m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05947
|
[1] | |||
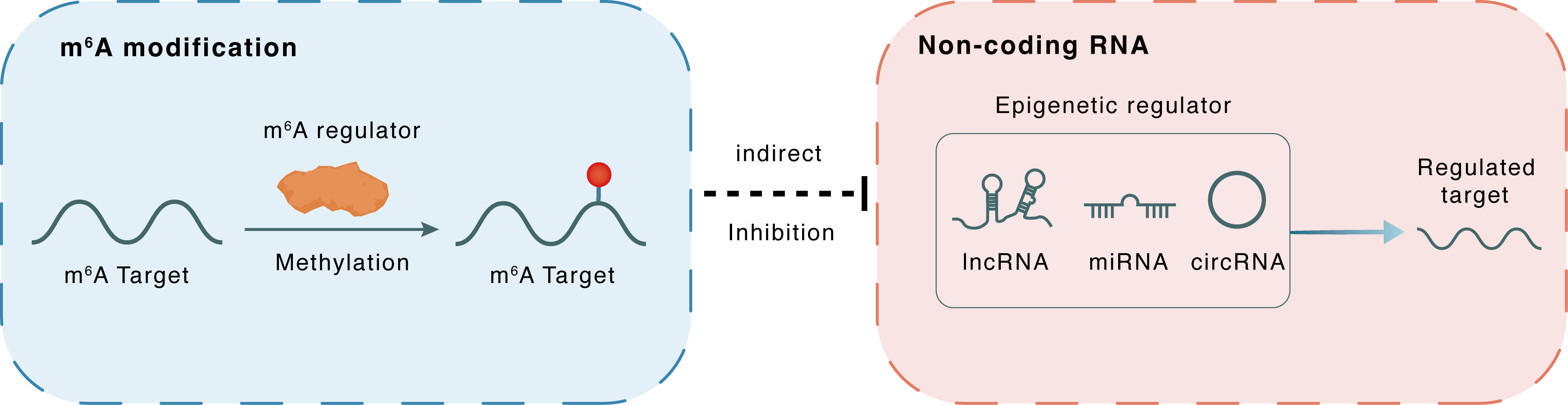 m6A modification
FAM225A
FAM225A
METTL3
Methylation
m6A modification
FAM225A
FAM225A
METTL3
Methylation
 : m6A sites
Indirect
Inhibition
Non-coding RNA
miR-1275
ITGB3
lncRNA miRNA circRNA : m6A sites
Indirect
Inhibition
Non-coding RNA
miR-1275
ITGB3
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Family with sequence similarity 225 member A (FAM225A) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | MicroRNA 1275 (MIR1275) | LncRNA | View Details | ||
| Regulated Target | Integrin subunit beta 3 (ITGB3) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Inhibition | |||
| Crosstalk Mechanism | m6A regulators indirectly modulate the functionality of ncRNAs through downstream signaling pathways | ||||
| Crosstalk Summary | Silencing METTL3 decreases Family with sequence similarity 225 member A (FAM225A) RNA stability, which serves as the ceRNA for sponging both miR-590-3p and MIR1275, increasing the levels of their target integrin beta3 (Integrin subunit beta 3 (ITGB3)), finally stimulating FAK/PI3K/Akt signaling. miR-590-3p has been reported as a tumor suppressor in cholangiocarcinoma and hepatocellular carcinoma and miR-1275 can inhibit NPC cell growth and suppress hepatocellular carcinoma cell proliferation. | ||||
| Responsed Disease | Nasopharyngeal carcinoma | ICD-11: 2B6B | |||
| Pathway Response | PI3K-Akt signaling pathway | hsa04151 | |||
| Cell Process | mRNA stability | ||||
| Cell proliferation | |||||
| Cell invasion | |||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Integrin subunit beta 3 (ITGB3) | 77 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| XEMILOFIBAN | Discontinued in Phase 3 | [2] | ||
| Synonyms |
Xemilofiban [INN]; 149820-74-6; UNII-P614JI3IYK; SC-54684A; SC 54684A; P614JI3IYK; SC-54684; CHEMBL76098; Ethyl-3S-((4-((4-(aminoiminomethyl)phenyl)amino)-1,4-dioxobutyl)amino)-4-pentynoate; C18H22N4O4; 4-Pentynoic acid, 3-((4-((4-(aminoiminomethyl)phenyl)amino)-1,4-dioxobutyl)amino)-, ethyl ester, (S)-; CS-551; AC1Q5MFX; AC1L1U8F; SCHEMBL50663; DTXSID60164410; ZINC1535967; BDBM50083460; KB-81502; LS-102305; ethyl(3s)-3-[3-[(p-amidinophenyl)carbamoyl]propionamido]-4-pentynoate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DMP-802 | Discontinued in Phase 1 | [3] | ||
| Synonyms |
CHEMBL117989; CHEMBL275611; SCHEMBL7748060; BDBM50075566; (S)-3-{2-[(R)-3-(4-Carbamimidoyl-phenyl)-4,5-dihydro-isoxazol-5-yl]-acetylamino}-2-(3,5-dimethyl-isoxazole-4-sulfonylamino)-propionic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-709780 | Terminated | [4] | ||
| Synonyms |
CHEMBL32960; BDBM50078448; l709780; N-[2-[2-(4-Piperidinyl)ethyl]-3-oxoisoindoline-5-ylcarbonyl]-beta-alanine; 3-{[3-Oxo-2-(2-piperidin-4-yl-ethyl)-2,3-dihydro-1H-isoindole-5-carbonyl]-amino}-propionic acid(L-709780); 3-{[3-Oxo-2-(2-piperidin-4-yl-ethyl)-2,3-dihydro-1H-isoindole-5-carbonyl]-amino}-propionic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro-43-8857 | Terminated | [3] | ||
| Synonyms |
