAGGATCACTTGAG | ||
| Transcript ID List | ENST00000586532.5; ENST00000464747.5; ENST00000477534.1; ENST00000397062.8; ENST00000430047.1; ENST00000449627.1; ENST00000397063.8; ENST00000588123.1; ENST00000448782.5; ENST00000423513.5; rmsk_751808; ENST00000446151.6; ENST00000421929.5; ENST00000462023.1 | ||
| External Link | RMBase: RNA-editing_site_81819 | ||
| mod ID: A2ISITE010128 | Click to Show/Hide the Full List | ||
| mod site | chr2:177243648-177243649:- | [15] | |
| Sequence | CAGGCATGGTGATGCATGCTACTTGGGAGGCCAAGGTGGGA | ||
| Transcript ID List | ENST00000397062.8; ENST00000423513.5; ENST00000421929.5; ENST00000448782.5; ENST00000462023.1; rmsk_751808; ENST00000588123.1; ENST00000464747.5; ENST00000430047.1; ENST00000446151.6; ENST00000397063.8; ENST00000586532.5; ENST00000477534.1; ENST00000449627.1 | ||
| External Link | RMBase: RNA-editing_site_81820 | ||
| mod ID: A2ISITE010129 | Click to Show/Hide the Full List | ||
| mod site | chr2:177243687-177243688:- | [15] | |
| Sequence | ACATAGCAAGAACCATCTCTACAAAAAATAAAAATTAACCA | ||
| Transcript ID List | ENST00000397062.8; ENST00000449627.1; ENST00000423513.5; ENST00000477534.1; ENST00000462023.1; ENST00000588123.1; ENST00000586532.5; rmsk_751808; ENST00000446151.6; ENST00000421929.5; ENST00000397063.8; ENST00000430047.1; ENST00000448782.5; ENST00000464747.5 | ||
| External Link | RMBase: RNA-editing_site_81821 | ||
| mod ID: A2ISITE010130 | Click to Show/Hide the Full List | ||
| mod site | chr2:177243730-177243731:- | [15] | |
| Sequence | AGGAGGATCAGTTGGGGCCAAGAATTTGAGACCAGCATGAG | ||
| Transcript ID List | ENST00000464747.5; ENST00000430047.1; ENST00000397062.8; ENST00000586532.5; ENST00000477534.1; ENST00000462023.1; ENST00000397063.8; ENST00000446151.6; rmsk_751808; ENST00000588123.1; ENST00000423513.5; ENST00000448782.5; ENST00000449627.1; ENST00000421929.5 | ||
| External Link | RMBase: RNA-editing_site_81822 | ||
| mod ID: A2ISITE010131 | Click to Show/Hide the Full List | ||
| mod site | chr2:177243750-177243751:- | [14] | |
| Sequence | GCACTTTGGGAGACCAAGGCAGGAGGATCAGTTGGGGCCAA | ||
| Transcript ID List | ENST00000588123.1; ENST00000421929.5; rmsk_751808; ENST00000477534.1; ENST00000397063.8; ENST00000430047.1; ENST00000449627.1; ENST00000464747.5; ENST00000446151.6; ENST00000397062.8; ENST00000448782.5; ENST00000586532.5; ENST00000423513.5; ENST00000462023.1 | ||
| External Link | RMBase: RNA-editing_site_81823 | ||
| mod ID: A2ISITE010132 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244065-177244066:- | [15] | |
| Sequence | TCCTGTGATCCACCTACCTCAGCCTCCTAAAGTGCTGGGAT | ||
| Transcript ID List | ENST00000397063.8; ENST00000397062.8; ENST00000448782.5; ENST00000464747.5; ENST00000477534.1; ENST00000586532.5; ENST00000462023.1; ENST00000446151.6; ENST00000423513.5; ENST00000449627.1; ENST00000430047.1; ENST00000421929.5; ENST00000588123.1 | ||
| External Link | RMBase: RNA-editing_site_81824 | ||
| mod ID: A2ISITE010133 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244110-177244111:- | [15] | |
| Sequence | AGACGGGGTTTCACCGTGTTAGCCAGGATGGTCTCAATCTC | ||
| Transcript ID List | ENST00000397062.8; ENST00000446151.6; ENST00000423513.5; ENST00000449627.1; ENST00000397063.8; ENST00000448782.5; ENST00000588123.1; ENST00000462023.1; ENST00000477534.1; ENST00000421929.5; ENST00000586532.5; ENST00000430047.1; ENST00000464747.5 | ||
| External Link | RMBase: RNA-editing_site_81825 | ||
| mod ID: A2ISITE010134 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244132-177244133:- | [15] | |
| Sequence | AATTTTTTTGTATTTTTAGTAGAGACGGGGTTTCACCGTGT | ||
| Transcript ID List | ENST00000462023.1; ENST00000421929.5; ENST00000586532.5; ENST00000477534.1; ENST00000397063.8; ENST00000446151.6; ENST00000397062.8; ENST00000423513.5; ENST00000464747.5; ENST00000449627.1; ENST00000430047.1; ENST00000588123.1; ENST00000448782.5 | ||
| External Link | RMBase: RNA-editing_site_81826 | ||
| mod ID: A2ISITE010135 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244135-177244136:- | [15] | |
| Sequence | GCTAATTTTTTTGTATTTTTAGTAGAGACGGGGTTTCACCG | ||
| Transcript ID List | ENST00000448782.5; ENST00000397062.8; ENST00000462023.1; ENST00000423513.5; ENST00000446151.6; ENST00000449627.1; ENST00000477534.1; ENST00000397063.8; ENST00000586532.5; ENST00000588123.1; ENST00000430047.1; ENST00000464747.5; ENST00000421929.5 | ||
| External Link | RMBase: RNA-editing_site_81827 | ||
| mod ID: A2ISITE010136 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244200-177244201:- | [15] | |
| Sequence | TCAAGCGATTCTCCTGCCTCAGCCTCCCGAGTAGCTGGGAC | ||
| Transcript ID List | ENST00000446151.6; ENST00000430047.1; ENST00000588123.1; ENST00000586532.5; ENST00000449627.1; ENST00000462023.1; ENST00000464747.5; ENST00000448782.5; ENST00000421929.5; ENST00000477534.1; ENST00000397063.8; ENST00000397062.8; ENST00000423513.5 | ||
| External Link | RMBase: RNA-editing_site_81828 | ||
| mod ID: A2ISITE010137 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244217-177244218:- | [15] | |
| Sequence | CCTCTGCCTCCCCGGGTTCAAGCGATTCTCCTGCCTCAGCC | ||
| Transcript ID List | ENST00000462023.1; ENST00000421929.5; ENST00000448782.5; ENST00000477534.1; ENST00000588123.1; ENST00000586532.5; ENST00000449627.1; ENST00000397062.8; ENST00000446151.6; ENST00000464747.5; ENST00000423513.5; ENST00000397063.8; ENST00000430047.1 | ||
| External Link | RMBase: RNA-editing_site_81829 | ||
| mod ID: A2ISITE010138 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244218-177244219:- | [15] | |
| Sequence | ACCTCTGCCTCCCCGGGTTCAAGCGATTCTCCTGCCTCAGC | ||
| Transcript ID List | ENST00000430047.1; ENST00000588123.1; ENST00000397062.8; ENST00000397063.8; ENST00000586532.5; ENST00000462023.1; ENST00000446151.6; ENST00000423513.5; ENST00000421929.5; ENST00000477534.1; ENST00000449627.1; ENST00000448782.5; ENST00000464747.5 | ||
| External Link | RMBase: RNA-editing_site_81830 | ||
| mod ID: A2ISITE010139 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244238-177244239:- | [15] | |
| Sequence | CGCGATCTTGGCTCACTGCAACCTCTGCCTCCCCGGGTTCA | ||
| Transcript ID List | ENST00000477534.1; ENST00000588123.1; ENST00000423513.5; ENST00000421929.5; ENST00000446151.6; ENST00000397062.8; ENST00000586532.5; ENST00000430047.1; ENST00000449627.1; ENST00000464747.5; ENST00000397063.8; ENST00000448782.5; ENST00000462023.1 | ||
| External Link | RMBase: RNA-editing_site_81831 | ||
| mod ID: A2ISITE010140 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244262-177244263:- | [15] | |
| Sequence | GTCACCCAGGCTGGAGTGCAATGGCGCGATCTTGGCTCACT | ||
| Transcript ID List | ENST00000430047.1; ENST00000421929.5; ENST00000397062.8; ENST00000462023.1; ENST00000449627.1; ENST00000448782.5; ENST00000397063.8; ENST00000464747.5; ENST00000588123.1; ENST00000477534.1; ENST00000586532.5; ENST00000446151.6; ENST00000423513.5 | ||
| External Link | RMBase: RNA-editing_site_81832 | ||
| mod ID: A2ISITE010141 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244631-177244632:- | [14] | |
| Sequence | GGCCTTTGGAAAATAGATTTAACCCTTGTTTATTGGAAGGA | ||
| Transcript ID List | ENST00000423513.5; ENST00000446151.6; ENST00000397062.8; ENST00000464747.5; ENST00000477534.1; ENST00000421929.5; ENST00000430047.1; ENST00000449627.1; ENST00000448782.5; ENST00000397063.8; ENST00000586532.5; ENST00000588123.1; ENST00000462023.1 | ||
| External Link | RMBase: RNA-editing_site_81833 | ||
| mod ID: A2ISITE010142 | Click to Show/Hide the Full List | ||
| mod site | chr2:177244822-177244823:- | [14] | |
| Sequence | TTTAGTAGAGATGGGGTTTCACCGTTAGCCAGGATGGTCTC | ||
| Transcript ID List | ENST00000464747.5; ENST00000397063.8; ENST00000421929.5; ENST00000477534.1; ENST00000430047.1; ENST00000397062.8; ENST00000462023.1; ENST00000449627.1; ENST00000446151.6; ENST00000423513.5; ENST00000588123.1; ENST00000448782.5; ENST00000586532.5 | ||
| External Link | RMBase: RNA-editing_site_81834 | ||
| mod ID: A2ISITE010143 | Click to Show/Hide the Full List | ||
| mod site | chr2:177246037-177246038:- | [14] | |
| Sequence | ACACCAAGGAGCAGTGGACAAGCTTCCCAGGCTCAAGGTCA | ||
| Transcript ID List | ENST00000423513.5; ENST00000397063.8; ENST00000477534.1; ENST00000464747.5; ENST00000430047.1; ENST00000449627.1; ENST00000588123.1; ENST00000421929.5; ENST00000397062.8; ENST00000462023.1; ENST00000448782.5; rmsk_751815; ENST00000446151.6; ENST00000586532.5 | ||
| External Link | RMBase: RNA-editing_site_81835 | ||
| mod ID: A2ISITE010144 | Click to Show/Hide the Full List | ||
| mod site | chr2:177246706-177246707:- | [15] | |
| Sequence | TTCAGGAGGCTGAGGTGGGAAGATTGCTTGAGCCCAGGAGG | ||
| Transcript ID List | ENST00000477534.1; ENST00000464747.5; ENST00000421929.5; ENST00000586532.5; ENST00000446151.6; ENST00000423513.5; ENST00000430047.1; ENST00000397062.8; ENST00000462023.1; rmsk_751817; ENST00000449627.1; ENST00000588123.1; ENST00000448782.5; ENST00000397063.8 | ||
