m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05888
|
[1] | |||
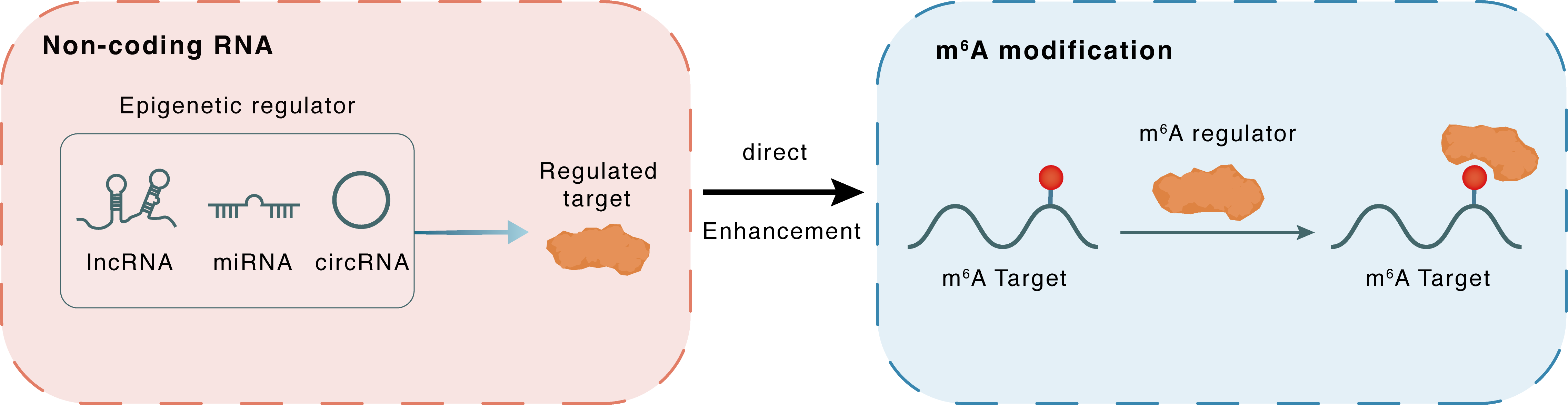 Non-coding RNA
HOXB-AS1
ELAVL1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
FUT4
FUT4
ELAVL1
Non-coding RNA
HOXB-AS1
ELAVL1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
FUT4
FUT4
ELAVL1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | ELAV-like protein 1 (ELAVL1) | READER | |||
| m6A Target | Alpha-(1,3)-fucosyltransferase 4 (FUT4) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | HOXB cluster antisense RNA 1 (HOXB-AS1) | LncRNA | View Details | ||
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | HOXB-AS1 obstruction suppressed MM cell proliferation, and stimulated cell apoptosis. In addition, HOXB-AS1 could modulate Alpha-(1,3)-fucosyltransferase 4 (FUT4) and FUT4-mediated Wnt/beta-catenin pathway. HOXB-AS1 enhanced the interaction between ELAVL1 and FUT4 so as to stabilize FUT4 messenger RNA. | ||||
| Responsed Disease | Multiple myeloma | ICD-11: 2A83 | |||
| Cell Process | Cell proliferation | ||||
| Cell apoptosis | |||||
| RNA stability | |||||
In-vitro Model |
U-1996 | Plasma cell myeloma | Homo sapiens | CVCL_M495 | |
| KM-3 | Childhood B acute lymphoblastic leukemia | Homo sapiens | CVCL_0011 | ||
| NCI-H929 | Plasma cell myeloma | Homo sapiens | CVCL_1600 | ||
| U266 (Human multiple myeloma cells) | |||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Alpha-(1,3)-fucosyltransferase 4 (FUT4) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| 99mTc-fanolesomab | Approved | [2] | ||
| Synonyms |
LeuTech; NeutroSpec; RB5-IgM; Technetium (99mTc) fanolesomab; Anti-SSEA-1; Technetium-99m-fanolesomab; 99mTc RB5-IgM; 99mTc anti-SSEA-1; 99mTc-labeled anti-CD15 antibody
Click to Show/Hide
|
|||
| External Link | ||||
| 2A83: Multiple myeloma | 259 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Talquetamab | Approved | [3] | ||
| External Link | ||||
| Dostarlimab | Approved | [4] | ||
| External Link | ||||
| Belantamab mafodotin | Approved | [5] | ||
| External Link | ||||
| Elranatamab | Approved | [6] | ||
| Synonyms |
PF-06863135
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [7] | ||
| External Link | ||||
| Metadoxine | Approved | [8] | ||
| Synonyms |
MG01CI; Metadoxine (oral sustained release, ADHD); Metadoxine (oral extended release, ADHD), Alcobra; Metadoxine (oral sustained release, ADHD), Alcobra
Click to Show/Hide
|
|||
| External Link | ||||
| Ixazomib | Approved | [9] | ||
| External Link | ||||
| Thalidomide | Approved | [10] | ||
| Synonyms |
Algosediv; Asmadion; Asmaval; Bonbrain; Bonbrrin; Calmore; Calmorex; Contergan; Corronarobetin; Distaval; Distaxal; Distoval; Ectiluran; Enterosediv; Gastrinide; Glupan; Glutanon; Grippex; Hippuzon; Imidene; Isomin; Kedavon; Kevadon; Neaufatin; Neosedyn; Neosydyn; Nerosedyn; Neufatin; Neurodyn; Neurosedin; Neurosedym; Neurosedyn; Nevrodyn; Nibrol; Noctosediv; Noxodyn; Pangul; Pantosediv; Polygripan; Profarmil; Psycholiquid; Psychotablets; Quetimid; Quietoplex; Sandormin; Sedalis; Sedimide; Sedin; Sedisperil; Sedoval; Shinnibrol; Sleepan; Slipro; Softenil; Softenon; Synovir; Talargan; Talidomida; Talidomide; Talimol; Talismol; Talizer; Telagan; Telargan; Telargean; Tensival; Thaled; Thalidomidum; Thalin; Thalinette; Thalomid; Thalomide; Theophilcholine; Valgis; Valgraine; Yodomin; Celgene Brand of Thalidomide; Talidomide [DCIT]; Thalidomide Celgene; Thalidomide Pharmion; Asidon 3; ENMD 0995; IN1061; Thalidomine USP26; Alpha-Phthalimidoglutarimide; E-217; Imida-lab; Imidan (peyta); N-Phthalimidoglutamic acid imide; N-Phthaloylglutamimide; N-Phthalylglutamic acid imide; Poly-Giron; Predni-Sediv; Pro-Bam M; Pro-ban M; Sedalis sedi-lab; Shin-naito S; THALIDOMIDE (AIDS INITIATIVE); Talidomida [INN-Spanish]; Thaled (TN); Thalidomide (soluble form); Thalidomidum [INN-Latin]; Thalomid (TM); Thalomid (TN); Thalomid, Thalidomide; Alpha-N-Phthalylglutaramide; Thalidomide [USAN:INN:BAN]; Alpha-(N-Phthalimido)glutarimide; N-Phthalyl-glutaminsaeure-imid; N-Phthalyl-glutaminsaeure-imid [German]; Thalidomide (+ and-); Thalidomide (JAN/USP/INN); N-(2,6-Dioxo-3-piperidyl)phthalimide; (+)-Thalidomide; (+-)-Thalidomide; (+/-)-THALIDOMIDE; (inverted question mark)-Thalidomide; 2,6-Dioxo-3-phthalimidopiperidine; 3-Phthalimidoglutarimide
Click to Show/Hide
|
|||
| External Link | ||||
| Motixafortide | Approved | [11] | ||
| External Link | ||||
| Bortezomib | Approved | [12] | ||
| Synonyms |
179324-69-7; Velcade; Bortezomib (PS-341); UNII-69G8BD63PP; N-[(1R)-1-(DIHYDROXYBORYL)-3-METHYLBUTYL]-N-(PYRAZIN-2-YLCARBONYL)-L-PHENYLALANINAMIDE; MLN-341; [(1R)-3-methyl-1-[[(2S)-3-phenyl-2-(pyrazine-2-carbonylamino)propanoyl]amino]butyl]boronic acid; [(1R)-3-Methyl-1-[[(2S)-1-oxo-3-phenyl-2-[(pyrazinylcarbonyl)amino]propyl]amino]butyl]boronic acid; CHEMBL325041; 69G8BD63PP; Boronic acid,; DPBA; PROSCRIPT BORONIC ACID; LPD 341; LPD-341; VELCADE (TN); Pyz-Phe-boroLeu; Bortezomib(JAN/USAN/INN); Velcade, MG-341, PS-341, Bortezomib; N-[(1R)-1-(dihydroxyboranyl)-3-methylbutyl]-Nalpha-(pyrazin-2-ylcarbonyl)-L-phenylalaninamide; Bortezomib (Proteasome inhibitor); Peptide boronate
Click to Show/Hide
|
|||
| External Link | ||||
| Daratumumab | Approved | [13] | ||
| External Link | ||||
| Lenalidomide | Approved | [14] | ||
| Synonyms |
Revamid; Revimid; Revlimid; Celgene brand of lenalidomide; Lenalidomide [USAN]; CC 5013; CC5013; CDC 501; IMiD3; IMiD3cpd; ALBB-015321; CC-5013; CDC-501; CDC-5013; ENMD-0997; IMID-5013; Revlimid (Celgene); Revlimid (TN); Thalidomide analog CC-5013; Lenalidomide (USAN/INN); CC-5013, Revlimid, Lenalidomide; 3-(4-Amino-1-oxo-1,3-dihydro-2H-isoindol-2-yl)piperidine-2,6-dione; 3-(4-Amino-1-oxoisoindolin-2-yl)piperidine-2,6-dione; 3-(7-Amino-3-oxo-1H-isoindol-2-yl)-piperidine-2,6-dione; 3-(7-amino-3-oxo-1H-isoindol-2-yl)piperidine-2,6-dione; Lenalidomide (Immunomodulator)