CHEMBL290497; SCHEMBL8381416; BDBM50003852; alpha,alpha'-[[4-[[Methyl(4-amidinobenzoyl)amino]acetyl]-o-phenylene]bis(oxy)]diacetic acid; (4-{2-[(4-Carbamimidoyl-benzoyl)-methyl-amino]-acetyl}-2-carboxymethoxy-phenoxy)-acetic acid; (5-{2-[(4-Carbamimidoyl-benzoyl)-methyl-amino]-acetyl}-2-carboxymethoxy-phenoxy)-acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SKF-107260 | Terminated | [5] | ||
| Synonyms |
F 107260; CHEMBL18734; [(7s,13s)-13-{3-[(diaminomethylidene)amino]propyl}-14-methyl-6,9,12,15-tetraoxo-6,7,8,9,10,11,12,13,14,15-decahydro-5h-dibenzo[c,p][1,2,5,8,11,14]dithiatetraazacycloheptadecin-7-yl]acetic acid; 136620-00-3; Skf 107260; F-107260; AC1L4UPG; AC1Q6GV5; Cyclo-S,S-(mba-(N(alpha)-Me)arg-gly-asp-man); CTK4C0386; DTXSID70159857; BDBM50036088; BDBM50230131; Cyclo-S,S-(2-mercaptobenzoyl-N(alpha)-methylarginyl-glycyl-aspartyl-2-mercaptophenylamide)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-738167 | Terminated | [3] | ||
| Synonyms |
CHEMBL298655; L 738167; 163212-43-9; AC1L4BHI; SCHEMBL609722; DTXSID30167534; YLFFZEQHDMFOEC-NRFANRHFSA-N; BDBM50058239; L-Alanine, N-((4-methylphenyl)sulfonyl)-3-(((5,6,7,8-tetrahydro-4-oxo-5-(2-(4-piperidinyl)ethyl)-4H-pyrazolo(1,5-a)(1,4)diazepin-2-yl)carbonyl)amino)-; 3-{[4-Oxo-5-(2-piperidin-4-yl-ethyl)-5,6,7,8-tetrahydro-4H-1,5,8a-triaza-azulene-2-carbonyl]-amino}-2-(toluene-4-sulfonylamino)-propionic acid (L-738167)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DMP-757 | Terminated | [6] | ||
| Synonyms |
CHEMBL65617; BDBM50285199; [(5S,11S,14S)-11-(3-Guanidino-propyl)-14-isopropyl-12-methyl-4,7,10,13,16-pentaoxo-3,6,9,12,15-pentaaza-bicyclo[15.3.1]henicosa-1(20),17(21),18-trien-5-yl]-acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro-43-5054 | Terminated | [3] | ||
| Synonyms |
CHEMBL117775; SCHEMBL7306316; BDBM50092124; 2-{2-[3-(4-Carbamimidoyl-benzoylamino)-propionylamino]-3-carboxy-propionylamino}-3-methyl-butyric acid (Ro 43-5054)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SC-47643 | Terminated | [7] | ||
| Synonyms |
CHEMBL129921; BDBM50035970; 3-(8-Guanidino-octanoylamino)-N-[2-(4-methoxy-phenyl)-ethyl]-succinamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-223245 | Terminated | [8] | ||
| Synonyms |
CHEMBL50106; SCHEMBL245523; BDBM50059133; {7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid; {(S)-7-[(1H-Benzoimidazol-2-ylmethyl)-methyl-carbamoyl]-4-methyl-3-oxo-2,3,4,5-tetrahydro-1H-benzo[e][1,4]diazepin-2-yl}-acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-739758 | Investigative | [3] | ||
| Synonyms |
CHEMBL78760; BDBM50078437; 3-{[5-(2-Piperidin-4-yl-ethyl)-thieno[2,3-b]thiophene-2-carbonyl]-amino}-2-(pyridine-3-sulfonylamino)-propionic acid(L-739758); (S)-3-{[5-(2-Piperidin-4-yl-ethyl)-thieno[2,3-b]thiophene-2-carbonyl]-amino}-2-(pyridine-3-sulfonylamino)-propionic acid; (2S)-2-[(3-Pyridinyl)sulfonylamino]-3-[[5-[2-(4-piperidinyl)ethyl]thieno[2,3-b]thiophen-2-yl]carbonylamino]propanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ISIS 25237 | Investigative | [9] | ||
| External Link | ||||
| ROXIFIBAN | Investigative | [3] | ||
| Synonyms |
UNII-Q476FMZ72G; DMP754; 170902-47-3; CHEMBL18301; Q476FMZ72G; DMP-754; Roxifiban [INN]; AC1L42MF; SCHEMBL344302; DTXSID70168969; BDBM50075579; methyl (2S)-2-(butoxycarbonylamino)-3-[[2-[(5R)-3-(4-carbamimidoylphenyl)-4,5-dihydro-1,2-oxazol-5-yl]acetyl]amino]propanoate; L-Alanine, 3-(((3-(4-(aminoiminomethyl)phenyl)-4,5-dihydro-5-isoxazolyl)acetyl)amino)-N-(butoxycarbonyl)-, methyl ester, (R)-; L-Alanine, 3-((((5R)-3-(4-(aminoiminomethyl)phenyl)-4,5-dihydro-5-isoxazolyl)acetyl)amino)-N-(butoxycarbonyl)-, methy
