m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05745
|
[1] | |||
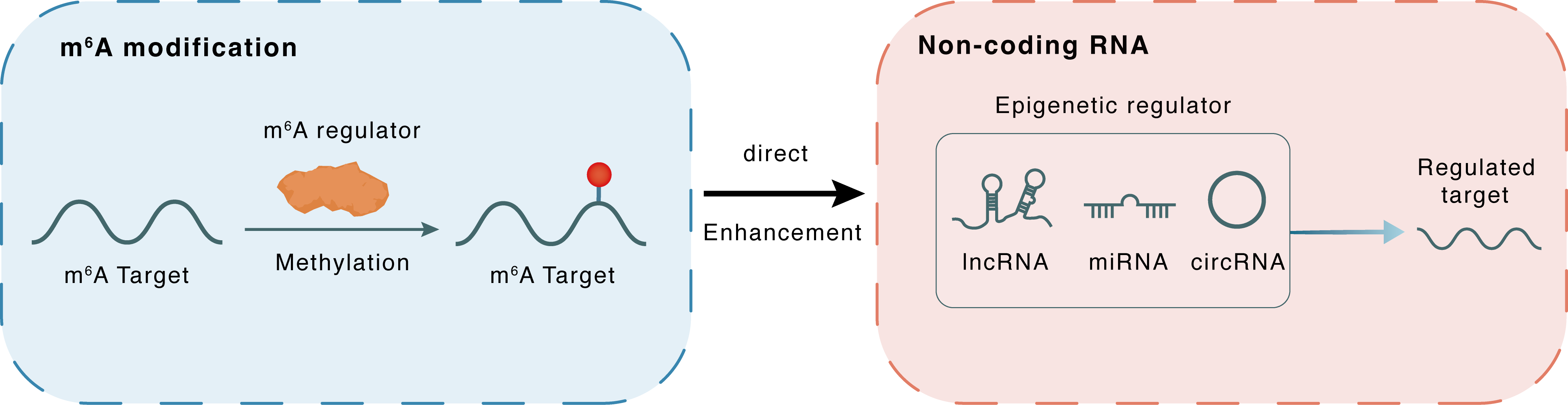 m6A modification
miR-27a-3p
miR-27a-3p
METTL3
Methylation
m6A modification
miR-27a-3p
miR-27a-3p
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR-27a-3p
PPARG
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR-27a-3p
PPARG
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | hsa-miR-27a-3p | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-27a-3p | microRNA | View Details | ||
| Regulated Target | Peroxisome proliferator-activated receptor gamma (PPARG) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | OMT exhibited a dose-dependent inhibitory effect on the volume of IH PPNL-resistant tumors, which was partially dependent on the regulation of m6A methylation transfer enzyme METTL3 and the hsa-miR-27a-3p/Peroxisome proliferator-activated receptor gamma (PPARG) axis, thereby inducing apoptosis. | ||||
| Responsed Disease | Infantile hemangioma | ICD-11: 2E81 | |||
| Responsed Drug | Oxymatrine | ||||
| Pathway Response | Apoptosis | hsa04210 | |||
| Cell Process | Cell apoptosis | ||||
In-vitro Model |
HemSCs/PPNL (Propranolol (PPNL)-resistant hemangioma stem cells) | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Peroxisome proliferator-activated receptor gamma (PPARG) | 143 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Pioglitazone | Approved | [2] | ||
| Synonyms |
111025-46-8; Actos; Pioglitazona; Pioglitazonum; Glustin; Zactos; 105355-27-9; Pioglitazonum [INN-Latin]; Pioglitazona [INN-Spanish]; Duetact; Pioglitazone [INN:BAN]; Pioglitazone [BAN:INN]; 5-(4-(2-(5-ethylpyridin-2-yl)ethoxy)benzyl)thiazolidine-2,4-dione; AD-4833; U 72107; CHEBI:8228; Pioglitazone (Actos); HSDB 7322; Actos (TN); 5-{4-[2-(5-ethylpyridin-2-yl)ethoxy]benzyl}-1,3-thiazolidine-2,4-dione; C19H20N2O3S; AD 4833; 5-[4-[2-(5-ETHYL-2-PYRIDYL)ETHOXY]BENZYL]-2,4-THIAZOLIDINEDIONE; U 72107A; Actost; Glustin (TN); HS-0047; Pioglitazone (INN); U-72107; U72,107A; Zactos (TN); (+-)-5-((4-(2-(5-ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione; (+/-)-5-[[4-[2-(5-Ethyl-2-pyridinyl)-ethoxy]phenyl]methyl]-2,4-thiazolidinedione; (+/-)-5-[p-[2-(ethyl-2-pyridyl)ethoxy]benzyl]-2,4-thiazolidinedione; 2,4-Thiazolidinedione, 5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-(9CI); 5-((4-(2-(5-Ethyl-2-pyridinyl)ethoxy)phenyl)methyl)-2,4-thiazolidinedione; 5-(4-(2-(5-ethyl-2-pyridyl)ethoxy)benzyl)-2,4-thiazolidinedione; 5-[4-[2-(5-Ethyl-2-pyridyl)ethoxy]benzyl]thiazolidine-2,4-dione; 5-[[4-[2-(5-Ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-thiazolidinedione; 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 5-[[4-[2-(5-ethylpyridin-2-yl)ethoxy]phenyl]methyl]thiazolidine-2,4-dione; 5-[[4-[2-[(5-ethyl-2-pyridyl)]ethoxy]phenyl]methyl]thiazolidine-2,4-dione; Linagliptin + pioglitazone; PCG1