Click to Show/Hide
|
|||
| External Link | ||||
| Isatuximab | Approved | [15] | ||
| External Link | ||||
| Dasatinib | Approved | [16] | ||
| Synonyms |
Sprycel (TN); BMS 354825; BMS-354825; BMS-354825, Sprycel, BMS354825, Dasatinib; BMS354825; Dasatinib (USAN); Dasatinib [USAN]; Dasatinib anhydrous; Dasatinib, BMS 354825; Dasatinibum; Sprycel; Spyrcel
Click to Show/Hide
|
|||
| External Link | ||||
| Panobinostat | Approved | [17] | ||
| Synonyms |
Faridak; LBH 589; LBH589; LBH-589; LBH-589B; NVP-LBH589; NVP-LBH-589; Panobinostat, NVP-LBH589, LBH589; (E)-N-HYDROXY-3-(4-{[2-(2-METHYL-1H-INDOL-3-YL)-ETHYLAMINO]-METHYL}-PHENYL)-ACRYLAMIDE; (E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]prop-2-enamide
Click to Show/Hide
|
|||
| External Link | ||||
| Siltuximab | Approved | [7] | ||
| External Link | ||||
| Elotuzumab | Approved | [18] | ||
| Synonyms |
BMS-901608
Click to Show/Hide
|
|||
| External Link | ||||
| Idecabtagene vicleucel | Approved | [19] | ||
| Synonyms |
Bb2121
Click to Show/Hide
|
|||
| External Link | ||||
| Sirolimus | Approved | [20] | ||
| Synonyms |
53123-88-9; Rapamune; Rapamycin (Sirolimus); AY-22989; Rapammune; sirolimusum; WY-090217; RAPA; Antibiotic AY 22989; AY 22989; UNII-W36ZG6FT64; CCRIS 9024; CHEBI:9168; SILA 9268A; W36ZG6FT64; HSDB 7284; C51H79NO13; NSC 226080; DE-109; NCGC00021305-05; DSSTox_CID_3582; DSSTox_RID_77091; DSSTox_GSID_23582; Cypher; Supralimus; Wy 090217; Perceiva; RAP; RPM; Rapamycin from Streptomyces hygroscopicus; SIIA 9268A; LCP-Siro; MS-R001; Rapamune (TN); Rapamycin (TN); Sirolimus (RAPAMUNE); Rapamycin C-7, analog 4; Sirolimus (USAN/INN); Sirolimus [USAN:BAN:INN]; Sirolimus, Rapamune,Rapamycin; Heptadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy; 23,27-Epoxy-3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine; 23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine; 23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29; 3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone; Sirolimus (MTOR inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Cerivastatin | Approved | [21] | ||
| Synonyms |
Baycol; cerivastatin acid; Lipobay; 145599-86-6; Cerivastatin [INN:BAN]; UNII-AM91H2KS67; CHEBI:3558; AM91H2KS67; HSDB 7357; (3R,5S,6E)-7-(4-(p-Fluorophenyl)-2,6-diisopropyl-5-(methoxymethyl)-3-pyridyl)-3,5-dihydroxy-6-heptenoic acid; (3R,5S,6E)-7-(4-(4-Fluorophenyl)-5-(methoxymethyl)-2,6-bis(1-methylethyl)-3-pyridinyl)-3,5-dihydroxy-6-heptenoic acid; 143201-11-0; 6-Heptenoic acid, 7-(4-(4-fluorophenyl)-5-(methoxymethyl)-2,6-bis(1-methylethyl)-3-pyridinyl)-3,5-dihydroxy-, (3R,5S,6E)-; Statins
Click to Show/Hide
|
|||
| External Link | ||||
| Teclistamab | Approved | [22] | ||
| External Link | ||||
| Futibatinib | Phase 1/2 | [23] | ||
| External Link | ||||
| Romiplostim | Approved | [24] | ||
| External Link | ||||
| MLN9708 | Approved | [25] | ||
| External Link | ||||
| Melphalan flufenamide | Approved | [26] | ||
| Synonyms |
J-1; Dipeptide-conjugated melphalan prodrug (iv formulation, ovary tumor), Oncopeptides
Click to Show/Hide
|
|||
| External Link | ||||
| Carfilzomib | Approved | [27] | ||
| Synonyms |
868540-17-4; Kyprolis; Carfilzomib (PR-171); PR-171; UNII-72X6E3J5AR; 72X6E3J5AR; CHEMBL451887; CHEBI:65347; NCGC00249613-01; DSSTox_RID_82886; DSSTox_CID_28616; DSSTox_GSID_48690; (S)-4-methyl-N-((S)-1-(((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamide; N-{(2S)-2-[(morpholin-4-ylacetyl)amino]-4-phenylbutanoyl}-L-leucyl-N-{(2S)-4-methyl-1-[(2R)-2-methyloxiran-2-yl]-1-oxopentan-2-yl}-L-phenylalaninamide
Click to Show/Hide
|
|||
| External Link | ||||
| Nivolumab | Approved | [7] | ||
| External Link | ||||
| Melphalan | Approved | [28] | ||
| Synonyms |
Alkeran; Levofalan; Levofolan; Levopholan; Melfalan; Melfalano; Melphalanum; Alanine Nitrogen Mustard; Phenylalanine mustard; Phenylalanine nitrogen mu stard; Phenylalanine nitrogen mustard; AY3360000; CB 3025; ALKERAN (TN); At-290; CB-3025; L-PAM; L-Phenylalanine mustard; L-Sarcolysin; L-Sarcolysine; L-Sarkolysin; Melfalano [INN-Spanish]; Melphalanum [INN-Latin]; SK-15673; TRANSGENIC MODEL EVALUATION (MELPHALAN); MELPHALAN (SEE ALSO TRANSGENIC MODEL EVALUATION (MELPHALAN)); P-L-Sarcolysin; P-L-sarcolysine; TRANSGENIC LEP (MELPHALAN) (SEE ALSO MELPHALAN); Melphalan (JP15/USP/INN); Melphalan [USAN:INN:BAN:JAN]; P-Bis(beta-chloroethyl)aminophenylalanine; P-N-Di(chloroethyl)aminophenylalanine; P-N-di(chloroethyl)aminophenylala nine; P-Di-(2-chloroethyl)amino-L-phenylalanine; P-N-Bis(2-chloroethyl)amino-L-phenylalanine; L-3-(p-(Bis(2-chloroethyl)amino)phenyl)alanine; L-3-(para-(Bis(2-chloroethyl)amino)phenyl)alanine; P-N,N-bis(2-chloroethyl)amino-L-phenylalanine; (2S)-2-amino-3-[4-[bis(2-chloroethyl)amino]phenyl]propanoic acid; (2s)-2-amino-3-(4-[bis(2-chloroethyl)amino]phenyl)propanoic acid; 3-(p-(Bis(2-chloroethyl)amino)phenyl)-L-alanine; 3-(p-(Bis(2-chloroethyl)amino)phenyl)alanine; 3-p-(Di(2-chloroethyl)amino)-phenyl-L-alanine; 4-(Bis(2-chloroethyl)amino)-L-phenylalanine; 4-[Bis(2-chloroethyl)amino]-L-phenylalanine; 4-[Bis-(2-chloroethyl)amino]-L-phenylalanine
Click to Show/Hide
|
|||
| External Link | ||||
| Trapidil | Phase 4 | [21] | ||
| Synonyms |
Trapymin; Rocornal; 15421-84-8; Avantrin; Trapymine; N,N-diethyl-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine; AR 12008; Trapidilum [INN-Latin]; UNII-EYG5Y6355E; EINECS 239-434-2; BRN 0186842; 7-Diethylamino-5-methyl-s-triazolo(1,5-a)pyrimidine; MLS000567667; EYG5Y6355E; N,N-Diethyl-5-methyl-(1,2,4)triazolo(1,5-a)pyrimidine-7-amine; (1,2,4)Triazolo(1,5-a)pyrimidin-7-amine, N,N-diethyl-5-methyl-; 5-Methyl-7-diethylamino-s-triazolo-(1,5-a)-pyrimidine; NCGC00016715-01; AR-12008; SMR000154170; SU10991