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RWJ-53419 | Investigative | [10] | ||
| Synonyms |
CHEMBL421547; BDBM50104598; 2-Benzyloxycarbonylamino-3-{[(R)-1-(3-piperidin-4-yl-propionyl)-piperidine-3-carbonyl]-amino}-propionic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-750034 | Investigative | [11] | ||
| Synonyms |
CHEMBL326492; BDBM50076058; 2-((S)-Benzenesulfonylamino)-3-(4-piperazin-1-yl-benzoylamino)-propionic acid; 2-Benzenesulfonylamino-3-(4-piperazin-1-yl-benzoylamino)-propionic acid(L-750034); (S)-2-Benzenesulfonylamino-3-(4-piperazin-1-yl-benzoylamino)-propionic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-756568 | Investigative | [3] | ||
| Synonyms |
CHEMBL96097; BDBM50092097; (S)-2-Benzenesulfonylamino-3-{[5-(piperidin-4-ylmethoxy)-1H-indole-2-carbonyl]-amino}-propionic acid; 2-Benzenesulfonylamino-3-{[5-(piperidin-4-ylmethoxy)-1H-indole-2-carbonyl]-amino}-propionic acid(L-756568)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SC-54701A | Investigative | [12] | ||
| Synonyms |
UNII-TN585Y92BI; SC-54701; CHEMBL311572; TN585Y92BI; Xemilofiban acid; Xemilofiban acid [MI]; SCHEMBL7080314; BDBM50031565; 4-Pentynoic acid, 3-((4-((4-(aminoiminomethyl)phenyl)amino)-1,4-dioxobutyl)amino)-, (3S)-; 149193-61-3; (S)-3-[3-(4-Carbamimidoyl-phenylcarbamoyl)-propionylamino]-pent-4-ynoic acid; 3-[3-(4-Carbamimidoyl-phenylcarbamoyl)-propionylamino]-pent-4-ynoic acid ethyl ester; 3-[3-(4-Carbamimidoyl-phenylcarbamoyl)-propionylamino]-pent-4-ynoic acid (SC-54701A)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-734115 | Investigative | [3] | ||
| Synonyms |
CHEMBL40502; BDBM50058236; N-(Butylsulfonyl)-3-[[4,5,6,7-tetrahydro-4-oxo-5-[2-(4-piperidinyl)ethyl]pyrazolo[1,5-a]pyrazin]-2-ylcarbonylamino]-L-alanine; (S)-2-(Butane-1-sulfonylamino)-3-{[4-oxo-5-(2-piperidin-4-yl-ethyl)-4,5,6,7-tetrahydro-pyrazolo[1,5-a]pyrazine-2-carbonyl]-amino}-propionic acid; 2-(Butane-1-sulfonylamino)-3-{[4-oxo-5-(2-piperidin-4-yl-ethyl)-4,5,6,7-tetrahydro-pyrazolo[1,5-a]pyrazine-2-carbonyl]-amino}-propionic acid(L-734115)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-207043 | Investigative | [5] | ||
| Synonyms |
CHEMBL606910
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ISIS 196103 | Investigative | [9] | ||
| External Link | ||||
| L-746233 | Investigative | [3] | ||
| Synonyms |
CHEMBL79294; BDBM50078442; 3-{[3-Oxo-2-(2-piperidin-4-yl-ethyl)-2,3-dihydro-1H-isoindole-5-carbonyl]-amino}-2-(pyridine-3-sulfonylamino)-propionic acid(L-746233); (2S)-2-[(3-Pyridinyl)sulfonylamino]-3-[[2-[2-(4-piperidinyl)ethyl]-3-oxoisoindolin-5-yl]carbonylamino]propanoic acid; (S)-3-{[3-Oxo-2-(2-piperidin-4-yl-ethyl)-2,3-dihydro-1H-isoindole-5-carbonyl]-amino}-2-(pyridine-3-sulfonylamino)-propionic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| L-767679 | Investigative | [3] | ||
| Synonyms |