| External Link | RMBase: RNA-editing_site_81836 | ||
| mod ID: A2ISITE010145 | Click to Show/Hide the Full List | ||
| mod site | chr2:177246720-177246721:- | [15] | |
| Sequence | TGTAATCTCAGCTATTCAGGAGGCTGAGGTGGGAAGATTGC | ||
| Transcript ID List | ENST00000449627.1; ENST00000462023.1; ENST00000430047.1; ENST00000588123.1; ENST00000423513.5; ENST00000464747.5; ENST00000397063.8; ENST00000421929.5; ENST00000397062.8; rmsk_751817; ENST00000446151.6; ENST00000586532.5; ENST00000477534.1; ENST00000448782.5 | ||
| External Link | RMBase: RNA-editing_site_81837 | ||
| mod ID: A2ISITE010146 | Click to Show/Hide the Full List | ||
| mod site | chr2:177246723-177246724:- | [15] | |
| Sequence | GCCTGTAATCTCAGCTATTCAGGAGGCTGAGGTGGGAAGAT | ||
| Transcript ID List | rmsk_751817; ENST00000430047.1; ENST00000423513.5; ENST00000449627.1; ENST00000421929.5; ENST00000477534.1; ENST00000397062.8; ENST00000464747.5; ENST00000448782.5; ENST00000588123.1; ENST00000586532.5; ENST00000397063.8; ENST00000446151.6; ENST00000462023.1 | ||
| External Link | RMBase: RNA-editing_site_81838 | ||
| mod ID: A2ISITE010147 | Click to Show/Hide the Full List | ||
| mod site | chr2:177246736-177246737:- | [15] | |
| Sequence | CATAGTGGCACATGCCTGTAATCTCAGCTATTCAGGAGGCT | ||
| Transcript ID List | rmsk_751817; ENST00000430047.1; ENST00000477534.1; ENST00000588123.1; ENST00000397063.8; ENST00000448782.5; ENST00000464747.5; ENST00000423513.5; ENST00000462023.1; ENST00000446151.6; ENST00000586532.5; ENST00000397062.8; ENST00000449627.1; ENST00000421929.5 | ||
| External Link | RMBase: RNA-editing_site_81839 | ||
| mod ID: A2ISITE010148 | Click to Show/Hide the Full List | ||
| mod site | chr2:177246753-177246754:- | [15] | |
| Sequence | GTAAAATATTAGCCAGGCATAGTGGCACATGCCTGTAATCT | ||
| Transcript ID List | ENST00000464747.5; ENST00000423513.5; ENST00000446151.6; ENST00000397063.8; ENST00000449627.1; ENST00000477534.1; ENST00000588123.1; ENST00000586532.5; ENST00000448782.5; ENST00000430047.1; rmsk_751817; ENST00000421929.5; ENST00000397062.8; ENST00000462023.1 | ||
| External Link | RMBase: RNA-editing_site_81840 | ||
| mod ID: A2ISITE010149 | Click to Show/Hide the Full List | ||
| mod site | chr2:177249095-177249096:- | [12] | |
| Sequence | TTCTGAGTAGCCGGGGCCACAGGTACGCGCCACCACGCCTG | ||
| Transcript ID List | ENST00000448782.5; ENST00000462023.1; ENST00000588123.1; ENST00000477534.1; ENST00000586532.5; ENST00000423513.5; ENST00000449627.1; ENST00000446151.6; ENST00000421929.5; ENST00000464747.5; ENST00000397063.8; ENST00000430047.1; ENST00000397062.8 | ||
| External Link | RMBase: RNA-editing_site_81841 | ||
| mod ID: A2ISITE010150 | Click to Show/Hide the Full List | ||
| mod site | chr2:177249097-177249098:- | [12] | |
| Sequence | CCTTCTGAGTAGCCGGGGCCACAGGTACGCGCCACCACGCC | ||
| Transcript ID List | ENST00000430047.1; ENST00000397063.8; ENST00000464747.5; ENST00000448782.5; ENST00000588123.1; ENST00000462023.1; ENST00000446151.6; ENST00000397062.8; ENST00000423513.5; ENST00000586532.5; ENST00000421929.5; ENST00000477534.1; ENST00000449627.1 | ||
| External Link | RMBase: RNA-editing_site_81842 | ||
| mod ID: A2ISITE010151 | Click to Show/Hide the Full List | ||
| mod site | chr2:177280422-177280423:- | [14] | |
| Sequence | TCTTTTTAACAAAGAAAAATACAGGATGCCCAGTTGGATTT | ||
| Transcript ID List | ENST00000588123.1; ENST00000464747.5; ENST00000586532.5; rmsk_751873 | ||
| External Link | RMBase: RNA-editing_site_81843 | ||
| mod ID: A2ISITE010152 | Click to Show/Hide the Full List | ||
| mod site | chr2:177289480-177289481:- | [14] | |
| Sequence | ACACTTACGGCATAAAGACAAGCTGCAGGGACTAGCCATCT | ||
| Transcript ID List | ENST00000430416.5; ENST00000627798.2; ENST00000456746.5; ENST00000464747.5; ENST00000625449.2; ENST00000586532.5; ENST00000397057.6 | ||
| External Link | RMBase: RNA-editing_site_81844 | ||
| mod ID: A2ISITE010153 | Click to Show/Hide the Full List | ||
| mod site | chr2:177289563-177289564:- | [14] | |
| Sequence | TTTGTAAAGCTCCCCACATAAATGGAGACCACTGCAACCTG | ||
| Transcript ID List | ENST00000430416.5; ENST00000397057.6; ENST00000456746.5; ENST00000586532.5; ENST00000625449.2; ENST00000627798.2; ENST00000464747.5 | ||
| External Link | RMBase: RNA-editing_site_81845 | ||
| mod ID: A2ISITE010154 | Click to Show/Hide the Full List | ||
| mod site | chr2:177289564-177289565:- | [14] | |
| Sequence | TTTTGTAAAGCTCCCCACATAAATGGAGACCACTGCAACCT | ||
| Transcript ID List | ENST00000456746.5; ENST00000397057.6; ENST00000625449.2; ENST00000430416.5; ENST00000586532.5; ENST00000464747.5; ENST00000627798.2 | ||
| External Link | RMBase: RNA-editing_site_81846 | ||
| mod ID: A2ISITE010155 | Click to Show/Hide the Full List | ||
| mod site | chr2:177289666-177289667:- | [14] | |
| Sequence | CTAATGGCTTCACCTGCCAAACATTTAAAACACACTGATGC | ||
| Transcript ID List | ENST00000397057.6; ENST00000625449.2; ENST00000456746.5; ENST00000627798.2; ENST00000430416.5; ENST00000464747.5; ENST00000586532.5 | ||
| External Link | RMBase: RNA-editing_site_81847 | ||
| mod ID: A2ISITE010156 | Click to Show/Hide the Full List | ||
| mod site | chr2:177291269-177291270:- | [14] | |
| Sequence | GGCTGGGCGCGGTGGCTCACACCTGTAATCCCAGCACTTTG | ||
| Transcript ID List | ENST00000430416.5; rmsk_751897; ENST00000456746.5; ENST00000586532.5; ENST00000464747.5; ENST00000625449.2; ENST00000627798.2; ENST00000397057.6 | ||
| External Link | RMBase: RNA-editing_site_81848 | ||
| mod ID: A2ISITE010157 | Click to Show/Hide the Full List | ||
| mod site | chr2:177298887-177298888:- | [13] | |
| Sequence | TAACCTTTGCCTCCGGTTTCAAGTGATTCTCCTGCCTCAAC | ||
| Transcript ID List | ENST00000430416.5; ENST00000627798.2; ENST00000397057.6; ENST00000464747.5; ENST00000586532.5; ENST00000456746.5; ENST00000625449.2 | ||
| External Link | RMBase: RNA-editing_site_81849 | ||
| mod ID: A2ISITE010158 | Click to Show/Hide the Full List | ||
| mod site | chr2:177305712-177305713:- | [14] | |
| Sequence | GGAAAGAGAAAAGGGAGAGAAAGAGGAAGAAAGGGAGTGCA | ||
| Transcript ID List | ENST00000430416.5; ENST00000464747.5; ENST00000586532.5; ENST00000625449.2; ENST00000456746.5; ENST00000397057.6; ENST00000627798.2 | ||
| External Link | RMBase: RNA-editing_site_81850 | ||
| mod ID: A2ISITE010159 | Click to Show/Hide the Full List | ||
| mod site | chr2:177312813-177312814:- | [14] | |
| Sequence | TTTTATTACTGGCTTTTCCTACTCAACTATTTGTGAGATTC | ||
| Transcript ID List | ENST00000625449.2; ENST00000464747.5; ENST00000430416.5; ENST00000443132.1; ENST00000627798.2; ENST00000586532.5; ENST00000397057.6; ENST00000456746.5 | ||
| External Link | RMBase: RNA-editing_site_81851 | ||
| mod ID: A2ISITE010160 | Click to Show/Hide the Full List | ||
| mod site | chr2:177313812-177313813:- | [14] | |
| Sequence | CTTCCAGCCCTTCTAATGGTAGGAGCATTAAGAGGGATGCT | ||
| Transcript ID List | MIMAT0025481; ENST00000443132.1; ENST00000586532.5; ENST00000397057.6; ENST00000627798.2; ENST00000430416.5; ENST00000615246.1; ENST00000625449.2; ENST00000464747.5; ENST00000456746.5 | ||
| External Link | RMBase: RNA-editing_site_81852 | ||
| mod ID: A2ISITE010161 | Click to Show/Hide the Full List | ||
| mod site | chr2:177319893-177319894:- | [13] | |
| Sequence | GGCAGAGGTTGCAGTGAGCCAAGATTGTGCCACTGCACTCC | ||
| Transcript ID List | ENST00000456746.5; ENST00000430416.5; ENST00000397057.6; ENST00000464747.5; rmsk_751968 | ||
| External Link | RMBase: RNA-editing_site_81853 | ||
| mod ID: A2ISITE010162 | Click to Show/Hide the Full List | ||
| mod site | chr2:177320117-177320118:- | [13] | |
| Sequence | GGCATAAAATTCGGCTGGGTATGGTGGCTCACACCTGTAAT | ||
| Transcript ID List | ENST00000430416.5; ENST00000456746.5; rmsk_751968; ENST00000397057.6; ENST00000464747.5 | ||
| External Link | RMBase: RNA-editing_site_81854 | ||
| mod ID: A2ISITE010163 | Click to Show/Hide the Full List | ||
| mod site | chr2:177321278-177321279:- | [13] | |
| Sequence | GTCTTGAACTTCTGGCCTCAAGTGATCCTCCCAACTCAGCC | ||
| Transcript ID List | ENST00000430416.5; ENST00000397057.6; ENST00000464747.5; ENST00000456746.5 | ||
| External Link | RMBase: RNA-editing_site_81855 | ||
| mod ID: A2ISITE010164 | Click to Show/Hide the Full List | ||
| mod site | chr2:177321977-177321978:- | [12] | |
| Sequence | AAAGTGCTAGGATTACAGGCATGAGCCACCACGCCCAGCCA | ||
| Transcript ID List | ENST00000430416.5; ENST00000456746.5; ENST00000464747.5; ENST00000397057.6; ENST00000447413.1 | ||
| External Link | RMBase: RNA-editing_site_81856 | ||
| mod ID: A2ISITE010165 | Click to Show/Hide the Full List | ||
| mod site | chr2:177331613-177331614:- | [13] | |
| Sequence | GTGCGGTGGCTCACCCCTTTAATCCCAGCACTTTGGAAGGC | ||
| Transcript ID List | ENST00000447413.1; ENST00000464747.5; rmsk_751995; ENST00000456746.5; ENST00000397057.6 | ||
| External Link | RMBase: RNA-editing_site_81857 | ||
| mod ID: A2ISITE010166 | Click to Show/Hide the Full List | ||
| mod site | chr2:177346259-177346260:- | [13] | |