Click to Show/Hide
|
|||
| External Link | ||||
| Ciltacabtagene autoleucel | Phase 3 | [29] | ||
| Synonyms |
JNJ-68284528
Click to Show/Hide
|
|||
| External Link | ||||
| ABC294640 | Phase 3 | [7] | ||
| Synonyms |
915385-81-8; Opaganib; ABC-294640; CHEMBL2158685; ABC 294640; UNII-DRG21OQ517; DRG21OQ517; Yeliva; SCHEMBL1548333; GTPL6624; CHEBI:124965; MolPort-044-560-286; HMS3402P05; BCP08959; EX-A1962; BDBM50393642; 3-(4-chlorophenyl)-N-(pyridin-4-ylmethyl)adamantane-1-carboxamide; AKOS027327311; CS-0877; SB17167; DB12764; API0017247; HY-16015; S7174; BRD-A70814879-003-01-8
Click to Show/Hide
|
|||
| External Link | ||||
| Tabalumab | Phase 3 | [30] | ||
| Synonyms |
LY2127399
Click to Show/Hide
|
|||
| External Link | ||||
| Marizomib | Phase 1 | [31] | ||
| Synonyms |
salinosporamide A; 437742-34-2; (-)-Salinosporamide A; UNII-703P9YDP7F; NPI-0052; NPI 0052; ML 858; 703P9YDP7F; CHEBI:48045; (1R,4R,5S)-4-(2-chloroethyl)-1-[(S)-(1S)-cyclohex-2-en-1-yl(hydroxy)methyl]-5-methyl-6-oxa-2-azabicyclo[3.2.0]heptane-3,7-dione; Marizomib [USAN:INN]; marizomibum; Marizomib (USAN/INN); Salinosporamide A (NPI-0052, Marizomib); SCHEMBL151667; CHEMBL371405; NGWSFRIPKNWYAO-SHTIJGAHSA-N; ZINC3990364; BDBM50398608; 2531AH; AKOS027323566; DB11762; Z-3093; D09640; 855517-10-1
Click to Show/Hide
|
|||
| External Link | ||||
| Galinpepimut-S | Phase 3 | [7] | ||
| External Link | ||||
| Ulocuplumab | Phase 3 | [32] | ||
| External Link | ||||
| Pelareorep | Phase 2 | [33] | ||
| External Link | ||||
| NVP-LAQ824 | Phase 3 | [21] | ||
| Synonyms |
Dacinostat; 404951-53-7; LAQ824; LAQ-824; LAQ824 (Dacinostat); UNII-V10P524501; (E)-N-hydroxy-3-[4-[[2-hydroxyethyl-[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]prop-2-enamide; CHEMBL356066; V10P524501; (2E)-N-hydroxy-3-[4-({(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino}methyl)phenyl]prop-2-enamide; Dacinostat [INN]; (E)-N-Hydroxy-3-[4-[[2-hydroxyethyl-[2-(1H-indol-3-yl)ethyl]amino]meth yl]phenyl]prop-2-enamide; (2E)-N-hydroxy-3-(4-{[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl}phenyl)prop-2-enamide; NVP-LAQ 824; NVP-LAQ824, Dacinostat, LAQ824; LBH539
Click to Show/Hide
|
|||
| External Link | ||||
| Plitidepsin | Phase 3 | [31] | ||
| Synonyms |
Aplidin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Oblimersen | Phase 3 | [34] | ||
| External Link | ||||
| T89 | Phase 3 | [35] | ||
| Synonyms |
2-Propanone, 1-(5-nitro-2H-tetrazol-2-yl)-; 131394-19-9; 1-(5-nitro-1,2,3,4-tetraazol-2-yl)acetone; ZERO/000247; AC1LOTQU; ACMC-20mu2z; 2-acetonyl-5-nitrotetrazole; CTK0C0970; DTXSID40361521; MolPort-001-904-996; ZINC1084291; STK760073; SBB000169; AKOS000558886; MCULE-5436124308; 1-(5-nitrotetrazol-2-yl)propan-2-one; 2-(2-Oxopropyl)-5-nitro-2H-tetrazole; ST026974; ST023819; BAS 01313849; 1-(5-Nitro-tetrazol-2-yl)-propan-2-one; EU-0085349; 1-(5-nitro-2H-tetrazol-2-yl)propan-2-one; SR-01000523987; SR-01000523987-1
Click to Show/Hide
|
|||
| External Link | ||||
| GMI-1271 | Phase 3 | [7] | ||
| Synonyms |
Uproleselan
Click to Show/Hide
|
|||
| External Link | ||||
| Selinexor | Phase 3 | [7] | ||
| Synonyms |
Xpovio; KPT 330; KPT-330; KPT-330(Selinexor); KPT330;Selinexor; 1393477-72-9; 31TZ62FO8F; CHEMBL3545185; SCHEMBL14678327; Selinexor (KPT-330); Selinexor [USAN:INN]; Tube706; UNII-31TZ62FO8F
Click to Show/Hide
|
|||
| External Link | ||||
| Imetelstat | Phase 2 | [36] | ||
| Synonyms |
Motesanib diphosphate; 857876-30-3; Motesanib (Diphosphate); Motesanib Diphosphate (AMG-706); UNII-T6Q3060U91; Motesanib diphosphate [USAN]; T6Q3060U91; Motesanib diphosphate (USAN); AMG-706 diphosphate; Motesanib phosphate (JAN); bis(phosphoric acid); Motesanib Diphosphate/AMG-706; CHEMBL2107357; DTXSID30235080; C22H29N5O9P2; MolPort-016-633-170; HMS3654K07; AOB87339; ABP000867; AKOS026750408; CS-0193; API0006359; AN-5306; SB16584; RL05311; AS-17019; HY-10229; SW218300-2; FT-0672543; X7466; D08947; W-5527; A841448
Click to Show/Hide
|
|||
| External Link | ||||
| CLR 131 | Phase 1 | [31] | ||
| External Link | ||||
| Descartes-11 | Phase 2 | [37] | ||
| External Link | ||||
| DKN-01 | Phase 2 | [38] | ||
| External Link | ||||
| RAPA-201 | Phase 2 | [39] | ||
| External Link | ||||
| BHQ880 | Phase 2 | [40] | ||
| Synonyms |
BHQ-880A; Anti-DKK1 monoclonal antibody (multiple myeloma), Novartis
Click to Show/Hide
|
|||
| External Link | ||||
| INCB00928 | Phase 2 | [41] | ||
| External Link | ||||
| Descartes-08 | Phase 2 | [42] | ||
| External Link | ||||
| GSK2857916 | Phase 2 | [7] | ||
| External Link | ||||
| Delanzomib | Phase 2 | [43] | ||
| Synonyms |
((R)-1-((2S,3R)-3-hydroxy-2-(6-phenylpicolinamido)butanamido)-3-methylbutan-2-yl)boronic acid; s1157; ZINC202536926; SB16695; SW219161-1; boronic acid, b-[(1r)-1-[[(2s,3r)-3-hydroxy-1-oxo-2-[[(6-phenyl-2-pyridinyl)carbonyl]amino]butyl]amino]-3-methylbutyl]-
Click to Show/Hide
|
|||
| External Link | ||||
| Myeloma cancer vaccine | Phase 2 | [44] | ||
| External Link | ||||
| CJM112 | Phase 2 | [7] | ||
| External Link | ||||
| Tanespimycin | Discontinued in Phase 3 | [45] | ||
| Synonyms |
CNF-101; [(3S,5S,6R,7S,8E,10R,11S,12E,14E)-6-Hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-21-(prop-2-enylamino)-17-azabicyclo[16.3.1]docosa-8,12,14,18,21-pentaen-10-yl] carbamate; [(3R,5S,6R,7S,8E,10S,11S,12Z,14E)-6-hydroxy-5,11-dimethoxy-3,7,9,15-tetramethyl-16,20,22-trioxo-21-(prop-2-enylamino)-17-azabicyclo[16.3.1]docosa-1(21),8,12,14,18-pentaen-10-yl] carbamate; 17AAG, Tanespimycin, Geldanamycin, NSC330507, 17-(Allylamino)-17-demethoxygeldanamycin, CP 127374