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Troglitazone | Approved | [3] | ||
| Synonyms |
Noscal; Prelay; Resulin; Rezulin; Romglizone; Romozin; Troglitazona; Troglitazonum; Parke Davis brand of troglitazone; CS 045; GR 92132X; CS-045; GR-92132X; Resulin (TN); Rezulin (TN); Romozin (TN); Warner-Lambert brand of troglitazone; Troglitazone [USAN:BAN:INN]; Troglitazone (JAN/USAN/INN); (+-)-all-rac-5-(p-((6-Hydroxy-2,5,7,8-tetramethyl-2-chromanyl)methoxy)benzyl)-2,4-thiazolidinedione; (+/-)-5-[4-[(6-hydroxy-2,5,7,8-tetramethylchroman-2-yl)methoxy]benzyl]-2,4-thiazolidinedione; 2,4-Thiazolidinedione, 5-[[4-[(3,4-dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)methoxy]phenyl]methyl]-(9CI); 5-(4-((6-hydroxy-2,5,7,8-tetramethylchroman-2-yl-methoxy)benzyl)-2,4-thiazolidinedione); 5-(4-(6-Hydroxy-2,5,7,8-tetramethylchroman-2-ylmethoxy)benzyl)thiazolidine-2,4-dione; 5-[(4-{[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-chromen-2-yl)methyl]oxy}phenyl)methyl]-1,3-thiazolidine-2,4-dione; 5-[[4-[(3,4-Dihydro-6-hydroxy-2,5,7,8-tetramethyl-2H-1-benzopyran-2-yl)methoxy]phenyl]methyl]-2,4-thiazolidinedione; 5-[[4-[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydrochromen-2-yl)methoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 5-{4-[(6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-2H-chromen-2-yl)methoxy]benzyl}-1,3-thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Rosiglitazone XR | Approved | [4] | ||
| Synonyms |
Avandia; Nyracta; Venvia; Rosiglitazone Maleate [USAN]; Rosiglitazone maleate; BRL 49653C; Avandia (TN); Avandiaadministration for 6-12 weeks; BRL 49653-C; BRL-49653C; SB-206846; SB-210232; Rosiglitazone maleate (JAN/USAN); (+-)-5-(p-(2-(Methyl-2-pyridylamino)ethoxy)benzyl)-2,4-thiazolidinedione maleate (1:1); (+-)-5-[[4-2-(methyl]-2-pyridinylamino)ethoxy]phenyl]methyl]-2,4-thiazolidinedione,(Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione, 5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-, (2Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione, 5-[[4-[2-(methyl-2-pyridinylamino) ethoxy]phenyl]methyl]-, (2Z)-2-butenedioate (1:1); 2,4-Thiazolidinedione,5-((4-(2-(methyl-2-pyridinylamino)ethoxy)phenyl)methyl)-,(2Z)-2-butenedioate; 5-[[4-[2-[methyl(pyridin-2-yl)amino]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 5-{[4-({2-[methyl(pyridin-2-yl)amino]ethyl}oxy)phenyl]methyl}-1,3-thiazolidine-2,4-dione (2Z)-but-2-enedioate