| Sequence | GTCTCGAACTCCTGGGCTCAAGCCATCTGCCCACCTCGGTT | ||
| Transcript ID List | ENST00000456746.5; ENST00000464747.5; ENST00000447413.1; ENST00000397057.6 | ||
| External Link | RMBase: RNA-editing_site_81858 | ||
| mod ID: A2ISITE010167 | Click to Show/Hide the Full List | ||
| mod site | chr2:177350738-177350739:- | [13] | |
| Sequence | AGCTGATCTTGTCTCACACAGACACACCACCCCAGCTGATC | ||
| Transcript ID List | ENST00000464747.5; ENST00000397057.6; ENST00000456746.5; ENST00000447413.1 | ||
| External Link | RMBase: RNA-editing_site_81859 | ||
| mod ID: A2ISITE010168 | Click to Show/Hide the Full List | ||
| mod site | chr2:177361502-177361503:- | [13] | |
| Sequence | CAGGTTTCACTATGTTACCCAGGCTGATCTCGAACTCCCGT | ||
| Transcript ID List | ENST00000397057.6; ENST00000447413.1; ENST00000464747.5; ENST00000456746.5 | ||
| External Link | RMBase: RNA-editing_site_81860 | ||
| mod ID: A2ISITE010169 | Click to Show/Hide the Full List | ||
| mod site | chr2:177361611-177361612:- | [13] | |
| Sequence | GACTCAACCTCCCAGGATCAAGCAATTCTCCCACCTCAGCC | ||
| Transcript ID List | ENST00000447413.1; ENST00000397057.6; ENST00000456746.5; ENST00000464747.5 | ||
| External Link | RMBase: RNA-editing_site_81861 | ||
| mod ID: A2ISITE010170 | Click to Show/Hide the Full List | ||
| mod site | chr2:177386069-177386070:- | [13] | |
| Sequence | AGGCTTGGTGGCACACACCTATAATCCCAGCTACTCAGGAG | ||
| Transcript ID List | ENST00000447413.1; ENST00000464747.5; ENST00000456746.5; ENST00000397057.6; rmsk_752112 | ||
| External Link | RMBase: RNA-editing_site_81862 | ||
5-methylcytidine (m5C)
N6-methyladenosine (m6A)
| In total 119 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE048440 | Click to Show/Hide the Full List | ||
| mod site | chr2:177227683-177227684:- | [16] | |
| Sequence | AACAAACTGTCTATTCTTAGACCAAATTGTGAGTGCAGAGA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD34; HeLa; A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501147 | ||
| mod ID: M6ASITE048441 | Click to Show/Hide the Full List | ||
| mod site | chr2:177227702-177227703:- | [16] | |
| Sequence | AACATAATCCCAGGTCTTGAACAAACTGTCTATTCTTAGAC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34; HeLa; A549 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501148 | ||
| mod ID: M6ASITE048442 | Click to Show/Hide the Full List | ||
| mod site | chr2:177227721-177227722:- | [16] | |
| Sequence | ATCACTTTTCCAAACTCAGAACATAATCCCAGGTCTTGAAC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | CD34; HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501149 | ||
| mod ID: M6ASITE048444 | Click to Show/Hide the Full List | ||
| mod site | chr2:177227728-177227729:- | [16] | |
| Sequence | AGAAGACATCACTTTTCCAAACTCAGAACATAATCCCAGGT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | CD34; HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501150 | ||
| mod ID: M6ASITE048445 | Click to Show/Hide the Full List | ||
| mod site | chr2:177227743-177227744:- | [16] | |
| Sequence | ATGGGGAATCTCGGGAGAAGACATCACTTTTCCAAACTCAG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | CD34; HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501151 | ||
| mod ID: M6ASITE048446 | Click to Show/Hide the Full List | ||
| mod site | chr2:177227788-177227789:- | [17] | |
| Sequence | TTTTCACGTGCTATCAAGGAACTTCCTTCCTCTGATGGTGT | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501152 | ||
| mod ID: M6ASITE048447 | Click to Show/Hide the Full List | ||
| mod site | chr2:177227947-177227948:- | [18] | |
| Sequence | TTTTTGCATTTTTAGTGGAGACAGGGTTTCACCATGTTGAC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501153 | ||
| mod ID: M6ASITE048448 | Click to Show/Hide the Full List | ||
| mod site | chr2:177229352-177229353:- | [17] | |
| Sequence | GTGGGCTTGAACTAAAAAGGACAAAATGGGAAGCAGCAATA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000443295.1 | ||
| External Link | RMBase: m6A_site_501154 | ||
| mod ID: M6ASITE048449 | Click to Show/Hide the Full List | ||
| mod site | chr2:177229425-177229426:- | [19] | |
| Sequence | CACATATAGCCTGAAGTAAAACAGGATTTTCAAAGTCTACC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000443295.1 | ||
| External Link | RMBase: m6A_site_501155 | ||
| mod ID: M6ASITE048450 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230335-177230336:- | [20] | |
| Sequence | AAAAAATTTTAAGAGCTGGTACTAATAAAGGATTATTATGA | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000397063.8; ENST00000458603.1; ENST00000397062.8; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501156 | ||
| mod ID: M6ASITE048451 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230403-177230404:- | [20] | |
| Sequence | TTTTTATAAATACTGTATGGACAAAAAATGGCATTTTTTAT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293T; Huh7 | ||
| Seq Type List | DART-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000446151.6; ENST00000397063.8; ENST00000397062.8; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501157 | ||
| mod ID: M6ASITE048452 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230426-177230427:- | [20] | |
| Sequence | TGCAAAACTAACCACTATGTACTTTTTTATAAATACTGTAT | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000446151.6; ENST00000397062.8; ENST00000397063.8; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501158 | ||
| mod ID: M6ASITE048453 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230433-177230434:- | [20] | |
| Sequence | AAATTATTGCAAAACTAACCACTATGTACTTTTTTATAAAT | ||
| Motif Score | 2.475107143 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000397063.8; ENST00000397062.8; ENST00000446151.6; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501159 | ||
| mod ID: M6ASITE048455 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230440-177230441:- | [18] | |
| Sequence | TAAAAAGAAATTATTGCAAAACTAACCACTATGTACTTTTT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000458603.1; ENST00000397062.8; ENST00000397063.8; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501160 | ||
| mod ID: M6ASITE048456 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230469-177230470:- | [20] | |
| Sequence | TAGATTTATATGATGATATGACATCTGGCTAAAAAGAAATT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000446151.6; ENST00000397063.8; ENST00000458603.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501161 | ||
| mod ID: M6ASITE048457 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230527-177230528:- | [18] | |
| Sequence | TAAGTGTAAATACTACAAAAACTTATTTATACTGTTCTTAT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000397063.8; ENST00000458603.1; ENST00000446151.6; ENST00000397062.8; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501162 | ||
| mod ID: M6ASITE048458 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230533-177230534:- | [20] | |
| Sequence | TCTGTGTAAGTGTAAATACTACAAAAACTTATTTATACTGT | ||
| Motif Score | 2.078666667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000446151.6; ENST00000464747.5; ENST00000397063.8; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501163 | ||
| mod ID: M6ASITE048459 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230536-177230537:- | [20] | |
| Sequence | GTTTCTGTGTAAGTGTAAATACTACAAAAACTTATTTATAC | ||
| Motif Score | 2.53247619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000458603.1; ENST00000397062.8; ENST00000397063.8; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501164 | ||
| mod ID: M6ASITE048460 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230571-177230572:- | [20] | |
| Sequence | ACTGTAAACAATTTCTTAGGACACCATTTGGGCTAGTTTCT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000464747.5; ENST00000446151.6; ENST00000397062.8; ENST00000397063.8 | ||
| External Link | RMBase: m6A_site_501165 | ||
| mod ID: M6ASITE048461 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230584-177230585:- | [20] | |
| Sequence | TAGTTTCACTTTAACTGTAAACAATTTCTTAGGACACCATT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000397063.8; ENST00000464747.5; ENST00000446151.6; ENST00000458603.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501166 | ||
| mod ID: M6ASITE048462 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230591-177230592:- | [20] | |
| Sequence | GAATCAGTAGTTTCACTTTAACTGTAAACAATTTCTTAGGA | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000397063.8; ENST00000446151.6; ENST00000397062.8; ENST00000458603.1; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501167 | ||
| mod ID: M6ASITE048463 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230597-177230598:- | [20] | |
| Sequence | TATGTTGAATCAGTAGTTTCACTTTAACTGTAAACAATTTC | ||
| Motif Score | 2.469291667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000397063.8; ENST00000446151.6; ENST00000464747.5; ENST00000458603.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501168 | ||
| mod ID: M6ASITE048464 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230642-177230643:- | [19] | |