Click to Show/Hide
|
|||
| External Link | ||||
| CLR-131 | Phase 2 | [7] | ||
| External Link | ||||
| CDX-301 | Phase 2 | [7] | ||
| External Link | ||||
| BI-505 | Phase 2 | [31] | ||
| External Link | ||||
| SurVaxM | Phase 2 | [46] | ||
| External Link | ||||
| LCAR-B38M CAR-T Cell | Phase 2 | [47] | ||
| External Link | ||||
| CART-19 cells | Phase 2 | [48] | ||
| External Link | ||||
| ALT-803 | Phase 2 | [7] | ||
| Synonyms |
IL-15 agonist/ IL-15R alpha-Fc fusion complex (cancer), Altor BioScience
Click to Show/Hide
|
|||
| External Link | ||||
| LCL161 | Phase 2 | [7] | ||
| External Link | ||||
| mab 1-7F9 | Phase 2 | [49] | ||
| External Link | ||||
| JNJ 63723283 | Phase 2 | [7] | ||
| External Link | ||||
| GSK3377794 | Phase 2 | [7] | ||
| External Link | ||||
| GL-0817 | Phase 2 | [50] | ||
| Synonyms |
912762-70-0; MolPort-002-506-765; tert-butyl N-[(2Z)-2-(2,4-dimethoxyphenyl)-2-(hydroxyimino)ethyl]carbamate; ZINC103473003; AKOS005256100; [2-(2,4-DIMETHOXY-PHENYL)-2-HYDROXYIMINO-ETHYL]-CARBAMIC ACID TERT-BUTYL ESTER
Click to Show/Hide
|
|||
| External Link | ||||
| ONC201 | Phase 2 | [7] | ||
| Synonyms |
Onc-201; TIC10; 1616632-77-9; UNII-9U35A31JAI; 9U35A31JAI; TIC10(ONC-201); 7-Benzyl-4-(2-methylbenzyl)-1,2,6,7,8,9-hexahydroimidazo[1,2-A]pyrido[3,4-E]pyrimidin-5(4H)-one; TIC 10 active isomer; ONC201(TIC10 isomer); TIC 10; GTPL9978; SCHEMBL16227974; EX-A669; ONC 201; AOB2892; MolPort-039-137-731; HY-15615A; 3388AH; s7963; ZINC169620396; AKOS025404904; NSC 350625; CS-3564; AS-16735; AK174891; KB-335104; FT-0700231; J-690224; 1342897-86-2; 2,4,6,7,8,9-Hexahydro-4-((2-methylphenyl)methyl)-7-phenylmethyl)imidazo)(1,2-a)pyrido(3,4-e)pyrimid
Click to Show/Hide
|
|||
| External Link | ||||
| Selinexor | Approved | [20] | ||
| External Link | ||||
| Thalidomide | Approved | [51] | ||
| External Link | ||||
| Bb2121 | Phase 2 | [7] | ||
| External Link | ||||
| PVX-410 | Phase 2 | [46] | ||
| External Link | ||||
| Rocilinostat | Phase 2 | [52] | ||
| Synonyms |
1316214-52-4; Rocilinostat (ACY-1215); 2-(Diphenylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide; UNII-WKT909C62B; ACY-63; WKT909C62B; 2-(Diphenylamino)-N-[7-(hydroxyamino)-7-oxoheptyl]-5-pyrimidinecarboxamide; AK151416; N-[7-(hydroxyamino)-7-oxoheptyl]-2-(N-phenylanilino)pyrimidine-5-carboxamide; 2-(diphenylamino)-N-[7-(hydroxyamino)-7-oxoheptyl]pyrimidine-5-carboxamide; Ricolinostat [USAN:INN]; AH4; Ricolinostat (USAN/INN); MLS006011181; SCHEMBL574580; GTPL7010
Click to Show/Hide
|
|||
| External Link | ||||
| NVP-AUY922 | Phase 2 | [53] | ||
| Synonyms |
Luminespib; 747412-49-3; AUY922; VER-52296; AUY-922; UNII-C6V1DAR5EB; AUY922 (NVP-AUY922); Luminespib (NVP-AUY922); NVP-AUY 922; 5-(2,4-dihydroxy-5-isopropylphenyl)-N-ethyl-4-(4-(morpholinomethyl)phenyl)isoxazole-3-carboxamide; C6V1DAR5EB; Luminespib (AUY-922, NVP-AUY922); CHEBI:83656; 5-[2,4-DIHYDROXY-5-ISOPROPYLPHENYL]-N-ETHYL-4-[4-(4-MORPHOLINYLMETHYL)PHENYL]-3-ISOXAZOLECARBOXAMIDE; AK174786; 5-[2,4-Dihydroxy-5-(1-Methylethyl)phenyl]-N-Ethyl-4-[4-(Morpholin-4-Ylmethyl)phenyl]isoxazole-3-Carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| ARRY-520 | Phase 2 | [31] | ||
| Synonyms |
Filanesib; 885060-09-3; UNII-8A49OSO368; Arry520; CHEMBL2347655; ARRY 520 trifluoroacetate; 8A49OSO368; ARRY 520; (2S)-2-(3-aminopropyl)-5-(2,5-difluorophenyl)-N-methoxy-N-methyl-2-phenyl-1,3,4-thiadiazole-3(2H)-carboxamide; Filanesib [INN]; Filanesib [USAN:INN]; C20H22F2N4O2S; 1385020-40-5; Filanesib(ARRY-520); SCHEMBL368043; DTXSID50237086; EX-A678; BCP07442; ZINC43204022; 3452AH; BDBM50431893; AKOS030526964; SB19209; RL05514; CS-0867; NCGC00381751-02; NCGC00381751-04
Click to Show/Hide
|
|||
| External Link | ||||
| BT-062 | Phase 2 | [7] | ||
| External Link | ||||
| Captisol-enabled Melphalan | Phase 2 | [54] | ||
| External Link | ||||
| IPH-2101 | Phase 2 | [55] | ||
| External Link | ||||
| Darinaparsin | Phase 2 | [56] | ||
| Synonyms |
ZIO-101
Click to Show/Hide
|
|||
| External Link | ||||
| Ficlatuzumab | Phase 2 | [57] | ||
| Synonyms |
AV-299
Click to Show/Hide
|
|||
| External Link | ||||
| GVAX | Phase 2 | [58] | ||
| External Link | ||||
| INK128 | Phase 2 | [59] | ||
| Synonyms |
1224844-38-5; Sapanisertib; INK-128; INK 128; INK 128 (MLN0128); TAK-228; UNII-JGH0DF1U03; JGH0DF1U03; 5-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)benzo[d]oxazol-2-amine; INK-0128; 3-(2-Amino-5-benzoxazolyl)-1-(1-methylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine; C15H15N7O; 5-(4-amino-1-propan-2-ylpyrazolo[3,4-d]pyrimidin-3-yl)-1,3-benzoxazol-2-amine; 5-(4-Amino-1-isopropyl-1H-pyrazolo[3,4-d]-pyrimidin-3-yl)benzo[d]oxazol-2-amine; Sapanisertib (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| GSK2110183 | Phase 2 | [60] | ||
| External Link | ||||
| Oprozomib | Phase 1 | [7] | ||
| External Link | ||||
| CC-220 | Phase 1 | [7] | ||
| Synonyms |
AC1OEW2H; NPD6561; MCULE-9299015048; BCP9000573; BCP0726000266
Click to Show/Hide
|
|||
| External Link | ||||
| Ygalo | Phase 2 | [7] | ||
| External Link | ||||
| Indatuximab ravtansine | Phase 2 | [61] | ||
| External Link | ||||
| REGN5459 | Phase 1/2 | [62] | ||
| External Link | ||||
| REGN5458 | Phase 1/2 | [63] | ||
| External Link | ||||
| ISB 1342 | Phase 1/2 | [64] | ||
| Synonyms |
GBR1342
Click to Show/Hide
|
|||
| External Link | ||||
| PBCAR269A | Phase 1/2 | [65] | ||
| External Link | ||||
| NEXI-002 | Phase 1/2 | [66] | ||
| External Link | ||||
| Tinostamustine | Phase 1/2 | [67] | ||
| Synonyms |