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | Ki = 14 nM | |||
| External Link | ||||
| Thiazolidinedione | Approved | [2] | ||
| Synonyms |
2295-31-0; 1,3-Thiazolidine-2,4-dione; thiazolidine-2,4-dione; 2,4-Dioxothiazolidine; 2,4(3H,5H)-Thiazoledione; USAF EK-5496; Thiazolidindione; UNII-AA68LXK93C; Thiazolidinedione-2,4; NSC 6745; EINECS 218-941-2; BRN 0110700; AA68LXK93C; AI3-61185; CHEBI:50992; NSC6745; ZOBPZXTWZATXDG-UHFFFAOYSA-N; MFCD00005478; 2,4-Thiazolidinedione, 99%; C3H3NO2S; thiazolidine-dione; 2,4-thiazolidindione; 2,5H)-Thiazoledione; PubChem17487
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Lobeglitazone | Approved | [5] | ||
| Synonyms |
Lobeglitazone sulfate; CKD-501; Dual PPARalpha/delta (type 2 diabetes), Chong Kun Dang
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Glitazone | Phase 4 | [6] | ||
| Synonyms |
74772-78-4; 5-(4-Hydroxybenzyl)thiazolidine-2,4-dione; 5-(4-Hydroxybenzyl)-2,4-thiazolidinedione; 5-(4-HYDROXYBENZYL)-1,3-THIAZOLIDINE-2,4-DIONE; U-90441; 2,4-dioxo-5-[(p-hydroxyphenyl)-methyl]thiazolidine; 5-[(4-Hydroxyphenyl)methyl]-2,4-thiazolidinedione; 2,4-Thiazolidinedione, 5-[(4-hydroxyphenyl)methyl]-; 5-(4-Hydroxybenzyl)thiazolidin-2,4-dione; ACMC-1BF0J; Pioglitazone Metabolite M1; SCHEMBL623021; SCHEMBL18174924; CTK5E0483; MolPort-006-394-329; NKOHRVBBQISBSB-UHFFFAOYSA-N; ZX-AT015682; KM1640; CH-087
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Rivoglitazone | Phase 3 | [7] | ||
| Synonyms |
CS-011; DE-101; Rivoglitazone (ophthalmic); Rivoglitazone (ophthalmic), Santen; CS-011 (ophthalmic), Santen
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 43 nM | |||
| External Link | ||||
| Balaglitazone | Phase 3 | [8] | ||
| Synonyms |
DRF-2593; NN-2344; DRF-2593-307; NNC-61-0645
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| FARGLITAZAR | Phase 3 | [9] | ||
| Synonyms |
GI-262570; GI262570; 196808-45-4; GI 262570; UNII-3433GY7132; CHEMBL107367; GI-262570X; 2-(2-BENZOYL-PHENYLAMINO)-3-{4-[2-(5-METHYL-2-PHENYL-OXAZOL-4-YL)-ETHOXY]-PHENYL}-PROPIONIC ACID; 3433GY7132; L-Tyrosine, N-(2-benzoylphenyl)-o-(2-(5-methyl-2-phenyl-4-oxazolyl)ethyl)-; L-Tyrosine, N-(2-benzoylphenyl)-o-(2-(5-methyl-2-phenyl-4-oxazolyl)ethyl); N-(o-Benzoylphenyl)-O-(2-(5-methyl-2-phenyl-4-oxazolyl)ethyl)-L-tyrosine; Farglitazar [USAN]; Farglitazar [USAN:INN]; L-tyrosine, N-(2-benzoylphenyl)-O-[2-(5-methyl-