| Sequence | AGTATAGAAAATAATACGAAACTTTAAAAAGCATTGGAGTG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T; Huh7 | ||
| Seq Type List | m6A-seq; DART-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397062.8; ENST00000458603.1; ENST00000446151.6; ENST00000397063.8; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501169 | ||
| mod ID: M6ASITE048466 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230647-177230648:- | [20] | |
| Sequence | AAACTAGTATAGAAAATAATACGAAACTTTAAAAAGCATTG | ||
| Motif Score | 2.084416667 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000397062.8; ENST00000464747.5; ENST00000397063.8; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501170 | ||
| mod ID: M6ASITE048467 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230665-177230666:- | [19] | |
| Sequence | TATGCAAAATCATAGCCAAAACTAGTATAGAAAATAATACG | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397063.8; ENST00000446151.6; ENST00000458603.1; ENST00000464747.5; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501171 | ||
| mod ID: M6ASITE048468 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230701-177230702:- | [20] | |
| Sequence | CTGTGATGTGAAATGCTCATACTTTATAAGTAATTCTATGC | ||
| Motif Score | 2.53247619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000397062.8; ENST00000458603.1; ENST00000397063.8; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501172 | ||
| mod ID: M6ASITE048469 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230722-177230723:- | [20] | |
| Sequence | CTATTATACTAAAAGCTCCTACTGTGATGTGAAATGCTCAT | ||
| Motif Score | 2.500660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000397063.8; ENST00000464747.5; ENST00000397062.8; ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501173 | ||
| mod ID: M6ASITE048470 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230743-177230744:- | [20] | |
| Sequence | TTCTGAGCTAGTTTTTTTGTACTATTATACTAAAAGCTCCT | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000397062.8; ENST00000397063.8; ENST00000458603.1; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501174 | ||
| mod ID: M6ASITE048471 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230768-177230769:- | [20] | |
| Sequence | ACTAGATTTAGGAGGATTTGACCTTTTCTGAGCTAGTTTTT | ||
| Motif Score | 2.839113095 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000397062.8; ENST00000458603.1; ENST00000397063.8; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501175 | ||
| mod ID: M6ASITE048472 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230788-177230789:- | [19] | |
| Sequence | GAAGCCAGATGTTAAGAAAAACTAGATTTAGGAGGATTTGA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000458603.1; ENST00000397062.8; ENST00000446151.6; ENST00000397063.8 | ||
| External Link | RMBase: m6A_site_501176 | ||
| mod ID: M6ASITE048473 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230846-177230847:- | [19] | |
| Sequence | GTGAATACTCCCTGCAGCAAACAAGAGATGGCAATGTTTTC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HEK293T; HepG2; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq; DART-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000458603.1; ENST00000397062.8; ENST00000464747.5; ENST00000397063.8 | ||
| External Link | RMBase: m6A_site_501177 | ||
| mod ID: M6ASITE048474 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230860-177230861:- | [20] | |
| Sequence | ACCTTATTCTCCTAGTGAATACTCCCTGCAGCAAACAAGAG | ||
| Motif Score | 2.53247619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000397062.8; ENST00000464747.5; ENST00000458603.1; ENST00000446151.6; ENST00000397063.8 | ||
| External Link | RMBase: m6A_site_501178 | ||
| mod ID: M6ASITE048475 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230880-177230881:- | [19] | |
| Sequence | CTACGTGATGAAGATGGAAAACCTTATTCTCCTAGTGAATA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; LCLs; MM6; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000458603.1; ENST00000446151.6; ENST00000397063.8; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501179 | ||
| mod ID: M6ASITE048477 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230898-177230899:- | [20] | |
| Sequence | CTCGAAGTTTTCAGCATGCTACGTGATGAAGATGGAAAACC | ||
| Motif Score | 2.05260119 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000464747.5; ENST00000458603.1; ENST00000397062.8; ENST00000397063.8 | ||
| External Link | RMBase: m6A_site_501180 | ||
| mod ID: M6ASITE048478 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230937-177230938:- | [19] | |
| Sequence | AGCCTTCACCTACTGAAAAAACAACTCAGCACCTTATATCT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; hNPCs; fibroblasts; GM12878; LCLs; MM6; Huh7; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000397062.8; ENST00000397063.8; ENST00000458603.1; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501181 | ||
| mod ID: M6ASITE048479 | Click to Show/Hide the Full List | ||
| mod site | chr2:177230962-177230963:- | [20] | |
| Sequence | CAAAGAAAAAGGAGAAAATGACAAAAGCCTTCACCTACTGA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000458603.1; ENST00000397062.8; ENST00000397063.8; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501182 | ||
| mod ID: M6ASITE048480 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231030-177231031:- | [19] | |
| Sequence | AAACTGGAAAATATAGTAGAACTAGAGCAAGATTTAGATCA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; hNPCs; hESCs; fibroblasts; GM12878; LCLs; MM6; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000449627.1; ENST00000458603.1; ENST00000446151.6; ENST00000464747.5; ENST00000397063.8; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501183 | ||
| mod ID: M6ASITE048481 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231048-177231049:- | [19] | |
| Sequence | CAGAATTGCAGAAAAAGAAAACTGGAAAATATAGTAGAACT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; hNPCs; hESCs; fibroblasts; GM12878; LCLs; MM6; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000446151.6; ENST00000464747.5; ENST00000449627.1; ENST00000397063.8; ENST00000458603.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501184 | ||
| mod ID: M6ASITE048482 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231157-177231158:- | [20] | |
| Sequence | CCTCCCTGTTGTTGACTTCAACGAAATGATGTCCAAAGAGC | ||
| Motif Score | 2.147845238 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000397062.8; ENST00000464747.5; ENST00000449627.1; ENST00000397063.8; ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501185 | ||
| mod ID: M6ASITE048483 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231163-177231164:- | [20] | |
| Sequence | CATTAACCTCCCTGTTGTTGACTTCAACGAAATGATGTCCA | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000397062.8; ENST00000449627.1; ENST00000397063.8; ENST00000458603.1; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501186 | ||
| mod ID: M6ASITE048484 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231228-177231229:- | [19] | |
| Sequence | GCTCATCTCACAAGAGATGAACTTAGGGCAAAAGCTCTCCA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000446151.6; ENST00000458603.1; ENST00000397062.8; ENST00000397063.8; ENST00000464747.5; ENST00000449627.1 | ||
| External Link | RMBase: m6A_site_501187 | ||
| mod ID: M6ASITE048485 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231239-177231240:- | [21] | |
| Sequence | GCCGCTTGGAGGCTCATCTCACAAGAGATGAACTTAGGGCA | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | brain; liver | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000397062.8; ENST00000458603.1; ENST00000449627.1; ENST00000464747.5; ENST00000397063.8 | ||
| External Link | RMBase: m6A_site_501188 | ||
| mod ID: M6ASITE048486 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231267-177231268:- | [22] | |
| Sequence | ACCCCATTCACAAAAGACAAACATTCAAGCCGCTTGGAGGC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | CD8T; A549; AML | ||
| Seq Type List | m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000464747.5; ENST00000397063.8; ENST00000458603.1; ENST00000449627.1; ENST00000397062.8; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501189 | ||
| mod ID: M6ASITE048488 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231271-177231272:- | [19] | |
| Sequence | GAAAACCCCATTCACAAAAGACAAACATTCAAGCCGCTTGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000397063.8; ENST00000464747.5; ENST00000397062.8; ENST00000446151.6; ENST00000458603.1; ENST00000449627.1 | ||
| External Link | RMBase: m6A_site_501190 | ||
| mod ID: M6ASITE048489 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231278-177231279:- | [20] | |
| Sequence | GTCATCGGAAAACCCCATTCACAAAAGACAAACATTCAAGC | ||
| Motif Score | 2.047297619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000397062.8; ENST00000449627.1; ENST00000446151.6; ENST00000397063.8; ENST00000458603.1; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501191 | ||