1236199-60-2; 1H-Benzimidazole-2-heptanamide, 5-(bis(2-chloroethyl)amino)-N-hydroxy-1-methyl-; 1H-Benzimidazole-2-heptanamide, 5-[bis(2-chloroethyl)amino]-N-hydroxy-1-methyl-; 29DKI2H2NY; 7-(5-(bis(2-chloroethyl)amino)-1-methyl-1H-benzo[d]imidazol-2-yl)-N-hydroxyheptanamide; 7-[5-[bis(2-chloroethyl)amino]-1-methylbenzimidazol-2-yl]-N-hydroxyheptanamide; 7-{5-[Bis(2-chloroethyl)amino]-1-methyl-1H-benzimidazol-2-yl}-N-hydroxyheptanamide; AKOS030526024; BCP20331; BDBM50569838; BE170657; CHEMBL3989941; CS-6484; D11182; DB15147; E76854; EDO-S 101; EDO-S101; EDO-S-101; EX-A1322; HY-101780; Minomustine; MS-27198; S8769; SB19172; SCHEMBL7915449; starbld0018955; Tinostamustine; Tinostamustine (USAN/INN); TINOSTAMUSTINE [INN]; Tinostamustine [USAN]; TINOSTAMUSTINE [WHO-DD]; Tinostamustine(EDO-S101); UNII-29DKI2H2NY
Click to Show/Hide
|
|||
| External Link | ||||
| Modakafusp alfa | Phase 1/2 | [68] | ||
| Synonyms |
TAK-573
Click to Show/Hide
|
|||
| External Link | ||||
| SL-401 | Phase 1/2 | [7] | ||
| External Link | ||||
| Milatuzumab-doxorubicin conjugate | Phase 1/2 | [69] | ||
| External Link | ||||
| CART-138 cells | Phase 1/2 | [70] | ||
| External Link | ||||
| CAR-T cells targeting CD30 | Phase 1/2 | [71] | ||
| External Link | ||||
| CD56 CAR T cells | Phase 1/2 | [72] | ||
| External Link | ||||
| NY-ESO-TCR | Phase 1/2 | [33] | ||
| External Link | ||||
| CAR-T cells targeting CD22 | Phase 1/2 | [71] | ||
| External Link | ||||
| Anti-CD38 CAR-T cells | Phase 1/2 | [73] | ||
| External Link | ||||
| MOR-202 | Phase 1/2 | [74] | ||
| Synonyms |
Anti-CD38 antibodies, MorphoSys; HuCAL-mAb1, MorphoSys; HuCAL-mAb2, MorphoSys; HuCAL-mAb3, MorphoSys
Click to Show/Hide
|
|||
| External Link | ||||
| BIW-8962 | Phase 1/2 | [75] | ||
| External Link | ||||
| Anti-BCMA CAR-T cells | Phase 1/2 | [73] | ||
| External Link | ||||
| CART-138 cells | Phase 1/2 | [76] | ||
| External Link | ||||
| Anti-NY-ESO-1 CAR-T cells | Phase 1/2 | [73] | ||
| External Link | ||||
| CC-92480 | Phase 1/2 | [77] | ||
| Synonyms |
CELMoD
Click to Show/Hide
|
|||
| External Link | ||||
| CD38 and BCMA CAR-T Cells | Phase 1/2 | [78] | ||
| External Link | ||||
| JCARH125 | Phase 1/2 | [79] | ||
| External Link | ||||
| CAR-T cells targeting CLL1 | Phase 1/2 | [71] | ||
| External Link | ||||
| BION-1301 | Phase 1/2 | [7] | ||
| External Link | ||||
| Atiprimod | Phase 1/2 | [21] | ||
| Synonyms |
Atiprimod [INN]; SKF 106615; SKF-106615; N,N-Diethyl-8,8-dipropyl-2-azaspiro(4.5)decane-2-propanamine; 3-(8,8-dipropyl-3-azaspiro[4.5]decan-3-yl)-N,N-diethylpropan-1-amine
Click to Show/Hide
|
|||
| External Link | ||||
| CAR-T cells targeting BCMA | Phase 1/2 | [71] | ||
| External Link | ||||
| AUTO2 | Phase 1/2 | [80] | ||
| External Link | ||||
| CART-19/BCMA | Phase 1/2 | [81] | ||
| External Link | ||||
| CD38 CAR T cells | Phase 1/2 | [72] | ||
| External Link | ||||
| P-BCMA-101 | Phase 1/2 | [82] | ||
| External Link | ||||
| JCAR017 | Phase 1 | [79] | ||
| External Link | ||||
| Anti-BCMA-CAR-transduced T cells | Phase 1/2 | [83] | ||
| External Link | ||||
| JNJ-68284528 | Phase 1/2 | [84] | ||
| External Link | ||||
| AR-42 | Phase 1/2 | [85] | ||
| Synonyms |
935881-37-1; OSU-HDAC42; AR 42; (S)-HDAC-42; (S)-N-Hydroxy-4-(3-methyl-2-phenylbutanamido)benzamide; UNII-E0GG29V0AQ; (S)-HDAC 42; E0GG29V0AQ; AR42; HDAC-42; AR-42 (HDAC-42); N-hydroxy-4-{[(2S)-3-methyl-2-phenylbutanoyl]amino}benzamide; N-hydroxy-4-[[(2S)-3-methyl-2-phenylbutanoyl]amino]benzamide; AR-42 (OSU-HDAC42); AC1OCFYN; cc-20; OSU-HDAC 42; MLS006011089; CHEMBL191482; SCHEMBL2348844; OSU-42; DTXSID4042678; NOCAS_42678; CTK8C1969; AOB3995; N-Hydroxy-4-(3-methyl-2-(S)phenyl-butyrylamino)benzamide; MolPort-009-019-571
Click to Show/Hide
|
|||
| External Link | ||||
| LGH-447 | Phase 1/2 | [86] | ||
| External Link | ||||
| CWP232291 | Phase 1/2 | [7] | ||
| External Link | ||||
| CD138 CAR T cells | Phase 1/2 | [72] | ||
| External Link | ||||
| ImMucin | Phase 1/2 | [87] | ||
| Synonyms |
MUC-1 peptide vaccine (cancer), Vaxil BioTherapeutics/Hadassah Medical
Click to Show/Hide
|
|||
| External Link | ||||
| CART-BCMA cells | Phase 1/2 | [70] | ||
| External Link | ||||
| CAR-T cells targeting CD19 | Phase 1/2 | [71] | ||
| External Link | ||||
| BCMA CAR-T | Phase 1/2 | [88] | ||
| External Link | ||||
| BCMA CAR T cells | Phase 1/2 | [72] | ||
| External Link | ||||
| CC-98633 | Phase 1 | [89] | ||
| External Link | ||||
| BCMA-CS1 cCAR | Phase 1 | [90] | ||
| External Link | ||||
| TNB-383B | Phase 1 | [91] | ||
| External Link | ||||
| 131I-labelled CLR1404 | Phase 1 | [92] | ||
| External Link | ||||
| GPRC5D CAR-T cell therapy | Phase 1 | [93] | ||
| External Link | ||||
| AMG 420 | Phase 1 | [7] | ||
| External Link | ||||
| BCMA-CD19 cCAR | Phase 1 | [94] | ||
| External Link | ||||
| CD38 CAR-T | Phase 1 | [95] | ||
| External Link | ||||
| RG6538 | Phase 1 | [96] | ||
| External Link | ||||
| UCARTCS1 | Phase 1 | [97] | ||
| External Link | ||||
| MB-104 | Phase 1 | [98] | ||
| External Link | ||||
| JNJ-79635322 | Phase 1 | [99] | ||
| Synonyms |
JNJ-5322
Click to Show/Hide
|
|||
| External Link | ||||
| VOB560 | Phase 1 | [100] | ||
| Synonyms |
S 65487
Click to Show/Hide
|
|||
| External Link | ||||
| CC-93269 | Phase 1 | [7] | ||
| Synonyms |
BCMA TCE
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 424 | Phase 1 | [101] | ||
| External Link | ||||
| 225Ac-labelled aCD38 | Phase 1 | [102] | ||
| External Link | ||||
| CC-99712 | Phase 1 | [103] | ||
| External Link | ||||
| MEDI2228 | Phase 1 | [104] | ||
| External Link | ||||
| AMG 701 | Phase 1 | [7] | ||
| External Link | ||||
| KP1237 | Phase 1 | [105] | ||
| Synonyms |
BHV-1100
Click to Show/Hide
|
|||
| External Link | ||||
| CTX120 | Phase 1 | [106] | ||