CHEMBL57886; L 767679; AC1L42P7; BDBM50054538; 182198-53-4; 3-[2-(1-Oxo-7-piperazin-1-yl-3,4-dihydro-1H-isoquinolin-2-yl)-acetylamino]-pent-4-ynoic acid(L-767679); (S)-3-[2-(1-Oxo-7-piperazin-1-yl-3,4-dihydro-1H-isoquinolin-2-yl)-acetylamino]-pent-4-ynoic acid; (3S)-3-[[2-(1-oxo-7-piperazin-1-yl-3,4-dihydroisoquinolin-2-yl)acetyl]amino]pent-4-ynoic acid; 4-Pentynoic acid, 3-(((3,4-dihydro-1-oxo-7-(1-piperazinyl)-2(1H)-isoquinolinyl)acetyl)amino)-, (3S)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C(RGDfF) | Investigative | [13] | ||
| Synonyms |
CHEMBL380434
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ac-Asp-Arg-Leu-Asp-Ser-OH | Investigative | [14] | ||
| Synonyms |
CHEMBL238484; Acetyl-Asp-Arg-Leu-Asp-Ser-OH; ZINC28869420
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[-Arg-Gly-Asp-Acpca21-] | Investigative | [15] | ||
| Synonyms |
CHEMBL534711
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C-[-Arg-Gly-Asp-Acpca32-] | Investigative | [15] | ||
| Synonyms |
CHEMBL556402
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(3-(carbamoyl)benzamido)propanoic acid | Investigative | [16] | ||
| Synonyms |
CHEMBL219603
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo-[-Arg-Gly-Asp-Amp22-] | Investigative | [17] | ||
| Synonyms |
CHEMBL406912
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo-[-Arg-Gly-Asp-Amp21-] | Investigative | [17] | ||
| Synonyms |
CHEMBL411863; BDBM50372589
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cypate-[(RGD)2-NH2]2 | Investigative | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C[-Arg-Gly-Asp-Acpca35-] | Investigative | [15] | ||
| Synonyms |
CHEMBL534713
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGDf-(S)-alpha-TfmV] | Investigative | [13] | ||
| Synonyms |
CHEMBL203693
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo-[-Arg-Gly-Asp-Amp26-] | Investigative | [17] | ||
| Synonyms |
CHEMBL406680
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| E[c(RGDyK)]2 | Investigative | [19] | ||
| Synonyms |
CHEMBL414385
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C-[-Arg-Gly-Asp-Acpca30-] | Investigative | [15] | ||
| Synonyms |
CHEMBL540622
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[-Arg-Gly-Asp-Acpca36-] | Investigative | [15] | ||
| Synonyms |
CHEMBL557157
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Gly-Arg-Gly-Asp-Ser | Investigative | [20] | ||
| Synonyms |
96426-21-0; GRGDS; glycyl-arginyl-glycyl-aspartyl-serine; H-Gly-Arg-Gly-Asp-Ser-OH; CHEMBL417553; L-Serine, N-(N-(N-(N2-glycyl-L-arginyl)glycyl)-L-alpha-aspartyl)-; GRGDS Peptide; C17H30N8O9; AC1L3XCA; NH2-Gly-Arg-Gly-Asp-Ser; SCHEMBL17440322; RGNVSYKVCGAEHK-GUBZILKMSA-N; HY-P0295; ZINC13455558; MFCD00076459; BDBM50414896; BDBM50006330; AKOS030622941; NCGC00167212-01; Gly-Arg-Gly-Asp-Ser, > LS-178299; FT-0772116; L-Serine,glycyl-L-arginylglycyl-L-a-aspartyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo-[-Arg-Gly-Asp-Amp25-] | Investigative | [17] | ||
| Synonyms |
CHEMBL270690
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C(RGDfMeF) | Investigative | [13] | ||
| Synonyms |
CHEMBL383747
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGDf-(R)-alpha-TfmV] | Investigative | [13] | ||
| Synonyms |
CHEMBL203077
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C(Arg-Gly-Asp-D-Phe-Val) | Investigative | [21] | ||