| mod ID: M6ASITE048490 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231287-177231288:- | [19] | |
| Sequence | TAAGTCCTGGTCATCGGAAAACCCCATTCACAAAAGACAAA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; MM6; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000458603.1; ENST00000449627.1; ENST00000446151.6; ENST00000397063.8; ENST00000464747.5; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501192 | ||
| mod ID: M6ASITE048491 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231327-177231328:- | [20] | |
| Sequence | GATGCCCAATGTGAGAACACACCAGAGAAAGAATTGCCTGT | ||
| Motif Score | 2.064285714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000449627.1; ENST00000397063.8; ENST00000397062.8; ENST00000446151.6; ENST00000464747.5; ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501193 | ||
| mod ID: M6ASITE048492 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231331-177231332:- | [19] | |
| Sequence | GCATGATGCCCAATGTGAGAACACACCAGAGAAAGAATTGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; H1A; H1B; hNPCs; hESCs; fibroblasts; GM12878; H1299; MM6; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000458603.1; ENST00000449627.1; ENST00000446151.6; ENST00000397062.8; ENST00000397063.8 | ||
| External Link | RMBase: m6A_site_501194 | ||
| mod ID: M6ASITE048493 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231355-177231356:- | [20] | |
| Sequence | ATCTCAGGGGCAGAGCACTCACGTGCATGATGCCCAATGTG | ||
| Motif Score | 2.021232143 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000449627.1; ENST00000464747.5; ENST00000397063.8; ENST00000397062.8; ENST00000458603.1; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501195 | ||
| mod ID: M6ASITE048494 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231419-177231420:- | [19] | |
| Sequence | TCAAACAGAATGGTCCTAAAACACCAGTACATTCTTCTGGG | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397062.8; ENST00000464747.5; ENST00000458603.1; ENST00000449627.1; ENST00000397063.8; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501196 | ||
| mod ID: M6ASITE048495 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231435-177231436:- | [19] | |
| Sequence | AGTGCCCCTGGAAGTGTCAAACAGAATGGTCCTAAAACACC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; fibroblasts; GM12878; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397063.8; ENST00000458603.1; ENST00000446151.6; ENST00000449627.1; ENST00000397062.8; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501197 | ||
| mod ID: M6ASITE048496 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231492-177231493:- | [20] | |
| Sequence | TCCAGCTATGGAGACACACTACTTGGCCTCAGTGATTCTGA | ||
| Motif Score | 2.500660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000397062.8; ENST00000446151.6; ENST00000397063.8; ENST00000449627.1; ENST00000464747.5; ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501198 | ||
| mod ID: M6ASITE048497 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231499-177231500:- | [19] | |
| Sequence | GGAATCTTCCAGCTATGGAGACACACTACTTGGCCTCAGTG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; fibroblasts; GM12878; Huh7; Jurkat; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000458603.1; ENST00000397063.8; ENST00000464747.5; ENST00000449627.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501199 | ||
| mod ID: M6ASITE048500 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231528-177231529:- | [19] | |
| Sequence | CCCAGTGTGGCATCACCAGAACACTCAGTGGAATCTTCCAG | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; fibroblasts; GM12878; CD8T; Huh7; Jurkat; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000397062.8; ENST00000458603.1; ENST00000449627.1; ENST00000397063.8; ENST00000446151.6; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501200 | ||
| mod ID: M6ASITE048501 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231556-177231557:- | [19] | |
| Sequence | TGACTCCGGCATTTCACTAAACACAAGTCCCAGTGTGGCAT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; fibroblasts; GM12878; H1299; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; endometrial | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397062.8; ENST00000446151.6; ENST00000458603.1; ENST00000449627.1; ENST00000397063.8; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501201 | ||
| mod ID: M6ASITE048502 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231574-177231575:- | [20] | |
| Sequence | AGCAGAATTCAATGATTCTGACTCCGGCATTTCACTAAACA | ||
| Motif Score | 3.28175 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000458603.1; ENST00000397063.8; ENST00000464747.5; ENST00000449627.1; ENST00000446151.6; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501202 | ||
| mod ID: M6ASITE048503 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231596-177231597:- | [21] | |
| Sequence | ACCAAAACCACCCTGAAAGCACAGCAGAATTCAATGATTCT | ||
| Motif Score | 2.830589286 | ||
| Cell/Tissue List | brain | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000449627.1; ENST00000464747.5; ENST00000397063.8; ENST00000397062.8; ENST00000458603.1; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501203 | ||
| mod ID: M6ASITE048504 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231610-177231611:- | [19] | |
| Sequence | TTGCAAAGCTTTCAACCAAAACCACCCTGAAAGCACAGCAG | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; CD34; HepG2; HEK293T; A549; U2OS; hNPCs; hESCs; fibroblasts; GM12878; LCLs; H1299; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397062.8; ENST00000397063.8; ENST00000464747.5; ENST00000458603.1; ENST00000449627.1; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501204 | ||
| mod ID: M6ASITE048505 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231669-177231670:- | [19] | |
| Sequence | TTAAGCCATTCACTCTCTGAACTTCTAAATGGGCCCATTGA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; A549; HepG2; U2OS; hNPCs; hESCs; fibroblasts; GM12878; LCLs; CD8T; H1299; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000458603.1; ENST00000464747.5; ENST00000446151.6; ENST00000397063.8; ENST00000397062.8; ENST00000449627.1 | ||
| External Link | RMBase: m6A_site_501205 | ||
| mod ID: M6ASITE048506 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231692-177231693:- | [20] | |
| Sequence | ACAGCATGCCCTCACCTGCTACTTTAAGCCATTCACTCTCT | ||
| Motif Score | 2.500660714 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000458603.1; ENST00000397063.8; ENST00000464747.5; ENST00000449627.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501206 | ||
| mod ID: M6ASITE048507 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231712-177231713:- | [22] | |
| Sequence | AGCTGAGCCCAGTATCAGCAACAGCATGCCCTCACCTGCTA | ||
| Motif Score | 2.173910714 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000397062.8; ENST00000397063.8; ENST00000464747.5; ENST00000458603.1; ENST00000449627.1; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501207 | ||
| mod ID: M6ASITE048508 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231796-177231797:- | [19] | |
| Sequence | CCCCAACCAGTTGACAGTGAACTCATTAAATTCAGATGCCA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; HepG2; U2OS; hESCs; fibroblasts; A549; GM12878; LCLs; CD8T; H1299; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX; AML | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000446151.6; ENST00000464747.5; ENST00000448782.5; ENST00000397063.8; ENST00000449627.1; ENST00000458603.1; ENST00000430047.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501208 | ||
| mod ID: M6ASITE048509 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231817-177231818:- | [19] | |
| Sequence | CAGCATCCTCTCCACAGAAGACCCCAACCAGTTGACAGTGA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; CD34; HEK293T; HepG2; U2OS; hESCs; fibroblasts; A549; GM12878; LCLs; H1299; Huh7; HEK293A-TOA; iSLK; MSC; TIME; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000430047.1; ENST00000458603.1; ENST00000397062.8; ENST00000397063.8; ENST00000449627.1; ENST00000448782.5; ENST00000464747.5; ENST00000586532.5; ENST00000446151.6 | ||
| External Link | RMBase: m6A_site_501209 | ||
| mod ID: M6ASITE048511 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231880-177231881:- | [22] | |
| Sequence | AATGGAAAAAGAAGTAGGTAACTGTAGTCCACATTTTCTTA | ||
| Motif Score | 2.590089286 | ||
| Cell/Tissue List | A549 | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000421929.5; ENST00000458603.1; ENST00000464747.5; ENST00000586532.5; ENST00000397063.8; ENST00000397062.8; ENST00000448782.5; ENST00000446151.6; ENST00000449627.1; ENST00000430047.1 | ||
| External Link | RMBase: m6A_site_501210 | ||
| mod ID: M6ASITE048512 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231931-177231932:- | [20] | |
| Sequence | AGCCAAACTGACAGAAGTTGACAATTATCATTTTTACTCAT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000449627.1; ENST00000446151.6; ENST00000397063.8; ENST00000397062.8; ENST00000421929.5; ENST00000458603.1; ENST00000430047.1; ENST00000464747.5; ENST00000586532.5; ENST00000448782.5 | ||