| External Link | ||||
| RG6296 | Phase 1 | [107] | ||
| Synonyms |
AFM-26
Click to Show/Hide
|
|||
| External Link | ||||
| WVT078 | Phase 1 | [108] | ||
| External Link | ||||
| M3258 | Phase 1 | [109] | ||
| Synonyms |
LMP7-IN-1; 2285330-15-4; NSC818432; NSC-818432; HY-111790; CS-0091883; ((R)-2-(Benzofuran-3-yl)-1-((1S,2R,4R)-7-oxabicyclo[2.2.1]heptane-2-carboxamido)ethyl)boronic acid
Click to Show/Hide
|
|||
| External Link | ||||
| FT538 | Phase 1 | [110] | ||
| External Link | ||||
| RG6234 | Phase 1 | [96] | ||
| External Link | ||||
| NAM-NK cells | Phase 1 | [111] | ||
| External Link | ||||
| PHE885 | Phase 1 | [112] | ||
| External Link | ||||
| ABBV-467 | Phase 1 | [113] | ||
| External Link | ||||
| ALLO-715 | Phase 1 | [114] | ||
| External Link | ||||
| TTI-622 | Phase 1 | [115] | ||
| External Link | ||||
| SEA-BCMA | Phase 1 | [116] | ||
| External Link | ||||
| FOR46 | Phase 1 | [117] | ||
| External Link | ||||
| CART-ddBCMA | Phase 1 | [118] | ||
| External Link | ||||
| TAK-169 | Phase 1 | [119] | ||
| External Link | ||||
| CT053 | Phase 1 | [120] | ||
| External Link | ||||
| RO6870810 | Phase 1 | [121] | ||
| Synonyms |
JQ-35, (S)-; (S)-JQ-35; 1349719-98-7; (S)-JQ35; Bet inhibitor TEN-010; UNII-TA3QN7788D; TA3QN7788D; TEN-010; Ro-6870810; (S)-2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)-N-(3-(4-methylpiperazin-1-yl)propyl)acetamide; SCHEMBL881288; TEN010; CHEMBL4297423; Rg 6146; EX-A3890; NSC816555; DB15151; NSC-816555; SB19675; HY-117286; CS-0064969; Q27896195; C1CN(C)CCN1CCCNC(=O)C[C@H]1C2=NN=C(C)N2C(SC(C)=C2C)=C2C(C=2C=CC(Cl)=CC=2)=N1; 6H-Thieno(3,2-f)(1,2,4)triazolo(4,3-a)(1,4)diazepine-6-acetamide, 4-(4-chlorophenyl)-2,3,9-trimethyl-N-(3-(4-methyl-1-piperazinyl)propyl)-, (6S)-
Click to Show/Hide
|
|||
| External Link | ||||
| HPN217 | Phase 1 | [122] | ||
| External Link | ||||
| TAK-573 | Phase 1 | [7] | ||
| External Link | ||||
| PT-112 | Phase 1 | [7] | ||
| External Link | ||||
| Anti-CD38 CAR-T cell therapy | Phase 1 | [7] | ||
| External Link | ||||
| Actimab-M | Phase 1 | [33] | ||
| Synonyms |
Actinium-225; Actinium, isotope of mass 225; 225Ac; AC1L4Z7V; 14265-85-1
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-BCMA CAR T cells | Phase 1 | [123] | ||
| External Link | ||||
| BB-MPI-03 | Phase 1 | [46] | ||
| External Link | ||||
| Anti-FGFR3 | Phase 1 | [124] | ||
| External Link | ||||
| Anti-CD19/BCMA CAR-T cells | Phase 1 | [125] | ||
| External Link | ||||
| MTV273 | Phase 1 | [7] | ||
| External Link | ||||
| Anti-BCMA CAR T cells | Phase 1 | [126] | ||
| External Link | ||||
| BCMA nanobody CAR-T cells | Phase 1 | [127] | ||
| External Link | ||||
| BCMA CART and huCART19 | Phase 1 | [128] | ||
| External Link | ||||
| SGN-CD48A | Phase 1 | [7] | ||
| External Link | ||||
| BCMA CART | Phase 1 | [128] | ||
| External Link | ||||
| Anti-BCMA CART Cells | Phase 1 | [129] | ||
| External Link | ||||
| APO-010 | Phase 1 | [130] | ||
| Synonyms |
MegaFasL; Anticancer therapeutics (FasL), APoxis; Cancer therapeutics (FasL), Apoxis; Soluble FasL (Mega-Ligand), Apoxis; Soluble FasL (Mega-Ligand), TopoTarget
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-BCMA CAR-T cell therapy | Phase 1 | [33] | ||
| External Link | ||||
| CC-115 | Phase 1 | [131] | ||
| Synonyms |
CC-115 (hydrochloride); CC-115 hydrochloride; 1300118-55-1; SCHEMBL1767621; BCP20709; HY-16962A; AKOS030526389
Click to Show/Hide
|
|||
| External Link | ||||
| P-BCMA-101 CAR-T cells | Phase 1 | [132] | ||
| External Link | ||||
| BCMA CAR-T Cells | Phase 1 | [133] | ||
| External Link | ||||
| CM-CS1 T-cell | Phase 1 | [134] | ||
| External Link | ||||
| Mivebresib | Phase 1 | [7] | ||
| Synonyms |
ABBV-075; 1445993-26-9; UNII-VR86R11J7J; VR86R11J7J; N-[4-(2,4-Difluorophenoxy)-3-(6-Methyl-7-Oxo-6,7-Dihydro-1h-Pyrrolo[2,3-C]pyridin-4-Yl)phenyl]ethanesulfonamide; N-(4-(2,4-difluorophenoxy)-3-(6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridin-4-yl)phenyl)ethanesulfonamide; 8NG; Mivebresib [INN]; ABBV-075 (Mivebresib); ABBV075; GTPL9117; SCHEMBL15068241; CHEMBL3987016; Mivebresib(ABBV-075 pound(c); MolPort-044-561-801; RDONXGFGWSSFMY-UHFFFAOYSA-N; BDBM220447; EX-A1082; s8400; ZINC146486516; AKOS030628486; CS-5815
Click to Show/Hide
|
|||
| External Link | ||||
| PF-06688992 | Phase 1 | [33] | ||
| External Link | ||||
| DFRF4539A | Phase 1 | [135] | ||
| External Link | ||||
| C-CAR088 | Phase 1 | [136] | ||
| External Link | ||||
| AMG 176 | Phase 1 | [7] | ||
| Synonyms |
JQNINBDKGLWYMU-GEAQBIRJSA-N; AMG-176; 1883727-34-1; AMG176; SCHEMBL17550216; EX-A2666; HY-101565; CS-0021721; Spiro[5,7-etheno-1H,11H-cyclobut[i][1,4]oxazepino[3,4-f][1,2,7]thiadiazacyclohexadecine-2(3H),1'(2'H)-naphthalen]-8(9H)-one, 6'-chloro-3',4',12,13,16,16a,17,18,18a,19-decahydro-16-methoxy-11,12-dimethyl-,10,10-dioxide, (1'S,11R,12S,14E,16S,16aR,18aR)-
Click to Show/Hide
|
|||
| External Link | ||||
| LCAR-B4822M CAR-T Cell | Phase 1 | [137] | ||
| External Link | ||||
| Natural killer cell therapy | Phase 1 | [138] | ||
| Synonyms |
Killer cell therapy; Natural killer cell therapy (autologous, multiple myeloma)
Click to Show/Hide
|
|||
| External Link | ||||
| VLX1570 | Phase 1 | [31] | ||
| Synonyms |
SCKXBVLYWLLALY-ZSIIYVIPSA-N; VLX-1570; 1431280-51-1; BCP18900
Click to Show/Hide
|
|||
| External Link | ||||
| STRO-001 | Phase 1 | [7] | ||
| External Link | ||||
| JNJ-64407564 | Phase 1 | [7] | ||
| External Link | ||||
| KITE-585 | Phase 1 | [7] | ||
| External Link | ||||
| ABBV-838 | Phase 1 | [33] | ||
| External Link | ||||
| CAR-T cells targeting BCMA | Phase 1 | [139] | ||
| External Link | ||||
| CAR138 T Cells | Phase 1 | [140] | ||
| External Link | ||||