| Synonyms |
CHEMBL383412
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C-[-Arg-Gly-Asp-Acpca31-] | Investigative | [15] | ||
| Synonyms |
CHEMBL534934
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RGDechi | Investigative | [21] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(3-(benzamido)-5-nitrobenzamido)propanoic acid | Investigative | [16] | ||
| Synonyms |
CHEMBL218226
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo(RGDfV) (control) | Investigative | [18] | ||
| Synonyms |
CHEMBL206344
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGDf-(R)-N-Me-alpha-TfmF] | Investigative | [13] | ||
| Synonyms |
CHEMBL381590
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ISONIPECOTAMIDE | Investigative | [22] | ||
| Synonyms |
Piperidine-4-carboxamide; 39546-32-2; 4-Piperidinecarboxamide; Hexahydroisonicotinamide; 4-carbamoylpiperidine; UNII-CZE2810T4X; CZE2810T4X; DPBWFNDFMCCGGJ-UHFFFAOYSA-N; Piperidine-4-carboxylic acid amide; MFCD00038012; 4-Piperdinecarboxamide; Isonipecotamide, 98%; piperidin-4-carboxamid; iso nipecotamide; iso-nipecotamide; Isonipecotinamide; Piperidine-4-carboxylicacidamide; NSC82318; EINECS 254-501-6; NSC 82318; ISONIPECTOAMIDE; ACMC-20aipw; PubChem9754; Isonipecotic acid amide; 4-piperidine-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGDf-(S)-alpha-TfmF] | Investigative | [13] | ||
| Synonyms |
CHEMBL204309
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CYCLORGDFV | Investigative | [23] | ||
| Synonyms |
cyclo(Arg-Gly-Asp-D-Phe-Val); CHEMBL411941; cyclo[Arg-Gly-Asp-D-Phe-Val]; Cyclo(-Arg-Gly-Asp-D-Phe-Val); 137813-35-5; c-[-Arg-Gly-Asp-fV-]; c[Arg-Gly-Asp-(R)-Phe-Val]; cyclo-(Arg-Gly-Asp-D-Phe-Val); Cyclo(Arg-Gly-Asp-D-Phe-Val-); cyclo(-Arg-Gly-Asp-D-Phe-Val-); BDBM50237601; ZINC17655303; NCGC00167283-01; [(2S,5R,8S,11S)-5-Benzyl-11-(3-guanidino-propyl)-8-isopropyl-3,6,9,12,15-pentaoxo-1,4,7,10,13pentaaza-cyclopentadec-2-yl]-acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo-[-Arg-Gly-Asp-Amp28-] | Investigative | [17] | ||
| Synonyms |
CHEMBL270683
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| E[c(RGDyK)]2-PTX conjugate | Investigative | [19] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cypate-[(RGD)3-NH2]1 | Investigative | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C[-Arg-Gly-Asp-Acpca19-] | Investigative | [15] | ||
| Synonyms |
CHEMBL539338
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Gly-Arg-Gly-Asp-Ser-Pro-Lys | Investigative | [24] | ||
| Synonyms |
Grgdspk; 111119-28-9; Glycyl-arginyl-glycyl-aspartyl-seryl-prolyl-lysine; CHEMBL58763; AC1L2XSM; SCHEMBL891576; DTXSID20149506; HY-P0322; BDBM50079446; ZINC38989354; MFCD00076462; AKOS027382824; N2-(1-(N-(N-(N-(N2-Glycyl-L-arginyl)glycyl)-L-alpha-aspartyl)-L-seryl)-L-prolyl)-L-lysine; NCGC00167196-01; FT-0773649; Gly-Arg-Gly-Asp-Ser-Pro-Lys, > glycyl- arginyl-glycyl-aspartyl-seryl-prolyl-lysine; L-Lysine,glycyl-L-arginylglycyl-L-a-aspartyl-L-seryl-L-prolyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGDf-(R)-alpha-TfmF] | Investigative | [13] | ||
| Synonyms |
CHEMBL381589
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGDf-(S)-N-Me-alpha-TfmF] | Investigative | [13] | ||
| Synonyms |
CHEMBL413574