| External Link | RMBase: m6A_site_501211 | ||
| mod ID: M6ASITE048513 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231941-177231942:- | [20] | |
| Sequence | CAAGTCCAGAAGCCAAACTGACAGAAGTTGACAATTATCAT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000397063.8; ENST00000430047.1; ENST00000421929.5; ENST00000446151.6; ENST00000397062.8; ENST00000458603.1; ENST00000464747.5; ENST00000449627.1; ENST00000448782.5; ENST00000586532.5 | ||
| External Link | RMBase: m6A_site_501212 | ||
| mod ID: M6ASITE048514 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231945-177231946:- | [19] | |
| Sequence | GTTCCAAGTCCAGAAGCCAAACTGACAGAAGTTGACAATTA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000448782.5; ENST00000586532.5; ENST00000464747.5; ENST00000449627.1; ENST00000397062.8; ENST00000397063.8; ENST00000430047.1; ENST00000421929.5; ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501213 | ||
| mod ID: M6ASITE048515 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231974-177231975:- | [19] | |
| Sequence | AAAATGACAAGCTGGTTGAGACTACCATGGTTCCAAGTCCA | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397063.8; ENST00000464747.5; ENST00000446151.6; ENST00000449627.1; ENST00000421929.5; ENST00000430047.1; ENST00000397062.8; ENST00000586532.5; ENST00000448782.5; ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501214 | ||
| mod ID: M6ASITE048516 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231988-177231989:- | [20] | |
| Sequence | GTGTCTTAATATTGAAAATGACAAGCTGGTTGAGACTACCA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000446151.6; ENST00000448782.5; ENST00000397063.8; ENST00000449627.1; ENST00000586532.5; ENST00000421929.5; ENST00000430047.1; ENST00000458603.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501215 | ||
| mod ID: M6ASITE048517 | Click to Show/Hide the Full List | ||
| mod site | chr2:177232452-177232453:- | [20] | |
| Sequence | GGTAGCCCCTGTTGATTTAGACGGTATGCAACAGGACATTG | ||
| Motif Score | 2.871321429 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000423513.5; ENST00000448782.5; ENST00000397062.8; ENST00000449627.1; ENST00000421929.5; ENST00000464747.5; ENST00000458603.1; ENST00000397063.8; ENST00000430047.1; ENST00000586532.5 | ||
| External Link | RMBase: m6A_site_501216 | ||
| mod ID: M6ASITE048518 | Click to Show/Hide the Full List | ||
| mod site | chr2:177232486-177232487:- | [19] | |
| Sequence | ATCAGGCTCAGTCACCTGAAACTTCTGTTGCTCAGGTAGCC | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000449627.1; ENST00000464747.5; ENST00000446151.6; ENST00000430047.1; ENST00000397063.8; ENST00000448782.5; ENST00000397062.8; ENST00000423513.5; ENST00000586532.5; ENST00000421929.5; ENST00000458603.1 | ||
| External Link | RMBase: m6A_site_501217 | ||
| mod ID: M6ASITE048519 | Click to Show/Hide the Full List | ||
| mod site | chr2:177232612-177232613:- | [19] | |
| Sequence | TCATCCCCTGTTGGTGGAAGACTCATAAATCAATGCCTTAT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000449627.1; ENST00000397062.8; ENST00000446151.6; ENST00000421929.5; ENST00000448782.5; ENST00000464747.5; ENST00000430047.1; ENST00000397063.8; ENST00000423513.5; ENST00000588123.1; ENST00000586532.5 | ||
| External Link | RMBase: m6A_site_501218 | ||
| mod ID: M6ASITE048520 | Click to Show/Hide the Full List | ||
| mod site | chr2:177233216-177233217:- | [19] | |
| Sequence | ATAACCTGGTTAATAGAAAAACTCCATCATAACTATAAAAT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000423513.5; ENST00000397063.8; ENST00000446151.6; ENST00000397062.8; ENST00000586532.5; ENST00000448782.5; ENST00000430047.1; ENST00000449627.1; ENST00000421929.5; ENST00000588123.1; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501219 | ||
| mod ID: M6ASITE048522 | Click to Show/Hide the Full List | ||
| mod site | chr2:177233275-177233276:- | [19] | |
| Sequence | GCATGCAGCTTTTGGCGCAGACATTCCCGTTTGTAGATGAC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000586532.5; ENST00000448782.5; ENST00000421929.5; ENST00000446151.6; ENST00000397063.8; ENST00000464747.5; ENST00000449627.1; ENST00000423513.5; ENST00000430047.1; ENST00000588123.1; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501220 | ||
| mod ID: M6ASITE048523 | Click to Show/Hide the Full List | ||
| mod site | chr2:177233307-177233308:- | [20] | |
| Sequence | TCCCAAATCAGATGCTTTGTACTTTGATGACTGCATGCAGC | ||
| Motif Score | 3.278136905 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000586532.5; ENST00000423513.5; ENST00000449627.1; ENST00000448782.5; ENST00000397063.8; ENST00000446151.6; ENST00000464747.5; ENST00000421929.5; ENST00000397062.8; ENST00000430047.1; ENST00000588123.1 | ||
| External Link | RMBase: m6A_site_501221 | ||
| mod ID: M6ASITE048524 | Click to Show/Hide the Full List | ||
| mod site | chr2:177233506-177233507:- | [19] | |
| Sequence | TTGGAGGAGCTAAGATCTGAACCCATGTGGTCTAGTTCAAA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000588123.1; ENST00000397063.8; ENST00000449627.1; ENST00000477534.1; ENST00000448782.5; ENST00000446151.6; ENST00000397062.8; ENST00000421929.5; ENST00000430047.1; ENST00000464747.5; ENST00000586532.5; ENST00000423513.5 | ||
| External Link | RMBase: m6A_site_501222 | ||
| mod ID: M6ASITE048525 | Click to Show/Hide the Full List | ||
| mod site | chr2:177234030-177234031:- | [19] | |
| Sequence | CCCAGCACATCCAGTCAGAAACCAGTGGATCTGCCAACTAC | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | HeLa; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000423513.5; ENST00000446151.6; ENST00000586532.5; ENST00000588123.1; ENST00000462023.1; ENST00000397062.8; ENST00000448782.5; ENST00000449627.1; ENST00000430047.1; ENST00000397063.8; ENST00000421929.5; ENST00000477534.1; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501223 | ||
| mod ID: M6ASITE048526 | Click to Show/Hide the Full List | ||
| mod site | chr2:177234078-177234079:- | [19] | |
| Sequence | AGTTACAACTAGATGAAGAGACAGGTGAATTTCTCCCAATT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; endometrial | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000449627.1; ENST00000446151.6; ENST00000421929.5; ENST00000423513.5; ENST00000464747.5; ENST00000448782.5; ENST00000430047.1; ENST00000397062.8; ENST00000462023.1; ENST00000397063.8; ENST00000586532.5; ENST00000477534.1; ENST00000588123.1 | ||
| External Link | RMBase: m6A_site_501224 | ||
| mod ID: M6ASITE048527 | Click to Show/Hide the Full List | ||
| mod site | chr2:177234094-177234095:- | [20] | |
| Sequence | AAAGCCTTTTTCGCTCAGTTACAACTAGATGAAGAGACAGG | ||
| Motif Score | 2.07285119 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | DART-seq | ||
| Transcript ID List | ENST00000448782.5; ENST00000588123.1; ENST00000446151.6; ENST00000423513.5; ENST00000397062.8; ENST00000397063.8; ENST00000462023.1; ENST00000430047.1; ENST00000464747.5; ENST00000449627.1; ENST00000421929.5; ENST00000586532.5; ENST00000477534.1 | ||
| External Link | RMBase: m6A_site_501225 | ||
| mod ID: M6ASITE048528 | Click to Show/Hide the Full List | ||
| mod site | chr2:177234136-177234137:- | [19] | |
| Sequence | CTTGAAAAGGAAAGACAAGAACAACTCCAAAAGGAGCAAGA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; endometrial; AML | ||
| Seq Type List | m6A-seq; miCLIP | ||
| Transcript ID List | ENST00000421929.5; ENST00000430047.1; ENST00000477534.1; ENST00000448782.5; ENST00000464747.5; ENST00000397063.8; ENST00000423513.5; ENST00000397062.8; ENST00000449627.1; ENST00000588123.1; ENST00000446151.6; ENST00000586532.5; ENST00000462023.1 | ||
| External Link | RMBase: m6A_site_501226 | ||
| mod ID: M6ASITE048529 | Click to Show/Hide the Full List | ||
| mod site | chr2:177234142-177234143:- | [19] | |
| Sequence | AAAAAACTTGAAAAGGAAAGACAAGAACAACTCCAAAAGGA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000462023.1; ENST00000464747.5; ENST00000449627.1; ENST00000477534.1; ENST00000446151.6; ENST00000586532.5; ENST00000421929.5; ENST00000430047.1; ENST00000397063.8; ENST00000397062.8; ENST00000588123.1; ENST00000423513.5; ENST00000448782.5 | ||
| External Link | RMBase: m6A_site_501227 | ||
| mod ID: M6ASITE048530 | Click to Show/Hide the Full List | ||
| mod site | chr2:177234157-177234158:- | [19] | |
| Sequence | GAGCTGGAAAAACAGAAAAAACTTGAAAAGGAAAGACAAGA | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; DART-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000477534.1; ENST00000448782.5; ENST00000397062.8; ENST00000421929.5; ENST00000449627.1; ENST00000588123.1; ENST00000423513.5; ENST00000446151.6; ENST00000462023.1; ENST00000430047.1; ENST00000397063.8; ENST00000586532.5 | ||
| External Link | RMBase: m6A_site_501228 | ||
| mod ID: M6ASITE048531 | Click to Show/Hide the Full List | ||
| mod site | chr2:177234166-177234167:- | [19] | |