| SGN-CD352A | Phase 1 | [7] | ||
| External Link | ||||
| CB-5083 | Phase 1 | [141] | ||
| External Link | ||||
| AEW-541 | Phase 1 | [142] | ||
| Synonyms |
AECDBHGVIIRMOI-UHFFFAOYSA-N; NVP-AEW541; 475489-16-8; 475488-34-7; AEW541; NVP-AEW 541; UNII-97QB5037VR; AEW 541; AVP-AEW541; 7-((1s,3s)-3-(azetidin-1-ylmethyl)cyclobutyl)-5-(3-(benzyloxy)phenyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; CHEMBL1614712; 97QB5037VR; 7-[TRANS-3-(1-AZETIDINYLMETHYL)CYCLOBUTYL]-5-[3-(PHENYLMETHOXY)PHENYL]-7H-PYRROLO[2,3-D]PYRIMIDIN-4-AMINE; C27H29N5O; 7H-PYRROLO[2,3-D]PYRIMIDIN-4-AMINE, 7-[CIS-3-(1-AZETIDINYLMETHYL)CYCLOBUTYL]-5-[3-(PHENYLMETHOXY)PHENYL]-
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-BCMA CAR-T cells | Phase 1 | [143] | ||
| External Link | ||||
| IM21 CART | Phase 1 | [144] | ||
| External Link | ||||
| Anti-CD38 CAR-T cells | Phase 1 | [145] | ||
| External Link | ||||
| ATA520 | Phase 1 | [33] | ||
| External Link | ||||
| GRN163 | Phase 1 | [21] | ||
| External Link | ||||
| GBR 1342 | Phase 1 | [7] | ||
| External Link | ||||
| ACTR-BCMA | Phase 1 | [7] | ||
| Synonyms |
ACTR087 + SEA-BCMA
Click to Show/Hide
|
|||
| External Link | ||||
| CC-122 | Phase 1 | [7] | ||
| Synonyms |
1015474-32-4; Avadomide; 3-(5-Amino-2-methyl-4-oxoquinazolin-3(4H)-yl)piperidine-2,6-dione; CC122; CC 122; 3-(5-amino-2-methyl-4-oxo-4H-quinazolin-3-yl)-piperidine-2,6-dione; 2,6-Piperidinedione, 3-(5-amino-2-methyl-4-oxo-3(4H)-quinazolinyl)-;2,6-Piperidinedione, 3-(5-amino-2-methyl-4-oxo-3(4H)-quinazolinyl)-; Avadomide [USAN]; Avadomide(CC-122); Avadomide (USAN/INN); SCHEMBL282749; US9694015, Compound A; CHEMBL3989934; BDBM76986; RSNPAKAFCAAMBH-UHFFFAOYSA-N; EX-A1191; BCP15938; s7892; AKOS025399378; SB18829; CS-5995
Click to Show/Hide
|
|||
| External Link | ||||
| PNK-007 | Phase 1 | [7] | ||
| External Link | ||||
| Anti-CD19 CART Cells | Phase 1 | [129] | ||
| External Link | ||||
| BFCR4350A | Phase 1 | [7] | ||
| Synonyms |
RG6160
Click to Show/Hide
|
|||
| External Link | ||||
| CART-BCMA | Phase 1 | [146] | ||
| External Link | ||||
| Citarinostat | Phase 1 | [7] | ||
| Synonyms |
ACY-241; 1316215-12-9; HDAC-IN-2; 2-((2-Chlorophenyl)(phenyl)amino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide; UNII-441P620G3P; 441P620G3P; Citarinostat [USAN]; Citarinostat (USAN); 2-(N-(2-chlorophenyl)anilino)-N-[7-(hydroxyamino)-7-oxoheptyl]pyrimidine-5-carboxamide; 2-((2-Chlorophenyl)phenylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)-5-pyrimidinecarboxamide; 2-[(2-Chlorophenyl)phenylamino]-N-[7-(hydroxyamino)-7-oxoheptyl]-5-pyrimidinecarboxamide; Citarinostat (ACY-241); SCHEMBL2225863; GTPL942
Click to Show/Hide
|
|||
| External Link | ||||
| NAM-NK cells + IL-2 | Phase 1 | [7] | ||
| External Link | ||||
| PF-06863135 | Phase 1 | [7] | ||
| External Link | ||||
| AMG 224 | Phase 1 | [7] | ||
| External Link | ||||
| CART-19 T cells | Phase 1 | [147] | ||
| External Link | ||||
| Bb21217 | Phase 1 | [148] | ||
| External Link | ||||
| CAR-T cells targeting CD56 | Clinical trial | [149] | ||
| External Link | ||||
| CAR-T cells targeting CD38 | Clinical trial | [149] | ||
| External Link | ||||
| CAR-T cells targeting CD138 | Clinical trial | [149] | ||
| External Link | ||||
| CAR-BCMA T cell | Clinical trial | [150] | ||
| External Link | ||||
| BCMA-UCART | Clinical trial | [151] | ||
| External Link | ||||
| CAR-T cells targeting BCMA | Clinical trial | [149] | ||
| External Link | ||||
| BCMA-CART | Clinical trial | [152] | ||
| External Link | ||||
| RG7598 | Discontinued in Phase 1 | [153] | ||
| External Link | ||||
| AS602868 | Discontinued in Phase 1 | [154] | ||
| Synonyms |
Angelicin; ISOPSORALEN; 523-50-2; 2H-Furo[2,3-H]chromen-2-one; Angecin; furo[2,3-h]chromen-2-one; Isopsoralin; Furo(2,3-h)coumarin; Angelecin; Angelicin (coumarin derivative); 2H-Furo[2,3-H]-1-benzopyran-2-one; 2-Oxo-(2H)-furo(2,3-h)-1-benzopyran; UNII-CZZ080D7BD; Angelicin (coumarin deriv); NSC 404563; Furo(5',4':7,8)coumarin; CCRIS 4276; HSDB 3554; 4-Hydroxy-5-benzofuranacrylic acid gamma-lactone; BRN 0153970; CZZ080D7BD; 2H-Furo(2,3-H)-1-benzopyran-2-one; CHEMBL53569; Furo[5',4':7,8]coumarin
Click to Show/Hide
|
|||
| External Link | ||||
| UCART-CLL1 | Preclinical | [155] | ||
| External Link | ||||
| 211At-labelled aLAT-1 | Preclinical | [156] | ||
| External Link | ||||
| P-BCMA-ALL01 | Preclinical | [157] | ||
| External Link | ||||
| zoxazolamine | Terminated | [158] | ||
| Synonyms |
61-80-3; 2-Amino-5-chlorobenzoxazole; 5-chlorobenzo[d]oxazol-2-amine; 5-Chloro-1,3-benzoxazol-2-amine; Flexin; 2-Benzoxazolamine, 5-chloro-; Contrazole; Deflexol; Flexilon; Zoxamin; Zoxine; USAF MA-12; McN-485; 5-Chloro-2-benzoxazolamine; Zossazolamina [DCIT]; C7H5ClN2O; Zoxazolaminum [INN-Latin]; Zoxazolamina [INN-Spanish]; NSC 24995; UNII-9DOW362Q29; BENZOXAZOLE, 2-AMINO-5-CHLORO-; CHEBI:35053; EINECS 200-519-4; MLS002639033; AI3-63120; YGCODSQDUUUKIV-UHFFFAOYSA-N; 9DOW362Q29; 2-Amino-5-chlorobenzoxazole, 97%
Click to Show/Hide
|
|||
| External Link | ||||
| AG490 | Terminated | [159] | ||
| Synonyms |
(E)-N-Benzyl-2-cyano-3-(3,4-dihydroxyphenyl)acrylamide; 133550-30-8; AG-490; Tyrphostin B42; Tyrphostin AG 490; AG 490; Tyrphostin AG490; tyrphostin AG-490; AG-490 (Tyrphostin B42); alpha-Cyano-(3,4-dihydroxy)-N-benzylcinnamide; N-Benzyl-2-cyano-3-(3,4-dihydroxyphenyl)acrylamide; 134036-52-5; (2E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide; CHEMBL56543; (E)-N-benzyl-2-cyano-3-(3,4-dihydroxyphenyl)prop-2-enamide; (2E)-2-CYANO-3-(3,4-DIHYDROXYPHENYL)-N-(PHENYLMETHYL)-2-PROPENAMIDE; SMR001230665
Click to Show/Hide
|
|||
| External Link | ||||
| G3139 + Dexamethasone | Investigative | [160] | ||