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cypate-[(RGD)4-NH2]2 | Investigative | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C[-Arg-Gly-Asp-Acpca34-] | Investigative | [15] | ||
| Synonyms |
CHEMBL534933
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo-[-Arg-Gly-Asp-Amp24-] | Investigative | [17] | ||
| Synonyms |
CHEMBL437072
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cypate-[(RGD)2-NH2]1 | Investigative | [18] | ||
| Synonyms |
CHEMBL438186
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(3,5-dichlorophenyl)imidodicarbonimidic diamide | Investigative | [25] | ||
| Synonyms |
CHEMBL40929; 1672-93-1; Imidodicarbonimidic diamide, N-(3,5-dichlorophenyl)-; 1-(diaminomethylidene)-2-(3,5-dichlorophenyl)guanidine; AC1LBJDA; Maybridge1_007061; SCHEMBL891475; CTK7D2196; CTK0E5441; DTXSID50339817; 1-(3,5-dichlorophenyl)biguanide; BUXACHZAYWAZJL-UHFFFAOYSA-N; MolPort-003-943-662; ZINC4370978; ZX-AN037609; ALBB-022022; CCG-43794; STL482580; RJF 00091; BDBM50100971; AKOS003623024; MCULE-3366928787; CCG-245923; Biguanidine, 1-(3,5-dichlorophenyl)-; NCGC00331378-01
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[-Arg-Gly-Asp-Acpca22-] | Investigative | [15] | ||
| Synonyms |
CHEMBL539850
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ST-1646 | Investigative | [17] | ||
| Synonyms |
CHEMBL392303
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo[RGDfK(cypate)] | Investigative | [18] | ||
| Synonyms |
CHEMBL407126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(3-(carbamoyl)benzamido)-3-phenylpropanoic acid | Investigative | [16] | ||
| Synonyms |
CHEMBL220128; BDBM50323318; 3-phenyl-3-(3-(1,2,3,4-tetrahydroisoquinolin-7-ylcarbamoyl)benzamido)propanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo-[-Arg-Gly-Asp-Amp27-] | Investigative | [17] | ||
| Synonyms |
CHEMBL272436
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGD-(R)-alpha-TfmfV] | Investigative | [13] | ||
| Synonyms |
CHEMBL377066
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C-[-Arg-Gly-Asp-Acpca33-] | Investigative | [15] | ||
| Synonyms |
CHEMBL534712
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cypate-[(RGD)3-NH2]2 | Investigative | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGD-(S)-alpha-TfmfV] | Investigative | [13] | ||
| Synonyms |
CHEMBL379056
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[-Arg-Gly-Asp-Acpca20-] | Investigative | [15] | ||
| Synonyms |
CHEMBL540618
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C[RGDf-(S,R)-alpha-Dfm-F] | Investigative | [13] | ||
| Synonyms |
CHEMBL379911
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| AcDRGDS | Investigative | [26] | ||
| Synonyms |
CHEMBL241297; Acetyl-Asp-Arg-Gly-Asp-Ser-OH
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cypate-[(RGD)4-NH2]1 | Investigative | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclo-[-Arg-Gly-Asp-Amp23-] | Investigative | [17] | ||
| Synonyms |
CHEMBL410050
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-265123 | Investigative | [27] | ||
| Synonyms |