| Sequence | AAAGAGTATGAGCTGGAAAAACAGAAAAAACTTGAAAAGGA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000462023.1; ENST00000477534.1; ENST00000448782.5; ENST00000397063.8; ENST00000430047.1; ENST00000588123.1; ENST00000421929.5; ENST00000586532.5; ENST00000423513.5; ENST00000446151.6; ENST00000449627.1; ENST00000464747.5; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501229 | ||
| mod ID: M6ASITE048533 | Click to Show/Hide the Full List | ||
| mod site | chr2:177263531-177263532:- | ||
| Sequence | CGCCGCGCCAGGGCCAAGGGACAGGTTGGAGCTGTTGATCT | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | HEK293T | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000588123.1; ENST00000464747.5; ENST00000446151.6; ENST00000586532.5; ENST00000397063.8; ENST00000448782.5; ENST00000462023.1; ENST00000430047.1; ENST00000397062.8; ENST00000477534.1; ENST00000421929.5 | ||
| External Link | RMBase: m6A_site_501230 | ||
| mod ID: M6ASITE048534 | Click to Show/Hide the Full List | ||
| mod site | chr2:177263555-177263556:- | [23] | |
| Sequence | TCCTGCTTTATAGCGTGCAAACCTCGCCGCGCCAGGGCCAA | ||
| Motif Score | 2.185083333 | ||
| Cell/Tissue List | MSC; iSLK; TIME; GSCs | ||
| Seq Type List | MeRIP-seq; m6A-seq | ||
| Transcript ID List | ENST00000446151.6; ENST00000397063.8; ENST00000588123.1; ENST00000421929.5; ENST00000462023.1; ENST00000464747.5; ENST00000448782.5; ENST00000430047.1; ENST00000586532.5; ENST00000397062.8 | ||
| External Link | RMBase: m6A_site_501231 | ||
| mod ID: M6ASITE048535 | Click to Show/Hide the Full List | ||
| mod site | chr2:177263712-177263713:- | [19] | |
| Sequence | AGTGCGGGCGAGGGCAGTGGACTCTGAGGCCGGAGTCGGCG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; A549; H1A; Jurkat; GSC-11; HEK293T; HEK293A-TOA; MSC; TREX; iSLK; TIME; HEC-1-A; GSCs | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000462023.1; ENST00000588123.1; ENST00000586532.5; ENST00000464747.5; ENST00000430047.1; ENST00000421929.5; ENST00000397062.8; ENST00000397063.8 | ||
| External Link | RMBase: m6A_site_501232 | ||
| mod ID: M6ASITE048536 | Click to Show/Hide the Full List | ||
| mod site | chr2:177264546-177264547:- | [19] | |
| Sequence | TTGGAGCTGCCGCCGCCGGGACTCCCGTCCCAGCAGGTGCT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; A549; GSC-11; HEK293T; HEK293A-TOA; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000586532.5; ENST00000397062.8; ENST00000588123.1; ENST00000462023.1; ENST00000430047.1; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501233 | ||
| mod ID: M6ASITE048537 | Click to Show/Hide the Full List | ||
| mod site | chr2:177264568-177264569:- | [19] | |
| Sequence | TCCACAGCTCATCATGATGGACTTGGAGCTGCCGCCGCCGG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; A549; GSC-11; HEK293T; HEK293A-TOA; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000430047.1; ENST00000586532.5; ENST00000464747.5; ENST00000588123.1; ENST00000397062.8; ENST00000462023.1 | ||
| External Link | RMBase: m6A_site_501234 | ||
| mod ID: M6ASITE048538 | Click to Show/Hide the Full List | ||
| mod site | chr2:177264621-177264622:- | [19] | |
| Sequence | GCCGCGCCTCGGCAGCCGGAACAGGGCCGCCGTCGGGGAGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; A549; U2OS; CD4T; GSC-11; HEK293A-TOA; MSC; TREX; TIME | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000588123.1; ENST00000586532.5; ENST00000462023.1; ENST00000397062.8; ENST00000464747.5; ENST00000430047.1 | ||
| External Link | RMBase: m6A_site_501236 | ||
| mod ID: M6ASITE048539 | Click to Show/Hide the Full List | ||
| mod site | chr2:177264789-177264790:- | [19] | |
| Sequence | CGGGGGAAGGAAGGGCCCGGACTCTTGCCCCGCCCTTGTGG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2; A549; GSC-11; HEK293A-TOA | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000588123.1; ENST00000586532.5; ENST00000464747.5; ENST00000430047.1 | ||
| External Link | RMBase: m6A_site_501238 | ||
| mod ID: M6ASITE048541 | Click to Show/Hide the Full List | ||
| mod site | chr2:177283625-177283626:- | [19] | |
| Sequence | GGGAAGAAAAATATGAAAAGACCTAAAGATGCACGTCCTCA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397057.6; ENST00000586532.5; ENST00000456746.5; ENST00000464747.5; ENST00000588123.1; ENST00000430416.5 | ||
| External Link | RMBase: m6A_site_501241 | ||
| mod ID: M6ASITE048542 | Click to Show/Hide the Full List | ||
| mod site | chr2:177283654-177283655:- | [19] | |
| Sequence | GAGAAAAGACCAAACATAGGACTGTTACAGGGAAGAAAAAT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000430416.5; ENST00000464747.5; ENST00000397057.6; ENST00000586532.5; ENST00000588123.1; ENST00000456746.5 | ||
| External Link | RMBase: m6A_site_501242 | ||
| mod ID: M6ASITE048543 | Click to Show/Hide the Full List | ||
| mod site | chr2:177283661-177283662:- | [19] | |
| Sequence | ACCTGACGAGAAAAGACCAAACATAGGACTGTTACAGGGAA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000397057.6; ENST00000586532.5; ENST00000430416.5; ENST00000464747.5; ENST00000456746.5; ENST00000588123.1 | ||
| External Link | RMBase: m6A_site_501243 | ||
| mod ID: M6ASITE048544 | Click to Show/Hide the Full List | ||
| mod site | chr2:177283666-177283667:- | [19] | |
| Sequence | AATGAACCTGACGAGAAAAGACCAAACATAGGACTGTTACA | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | HeLa; HepG2 | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000586532.5; ENST00000430416.5; ENST00000456746.5; ENST00000397057.6; ENST00000464747.5; ENST00000588123.1 | ||
| External Link | RMBase: m6A_site_501244 | ||
| mod ID: M6ASITE048545 | Click to Show/Hide the Full List | ||
| mod site | chr2:177283681-177283682:- | [19] | |
| Sequence | TGTTAGTGTCAAGGGAATGAACCTGACGAGAAAAGACCAAA | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000586532.5; ENST00000464747.5; ENST00000588123.1; ENST00000397057.6; ENST00000456746.5; ENST00000430416.5 | ||
| External Link | RMBase: m6A_site_501245 | ||
| mod ID: M6ASITE048546 | Click to Show/Hide the Full List | ||
| mod site | chr2:177286884-177286885:- | [21] | |
| Sequence | GAGTCCTGCTGTATTTTGTGACATCCACATGCAGCATCCCA | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | kidney | ||
| Seq Type List | m6A-REF-seq | ||
| Transcript ID List | ENST00000430416.5; ENST00000464747.5; ENST00000586532.5; ENST00000456746.5; ENST00000588123.1; ENST00000397057.6 | ||
| External Link | RMBase: m6A_site_501246 | ||
| mod ID: M6ASITE048547 | Click to Show/Hide the Full List | ||
| mod site | chr2:177287451-177287452:- | [23] | |
| Sequence | CCACTTCTTTATGCCTCAAGACATCTCATATGTATTTATAT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | iSLK; MSC; TIME | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000430416.5; ENST00000456746.5; ENST00000588123.1; ENST00000627798.2; ENST00000586532.5; ENST00000464747.5; ENST00000397057.6; ENST00000625449.2 | ||
| External Link | RMBase: m6A_site_501247 | ||
| mod ID: M6ASITE048548 | Click to Show/Hide the Full List | ||
| mod site | chr2:177287515-177287516:- | [23] | |
| Sequence | CCAAGAATAGCACAAATTGAACTTTTACATTCTTCATAATA | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | iSLK; MSC; TIME; TREX | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000430416.5; ENST00000588123.1; ENST00000464747.5; ENST00000627798.2; ENST00000625449.2; ENST00000456746.5; ENST00000586532.5; ENST00000397057.6 | ||
| External Link | RMBase: m6A_site_501248 | ||
| mod ID: M6ASITE048549 | Click to Show/Hide the Full List | ||
| mod site | chr2:177287575-177287576:- | [23] | |
| Sequence | ACTGAGCAAGCCAGACACAGACTTGAATGTCATCACAATCT | ||
| Motif Score | 3.319380952 | ||
| Cell/Tissue List | iSLK; MSC; TIME; TREX | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000397057.6; ENST00000588123.1; ENST00000430416.5; ENST00000625449.2; ENST00000586532.5; ENST00000456746.5; ENST00000464747.5; ENST00000627798.2 | ||
| External Link | RMBase: m6A_site_501249 | ||
| mod ID: M6ASITE048551 | Click to Show/Hide the Full List | ||
| mod site | chr2:177287581-177287582:- | [23] | |
| Sequence | GCTTCCACTGAGCAAGCCAGACACAGACTTGAATGTCATCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | iSLK; MSC; TIME; TREX | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000627798.2; ENST00000397057.6; ENST00000588123.1; ENST00000586532.5; ENST00000625449.2; ENST00000456746.5; ENST00000430416.5 | ||
| External Link | RMBase: m6A_site_501250 | ||
| mod ID: M6ASITE048552 | Click to Show/Hide the Full List | ||
| mod site | chr2:177310878-177310879:- | [19] | |
| Sequence | ATAAAAACAGACTAATACAAACAGGTAGCTCTAAATTGTGA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000443132.1; ENST00000430416.5; ENST00000456746.5; ENST00000627798.2; ENST00000464747.5; ENST00000625449.2; ENST00000586532.5; ENST00000397057.6 | ||
| External Link | RMBase: m6A_site_501251 | ||
| mod ID: M6ASITE048553 | Click to Show/Hide the Full List | ||
| mod site | chr2:177310892-177310893:- | [19] | |