| External Link | ||||
| GNE-652 | Investigative | [161] | ||
| Synonyms |
PIM kinase inhibitor (multiple myeloma), Genentech
Click to Show/Hide
|
|||
| External Link | ||||
| CCX-721 | Investigative | [161] | ||
| Synonyms |
CCR1 antagonist (myeloma), ChemoCentryx
Click to Show/Hide
|
|||
| External Link | ||||
| JB-1 | Investigative | [21] | ||
| Synonyms |
JB1
Click to Show/Hide
|
|||
| External Link | ||||
| BIBF100 | Investigative | [21] | ||
| External Link | ||||
| BAY11-7082 | Investigative | [162] | ||
| Synonyms |
bay 11-7082; 19542-67-7; (E)-3-Tosylacrylonitrile; Bay 11-7821; (E)-3-(p-Toluenesulfonyl)acrylonitrile; BAY-11-7082; UNII-4Y5G2A4F6O; BAY-11-7821; (E)-3-(4-Methylphenyl)sulfonylprop-2-enenitrile; BAY-117082; BAY-117821; CHEMBL403183; 4Y5G2A4F6O; 3-(4-methylphenylsulfonyl)-2-propenenitrile; CHEBI:85928; 3-[(4-methylphenyl)sulfonyl]prop-2-enenitrile; AK129348; (E)-3-(4-Methylphenylsulfonyl)-2-propenenitrile; (E)3-[(4-Methylphenyl)sulfonyl]-2-propenenitrile; J-501956; (2E)-3-[(4-methylphenyl)sulfonyl]prop-2-enenitrile
Click to Show/Hide
|
|||
| External Link | ||||
| UCL-67022 | Investigative | [163] | ||
| Synonyms |
HDAC inhibitor (multiple myeloma), ST Barts/UCL
Click to Show/Hide
|
|||
| External Link | ||||
| AbGn-150 | Investigative | [161] | ||
| Synonyms |
Antibody-150
Click to Show/Hide
|
|||
| External Link | ||||
| ACHP | Investigative | [21] | ||
| External Link | ||||
| SU5402 | Investigative | [21] | ||
| Synonyms |
215543-92-3; SU 5402; SU-5402; 3-[3-(2-Carboxyethyl)-4-methylpyrrol-2-methylidenyl]-2-indolinone; (Z)-3-(4-methyl-2-((2-oxoindolin-3-ylidene)methyl)-1H-pyrrol-3-yl)propanoic acid; CHEMBL89363; 3-[(3-(2-CARBOXYETHYL)-4-METHYLPYRROL-2-YL)METHYLENE]-2-INDOLINONE; J-502595; 3-{[3-(2-carboxyethyl)-4-methylpyrrol-2-yl]methylene}-2-indolinone; 3-[4-methyl-2-[(Z)-(2-oxo-1H-indol-3-ylidene)methyl]-1H-pyrrol-3-yl]propanoic acid; (Z)-3-(4-Methyl-2-((2-oxoindolin-3-ylidene)-methyl)-1H-pyrrol-3-yl)propanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| TP-110 | Investigative | [161] | ||
| Synonyms |
Tyropeptin A derivatives, Microbial Chemistry Research Foundation; Proteasome inhibitors (cancer), Microbial Chemistry Research Foundation
Click to Show/Hide
|
|||
| External Link | ||||
| TMPyP4 | Investigative | [21] | ||
| Synonyms |
TMPP; AC1L1HPJ; N-Methylpyridylylporphyrin; AC1Q29RK; CHEMBL65606; ABCGFHPGHXSVKI-UHFFFAOYSA-O; BDBM50107609; 4,4',4'',4'''-(5,10,15,20-porphyrintetrayl)tetrakis(1-methylpyridinium); J2.096.016G; 5,10,15,20-tetra(1-methyl-4-pyridyl)-porphyrin; 5,10,15,20-tetrakis(1-methylpyridin-1-ium-4-yl)-21,22-dihydroporphyrin
Click to Show/Hide
|
|||
| External Link | ||||
| Sant7 | Investigative | [21] | ||
| Synonyms |
beta-Methylene tad; BETA-METHYLENE-THIAZOLE-4-CARBOXYAMIDE-ADENINE DINUCLEOTIDE; 102977-57-1; beta-Tad; NSC617998; 1lrt; NSC 617998; beta-Methylene thiazole-4-carboxamide adenine dinucleotide; .beta.-Methylene TAD; AC1L2TER; Adenosine, 5'-(hydrogen (phosphonomethyl)phosphonate), 5'-ester with 2-beta-D-ribofuranosyl-4-thiazolecarboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| PS-1145 | Investigative | [21] | ||
| Synonyms |
431898-65-6; PS 1145; CHEMBL79004; N-(6-Chloro-9H-beta-carbolin-8-yl)-nicotinamide; MLS006010310; ZINC9090; SCHEMBL1420453; CTK1D2755; KS-00000TTM; CHEBI:94801; DTXSID00433238; EX-A786; MolPort-042-665-715; JZRMBDHPALEPDM-UHFFFAOYSA-N; HMS3229F21; BCP24095; s7691; BDBM50130248; AKOS030526812; CS-5415; ACN-053038; NCGC00165873-02; NCGC00165873-01; SMR004701376; HY-18008; AK547800; FT-0700478; J-690297; BRD-K93023739-001-02-9; N-{6-chloro-9H-pyrido[3,4-b]indol-8-yl}pyridine-3-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| FM-101 | Investigative | [164] | ||
| External Link | ||||
| NVP-ADW742 | Investigative | [21] | ||
| Synonyms |
475488-23-4; ADW-742; 475489-15-7; UNII-MXS2N5862L; ADW742; MXS2N5862L; CHEMBL399021; 5-(3-(Benzyloxy)phenyl)-7-((1r,3r)-3-(pyrrolidin-1-ylmethyl)-cyclobutyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; 5-(3-(Benzyloxy)phenyl)-7-(cis-3-(pyrrolidin-1-ylmethyl)cyclobutyl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine; C28H31N5O; 5-(3-Benzyloxyphenyl)-7-[trans-3-[(pyrrolidin-1-yl)methyl]cyclobutyl]-7H-pyrrolo[2,3-d]pyrimidin-4-amine
Click to Show/Hide
|
|||
| External Link | ||||
| CGEN-928 | Investigative | [161] | ||
| Synonyms |
Anti-CGEN-928 polyclonal antibody (multiple myeloma treatment); Anti-TM21 polyclonal antibody (multiple myeloma treatment), Compugen; CGEN-928 (multiple myeloma treatment), Compugen; Anti-CGEN-928 polyclonal antibody (multiple myeloma treatment), Compugen
Click to Show/Hide
|
|||
| External Link | ||||
| Tubacin | Investigative | [165] | ||
| Synonyms |
537049-40-4; AC1O7Y2P; CHEMBL356769; 1350555-93-9; N1-(4-((2R,4R,6S)-4-(((4,5-Diphenyloxazol-2-yl)thio)methyl)-6-(4-(hydroxymethyl)phenyl)-1,3-dioxan-2-yl)phenyl)-N8-hydroxyoctanediamide; Tubacin (BML-GR362); Octanediamide, N1-(4-((2R,4R,6S)-4-(((4,5-diphenyl-2-oxazolyl)thio)methyl)-6-(4-(hydroxymethyl)phenyl)-1,3-dioxan-2-yl)phenyl)-N8-hydroxy-, rel-; N-[4-[(2R,4R,6S)-4-[[(4,5-Diphenyl-2-oxazolyl)thio]methyl]-6-[4-(hydroxymethyl)phenyl]-1,3-dioxan-2-yl]phenyl]-N'-hydroxyoctanediamide; SCHEMBL4741166
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-HM1.24 | Investigative | [161] | ||
| External Link | ||||
| Telomestatin | Investigative | [21] | ||
| Synonyms |
YVSQVYZBDXIXCC-UHFFFAOYSA-N; AC1L9EVW; CHEMBL443683; CHEBI:29689; Telomestatin (TMS); gm95; SureCN10025441; SCHEMBL10025441; CHEBI:601758; BDBM213233; DCL000983
Click to Show/Hide
|
|||
| External Link | ||||
| G3139 + Thalidomide | Investigative | [160] | ||
| External Link | ||||
References