CHEMBL288493; SCHEMBL244383; HODBWQCCKYDYPY-NRFANRHFSA-N; {3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid; BDBM50078714; {(S)-3-[3-(Pyridin-2-ylamino)-propoxy]-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-10-yl}-acetic acid; (S)-10,11-Dihydro-3-[3-(pyridin-2-ylamino)-1-propyloxy]-5H-dibenzo[a,d]cycloheptene-10-acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C(-GRGDfL-) | Investigative | [28] | ||
| Synonyms |
CHEMBL235999
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B6B: Nasopharyngeal carcinoma | 20 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Lotilaner | Approved | [29] | ||
| External Link | ||||
| Toripalimab | Approved in China | [30] | ||
| External Link | ||||
| Tabelecleucel | Phase 2 | [31] | ||
| Synonyms |
Tab-cel
Click to Show/Hide
|
|||
| External Link | ||||
| A167 | Phase 3 | [32] | ||
| Synonyms |
KL-A167
Click to Show/Hide
|
|||
| External Link | ||||
| MLN4924 | Phase 1 | [33] | ||
| Synonyms |
Pevonedistat; 905579-51-3; MLN-4924; MLN 4924; UNII-S3AZD8D215; S3AZD8D215; Sulfamic acid [(1S,2S,4R)-4-[4-[[(1S)-2,3-dihydro-1H-inden-1-yl]amino]-7H-pyrrolo[2,3-d]pyrimidin-7-yl]-2-hydroxycyclopentyl]methyl ester; ((1S,2S,4R)-4-(4-(((S)-2,3-dihydro-1H-inden-1-yl)amino)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)-2-hydroxycyclopentyl)methyl sulfamate; C21H25N5O4S; [(1s,2s,4r)-4-{4-[(1s)-2,3-Dihydro-1h-Inden-1-Ylamino]-7h-Pyrrolo[2,3-D]pyrimidin-7-Yl}-2-Hydroxycyclopentyl]methyl Sulfamate; Pevonedistat [USAN:INN]; 3gzn
Click to Show/Hide
|
|||
| External Link | ||||
| TT10 | Phase 3 | [34] | ||
| Synonyms |
EBVST; GVYPHJQPOHDZEI-UHFFFAOYSA-N; TT-10; 2230640-94-3
Click to Show/Hide
|
|||
| External Link | ||||
| DE-766 | Phase 3 | [35] | ||
| External Link | ||||
| Avastin+/-Tarceva | Phase 3 | [36] | ||
| Synonyms |
Juglone; 481-39-0; 5-Hydroxy-1,4-naphthoquinone; 5-Hydroxy-1,4-naphthalenedione; Regianin; Juglon; Nucin; 5-Hydroxynaphthalene-1,4-dione; Walnut extract; 5-Hydroxynaphthoquinone; Akhnot; Yuglon; 8-Hydroxy-1,4-naphthoquinone; CI Natural Brown 7; 1,4-NAPHTHALENEDIONE, 5-HYDROXY-; 5-Hydroxy-p-naphthoquinone; 1,4-Naphthoquinone, 5-hydroxy-; CI 75500; Juglane; Jugnlon; Iuglon; Caswell No 515AA; 1,4-Naphthoquinone, 8-hydroxy-; 5-Hydroxy-1,4-naphthosemiquinone; 5-Hydroxy-1,4-naftochinon; UNII-W6Q80SK9L6; NSC 622948; NSC 153189; CCRIS
Click to Show/Hide
|
|||
| External Link | ||||
| MK-2206 | Phase 2 | [37] | ||
| Synonyms |
1032349-93-1; UNII-51HZG6MP1K; MK 2206; 51HZG6MP1K; CHEMBL1079175; CHEBI:67271; NCGC00186465-01; 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-2H-[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3-one; DSSTox_RID_83143; DSSTox_CID_28874; 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-1,2,4-Triazolo[3,4-f][1,6]naphthyridin-3(2H)-one; DSSTox_GSID_48948; 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl[1,2,4]triazolo[3,4-f][1,6]naphthyridin-3(2H)-one; 1,2,4-Triazolo[3,4-f][1,6]naphthyridin-3(2H)-one, 8-[4-(1-aminocyclobutyl)phenyl]-9-phenyl-;
Click to Show/Hide
|
|||
| External Link | ||||
| R-roscovitine | Phase 2 | [38] | ||
| Synonyms |