| Sequence | ATCTTTATAGCGGTATAAAAACAGACTAATACAAACAGGTA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000443132.1; ENST00000627798.2; ENST00000397057.6; ENST00000456746.5; ENST00000586532.5; rmsk_751950; ENST00000464747.5; ENST00000430416.5; ENST00000625449.2 | ||
| External Link | RMBase: m6A_site_501252 | ||
| mod ID: M6ASITE048554 | Click to Show/Hide the Full List | ||
| mod site | chr2:177310965-177310966:- | [19] | |
| Sequence | GGCCTCCCCAGACATGTGGAACTGTGAGTCAATTAAATCTC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | HeLa; HepG2; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397057.6; ENST00000586532.5; ENST00000443132.1; ENST00000627798.2; rmsk_751950; ENST00000456746.5; ENST00000625449.2; ENST00000430416.5; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501253 | ||
| mod ID: M6ASITE048555 | Click to Show/Hide the Full List | ||
| mod site | chr2:177310974-177310975:- | [19] | |
| Sequence | GTTTCCTGAGGCCTCCCCAGACATGTGGAACTGTGAGTCAA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000443132.1; ENST00000464747.5; ENST00000627798.2; ENST00000625449.2; ENST00000586532.5; ENST00000430416.5; rmsk_751950; ENST00000456746.5; ENST00000397057.6 | ||
| External Link | RMBase: m6A_site_501254 | ||
| mod ID: M6ASITE048556 | Click to Show/Hide the Full List | ||
| mod site | chr2:177311287-177311288:- | [19] | |
| Sequence | GTTGCCCAGGCTGGTCTCAAACTCCTGAGCTCAGGCCATCT | ||
| Motif Score | 2.627720238 | ||
| Cell/Tissue List | HeLa; TREX | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000430416.5; ENST00000443132.1; ENST00000464747.5; ENST00000397057.6; ENST00000456746.5; ENST00000627798.2; ENST00000586532.5; ENST00000625449.2 | ||
| External Link | RMBase: m6A_site_501255 | ||
| mod ID: M6ASITE048557 | Click to Show/Hide the Full List | ||
| mod site | chr2:177321933-177321934:- | [23] | |
| Sequence | TATAACTTTTATATCATTAGACACATGGAATATGGGCCTCC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | TREX | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000447413.1; rmsk_751975; ENST00000456746.5; ENST00000464747.5; ENST00000397057.6; ENST00000430416.5 | ||
| External Link | RMBase: m6A_site_501256 | ||
| mod ID: M6ASITE048558 | Click to Show/Hide the Full List | ||
| mod site | chr2:177323154-177323155:- | [19] | |
| Sequence | GTTCATAAGGAGGAGCACAAACAGAAAGTTCAGTGGCCCCA | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa; HepG2; HEK293T; H1A; H1B; hNPCs; MM6; Huh7; TIME; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000456746.5; ENST00000430416.5; ENST00000397057.6; ENST00000447413.1; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501257 | ||
| mod ID: M6ASITE048559 | Click to Show/Hide the Full List | ||
| mod site | chr2:177323197-177323198:- | [19] | |
| Sequence | GGGCCCTTGGCACTGTGAGAACAGTGGAGGAGGAGGGAGCA | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | HeLa; HEK293T; H1A; H1B; hNPCs; MM6; Huh7; peripheral-blood; HEK293A-TOA; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000397057.6; ENST00000464747.5; ENST00000447413.1; ENST00000456746.5 | ||
| External Link | RMBase: m6A_site_501258 | ||
| mod ID: M6ASITE048560 | Click to Show/Hide the Full List | ||
| mod site | chr2:177323223-177323224:- | [19] | |
| Sequence | AGGAAGGCAAGCAAGCGAGGACTGATGGGCCCTTGGCACTG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HEK293T; H1A; H1B; hNPCs; MM6; Huh7; peripheral-blood; HEK293A-TOA; TREX; HEC-1-A | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000447413.1; ENST00000464747.5; ENST00000456746.5; ENST00000397057.6 | ||
| External Link | RMBase: m6A_site_501259 | ||
| mod ID: M6ASITE048562 | Click to Show/Hide the Full List | ||
| mod site | chr2:177323307-177323308:- | [19] | |
| Sequence | AAGACATGAATGGATGAAGAACCAAGTGCGTGAGGGCACCT | ||
| Motif Score | 2.930744048 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000447413.1; ENST00000464747.5; ENST00000456746.5; ENST00000397057.6 | ||
| External Link | RMBase: m6A_site_501260 | ||
| mod ID: M6ASITE048563 | Click to Show/Hide the Full List | ||
| mod site | chr2:177323324-177323325:- | [19] | |
| Sequence | GAAGGATGGAGATTTGGAAGACATGAATGGATGAAGAACCA | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000456746.5; ENST00000447413.1; ENST00000397057.6; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501261 | ||
| mod ID: M6ASITE048564 | Click to Show/Hide the Full List | ||
| mod site | chr2:177323352-177323353:- | [19] | |
| Sequence | AAACAGAAAGTACATGATGGACTTCATTGAAGGATGGAGAT | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | HeLa; HEK293T | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000464747.5; ENST00000397057.6; ENST00000447413.1; ENST00000456746.5 | ||
| External Link | RMBase: m6A_site_501262 | ||
| mod ID: M6ASITE048565 | Click to Show/Hide the Full List | ||
| mod site | chr2:177323370-177323371:- | [19] | |
| Sequence | TCTTTTGCTTGGGAGCAGAAACAGAAAGTACATGATGGACT | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | HeLa | ||
| Seq Type List | m6A-seq | ||
| Transcript ID List | ENST00000447413.1; ENST00000456746.5; ENST00000397057.6; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501263 | ||
| mod ID: M6ASITE048566 | Click to Show/Hide the Full List | ||
| mod site | chr2:177342058-177342059:- | [18] | |
| Sequence | GGAGGAACAGATTTGAGAAGACACTATTCTTACTGAGTATC | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000456746.5; ENST00000611493.1; ENST00000447413.1; ENST00000464747.5; ENST00000397057.6 | ||
| External Link | RMBase: m6A_site_501264 | ||
| mod ID: M6ASITE048567 | Click to Show/Hide the Full List | ||
| mod site | chr2:177342072-177342073:- | [18] | |
| Sequence | ACACAAGAAAAGGAGGAGGAACAGATTTGAGAAGACACTAT | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000611493.1; ENST00000397057.6; ENST00000456746.5; ENST00000447413.1; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501265 | ||
| mod ID: M6ASITE048568 | Click to Show/Hide the Full List | ||
| mod site | chr2:177342118-177342119:- | [18] | |
| Sequence | AAATGAGGAAATATAGAAGGACTATGACTAGATGGGGATGC | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000456746.5; ENST00000611493.1; ENST00000447413.1; ENST00000397057.6; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501266 | ||
| mod ID: M6ASITE048569 | Click to Show/Hide the Full List | ||
| mod site | chr2:177342151-177342152:- | [18] | |
| Sequence | ACTATTGAATGACCACTTGGACACAGGGGATGAAAATGAGG | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | Huh7 | ||
| Seq Type List | MeRIP-seq | ||
| Transcript ID List | ENST00000611493.1; ENST00000447413.1; ENST00000397057.6; ENST00000464747.5; ENST00000456746.5 | ||
| External Link | RMBase: m6A_site_501267 | ||
| mod ID: M6ASITE048570 | Click to Show/Hide the Full List | ||
| mod site | chr2:177342182-177342183:- | [24] | |
| Sequence | ATTCAAGAGACATTTGAAAGACAGAATTGGTACTATTGAAT | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | GM12878; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000447413.1; ENST00000456746.5; ENST00000611493.1; ENST00000397057.6; ENST00000464747.5 | ||
| External Link | RMBase: m6A_site_501268 | ||
| mod ID: M6ASITE048571 | Click to Show/Hide the Full List | ||
| mod site | chr2:177342193-177342194:- | [24] | |
| Sequence | GAAATGGAAGGATTCAAGAGACATTTGAAAGACAGAATTGG | ||
| Motif Score | 2.897386905 | ||
| Cell/Tissue List | GM12878; Huh7 | ||
| Seq Type List | m6A-seq; MeRIP-seq | ||
| Transcript ID List | ENST00000447413.1; ENST00000456746.5; ENST00000464747.5; ENST00000611493.1; ENST00000397057.6 | ||
| External Link | RMBase: m6A_site_501269 | ||
2'-O-Methylation (2'-O-Me)
| In total 1 m6A sequence/site(s) in this target gene | |||
| mod ID: 2OMSITE000236 | Click to Show/Hide the Full List | ||
| mod site | chr2:177323317-177323318:- | [25] | |
| Sequence | GGAGATTTGGAAGACATGAATGGATGAAGAACCAAGTGCGT | ||
| Cell/Tissue List | HEK293 | ||
| Seq Type List | RiboMeth-seq | ||
| Transcript ID List | ENST00000456746.5; ENST00000464747.5; ENST00000447413.1; ENST00000397057.6 | ||
| External Link | RMBase: Nm_site_3273 | ||
Pseudouridine (Pseudo)
| In total 2 m6A sequence/site(s) in this target gene | |||
| mod ID: PSESITE000144 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231720-177231721:- | [26] | |
| Sequence | GCTTTCATAGCTGAGCCCAGTATCAGCAACAGCATGCCCTC | ||
| Transcript ID List | ENST00000397063.8; ENST00000449627.1; ENST00000464747.5; ENST00000458603.1; ENST00000397062.8; ENST00000446151.6; ENST00000448782.5 | ||
| External Link | RMBase: Pseudo_site_2761 | ||
| mod ID: PSESITE000145 | Click to Show/Hide the Full List | ||
| mod site | chr2:177231927-177231928:- | [27] | |
| Sequence | AAACTGACAGAAGTTGACAATTATCATTTTTACTCATCTAT | ||
| Transcript ID List | ENST00000449627.1; ENST00000421929.5; ENST00000586532.5; ENST00000397063.8; ENST00000446151.6; ENST00000397062.8; ENST00000430047.1; ENST00000458603.1; ENST00000464747.5; ENST00000448782.5 | ||
| External Link | RMBase: Pseudo_site_2762 | ||
References