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 0.3388 nM | |||
| External Link | ||||
| Rosiglitazone + simvastatin | Phase 3 | [4] | ||
| MOA | Agonist | |||
| External Link | ||||
| Leriglitazone | Phase 3 | [10] | ||
| Synonyms |
146062-44-4; 1-Hydroxypioglitazone; 2,4-Thiazolidinedione, 5-((4-(2-(5-(1-hydroxyethyl)-2-pyridinyl)ethoxy)phenyl)methyl)-; 2,4-Thiazolidinedione, 5-[[4-[2-[5-(1-hydroxyethyl)-2-pyridinyl]ethoxy]phenyl]methyl]-; 5-(4-(2-(5-(1-hydroxyethyl)pyridin-2-yl)ethoxy)benzyl)thiazolidine-2,4-dione; 5-(4-{2-[5-(1-hydroxyethyl)pyridin-2-yl]ethoxy}benzyl)-1,3-thiazolidine-2,4-dione; 5-[[4-[2-[5-(1-Hydroxyethyl)-2-pyridinyl]ethoxy]phenyl]methyl]-2,4-thiazolidinedione; 5-[[4-[2-[5-(1-hydroxyethyl)pyridin-2-yl]ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 5-[4-[2-[5-(1-hydroxyethyl)-2-pyridyl]ethoxy]benzyl]-2,4-thiazolidinedione; A1-01710; AKOS015856344; ALL-AMBO-5-((4-(2-(5-(1-HYDROXYETHYL)PYRIDIN-2-YL)ETHOXY)PHENYL)METHYL)-1,3-THIAZOLE-2,4(3H,5H)-DIONE; BDBM50530214; CHEBI:82937; CHEMBL1267; CS-0067030; D11603; DB15021; DTXSID30399914; FT-0643388; HY-117727; Hydroxy Pioglitazone (M-IV); Hydroxy Pioglitazone-D5 (Major) (M-IV); Hydroxypioglitazone; J-008187; K824X25AYA; Leriglitazone; Leriglitazone (USAN/INN); LERIGLITAZONE [INN]; Leriglitazone [USAN]; MIN-102; OXVFDZYQLGRLCD-UHFFFAOYSA-N; Pioglitazone, hydroxy; Q27156475; SCHEMBL4098326; UNII-K824X25AYA; WHO 10868; ZINC01482947
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Ragaglitazar | Phase 3 | [11] | ||
| Synonyms |
DRF-2725; (2S)-2-ETHOXY-3-{4-[2-(10H-PHENOXAZIN-10-YL)ETHOXY]PHENYL}PROPANOIC ACID; CHEMBL24038; (2S)-2-ethoxy-3-[4-(2-phenoxazin-10-ylethoxy)phenyl]propanoic acid; DRF; 1nyx; DRF2725; AC1L9KVW; 2-Ethoxy-3-[4-(2-phenoxazin-10-yl-ethoxy)-phenyl]-propionic acid; GTPL2664; SCHEMBL4822459; WMUIIGVAWPWQAW-DEOSSOPVSA-N; NN-622; BDBM50109551; DB07675; NNC-61-0029; (-)-DRF-2725; (S)-2-Ethoxy-3-[4-(2-phenoxazin-10-yl-ethoxy)-phenyl]-propionic acid; (2S)-2-ethoxy-3-{4-[2-(phenoxazin-10-yl)ethoxy]phenyl}propanoic acid
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 90 nM | |||
| External Link | ||||
| CS-038 | Phase 3 | [12] | ||
| Synonyms |
Chiglitazar; CS-00098; PPAR alpha/gamma agonist (diabetes), Chipscreen Biosciences
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| TESAGLITAZAR | Phase 3 | [13] | ||
| Synonyms |