Seliciclib; roscovitine; 186692-46-6; (R)-roscovitine; CYC202; CYC-202; CYC 202; 2-(R)-(1-Ethyl-2-hydroxyethylamino)-6-benzylamino-9-isopropylpurine; UNII-0ES1C2KQ94; Roscovitine (Seliciclib,CYC202); NSC 701554; AL-39256; CHEMBL14762; 0ES1C2KQ94; CHEBI:45307; NSC701554; NSC-701554; (2R)-2-[[6-(benzylamino)-9-isopropyl-purin-2-yl]amino]butan-1-ol; (R)-2-((6-(Benzylamino)-9-isopropyl-9H-purin-2-yl)amino)butan-1-ol; (2R)-2-[[6-(benzylamino)-9-propan-2-ylpurin-2-yl]amino]butan-1-ol; RRC; Rosco; M02443; BMK1-E12; CYC202, Seliciclib, R-roscovitine, Roscovitine; (2r)-2-{[6-(benzylamino)-9-isopropyl-9h-purin-2-yl]amino}-1-butanol; 2-(R)-[[9-(1-Methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-1-butanol; 2-[[9-(1-Methylethyl)-6-[(phenylmethyl)amino]-9H-purin-2-yl]amino]-(R)-1-butanol; 6-(Benzylamino)-2(R)-[[1-(hydroxymethyl)propyl]amino]-9-isopropylpurine
Click to Show/Hide
|
|||
| External Link | ||||
| Epstein-barr virus-specific immunotherapy | Phase 2 | [39] | ||
| Synonyms |
Epstein-barr virus-specific immunotherapy (nasopharyngeal carcinoma)
Click to Show/Hide
|
|||
| External Link | ||||
| LMP1-CAR-T cells | Phase 1/2 | [40] | ||
| External Link | ||||
| APG-1387 | Phase 1 | [41] | ||
| Synonyms |
AKLBERUGKZNEJY-RTEPGWBGSA-N; SCHEMBL15490706
Click to Show/Hide
|
|||
| External Link | ||||
| GSK618334 | Phase 1 | [42] | ||
| Synonyms |
Fingolimod hydrochloride; 162359-56-0; FTY720; Fingolimod HCl; Gilenya; Gilenia; Fty-720; Fty 720; Fingolimod (FTY720) HCl; Fingolimod (hydrochloride); 2-Amino-2-(4-octylphenethyl)propane-1,3-diol hydrochloride; 2-Amino-2-(2-(4-octylphenyl)ethyl)-1,3-propanediol hydrochloride; UNII-G926EC510T; Fingolimod hydrochloride [USAN]; CHEBI:63112; G926EC510T; 2-amino-2-[2-(4-octylphenyl)ethyl]propane-1,3-diol hydrochloride; AK-33554; 2-AMINO-2-[2-(4-OCTYL-PHENYL)-ETHYL]-PROPANE-1,3-DIOL HCL; Fingolimod-d4 Hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| PD173074 | Investigative | [43] | ||
| External Link | ||||
| Ad5f35-LMPd1-2-transduced autologous dendritic cells | Investigative | [44] | ||
| Synonyms |
AddLMP1-I-LMP2; LMP1/LMP2 cytotoxic T-lymphocytes vaccine (nasopharyngeal cancer), Baylor College of Medicine; Ad5f35-LMPd1-2-transduced autologous dendritic cells (EBV-associated cancer); Ad5f35-LMPd1-2-transduced autologous dendritic cells (EBV-associated cancer),NCI
Click to Show/Hide
|
|||
| External Link | ||||
| THZ1 | Investigative | [45] | ||
| Synonyms |
HY-80013
Click to Show/Hide
|
|||
| External Link | ||||
| SAIT301 | Investigative | [35] | ||
| External Link | ||||
| HITOPK-032 | Investigative | [46] | ||
| Synonyms |
HI-TOPK-32; HI-TOPK-032; C20H11N5OS; N-(12-cyanoindolizino[2,3-b]quinoxalin-2-yl)thiophene-2-carboxamide; 799819-78-6; N-(12-cyano-2-indolizino[2,3-b]quinoxalinyl)-2-thiophenecarboxamide; AC1LYXW6; HI-TOPC-032; SCHEMBL15270774; DTXSID00365681; AOB1064; SYN5181; MolPort-002-572-638; ACN-S001950; ZINC2320807; STK548195; AKOS005476059; CS-6900; MCULE-6063579279; ACN-001741; AS-16541; HY-101550; B5640; J3.626.778J
Click to Show/Hide
|
|||
| External Link | ||||
| Ro5203280 | Investigative | [47] | ||
| Synonyms |
Ro3280; Ro 3280; PharmaGSID_48511; 4-((9-Cyclopentyl-7,7-difluoro-5-methyl-6-oxo-6,7,8,9-tetrahydro-5H-pyrimido[4,5-b][1,4]diazepin-2-yl)amino)-3-methoxy-N-(1-methylpiperidin-4-yl)benzamide; DJNZZLZKAXGMMC-UHFFFAOYSA-N; 79C; DSSTox_RID_82762; DSSTox_CID_28485; DSSTox_GSID_48511; SCHEMBL1559146; GTPL9403; DTXSID4048511; AOB5394
Click to Show/Hide
|
|||
| External Link | ||||
References