251565-85-2; Galida; (S)-2-Ethoxy-3-(4-(4-((methylsulfonyl)oxy)phenethoxy)phenyl)propanoic acid; AZ 242; AZ-242; AR-H039242XX; (S)-2-Ethoxy-3-{4-[2-(4-methanesulfonyloxyphenyl)ethoxy]phenyl}propionic acid; UNII-6734037O3L; (S)-2-Ethoxy-3-{4-[2-(4-methanesulfonyloxy-phenyl)-ethoxy]-phenyl}-propionic acid; (2S)-2-ethoxy-3-[4-[2-(4-methylsulfonyloxyphenyl)ethoxy]phenyl]propanoic acid; BR-44608; 6734037O3L; (S)-2-Ethoxy-3-[4-[2-(4-methanesulfonyloxyphenyl)ethoxy]phenyl]propanoic acid
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 13 nM | |||
| External Link | ||||
| MURAGLITAZAR | Phase 3 | [14] | ||
| Synonyms |
331741-94-7; Pargluva; BMS-298585; UNII-W1MKM70WQI; BMS 298585; W1MKM70WQI; CHEMBL186179; N-[(4-Methoxyphenoxy)carbonyl]-N-[[4-[2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]glycine; N-((4-methoxyphenoxy)carbonyl)-N-((4-(2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy)phenyl)methyl)glycine; BMS298585; Muraglitazar [USAN:INN]; CCRIS 9258; AC1L4FVP; Muraglitazar (USAN/INN); DSSTox_CID_31508; DSSTox_RID_97393; DSSTox_GSID_57719; SCHEMBL676469; DTXSID9057719; CTK8E8901; MolPort-006-395-259; IRLWJILLXJGJTD-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 3 nM | |||
| External Link | ||||
| ZYH-1 | Phase 3 | [15] | ||
| Synonyms |
Metabolism disorder treatment, Zydus-Cadila; PPAR alpha/gamma modulator (dyslipdemia), Zydus-Cadila
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Imiglitazar | Phase 3 | [16] | ||
| Synonyms |
TAK-559
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 4 nM | |||
| External Link | ||||
| Rosiglitazone + metformin | Phase 3 | [4] | ||
| Synonyms |
Orantinib; TSU-68; 252916-29-3; SU-6668; SU6668; SU 6668; 210644-62-5; UNII-9RL37ZZ665; Orantinib (TSU-68); NSC 702827; TSU68; CHEMBL274654; 9RL37ZZ665; TSU 68; PDGFR Tyrosine Kinase Inhibitor VI, SU6668; 2,4-Dimethyl-5-[(1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl]-pyrrole-3-propanoic acid; J-502593; Orantinibum; 3-(2,4-dimethyl-5-{[(3Z)-2-oxo-1H-indol-3-ylidene]methyl}-1H-pyrrol-3-yl)propanoic acid; 3-[2,4-dimethyl-5-[(Z)-(2-oxo-1H-indol-3-ylidene)methyl]-1H-pyrrol-3-yl]propanoic acid; Orantinib [INN]
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| MBX-102 | Phase 2/3 | [17] | ||
| Synonyms |
Arhalofenate; MBX 102; 24136-23-0; UNII-1P01UJR9X1; JNJ-39659100; 1P01UJR9X1; CB-102; M-102; Arhalofenate [USAN:INN]; Arhalofenate (USAN/INN); SCHEMBL3302781; CHEMBL2103824; BJBCSGQLZQGGIQ-QGZVFWFLSA-N; ZINC2012859; BDBM50093473; AKOS032945719; DB11811; CS-6812; SB16866; (-)-2-(Acetylamino)ethyl (2R)-(4-chlorophenyl)(3-(trifluoromethyl)phenoxy)acetate; CJ-31172; Benzeneacetic acid, 4-chloro-alpha-(3-(trifluoromethyl)phenoxy)-, 2-(acetylamino)ethyl ester, (-)-; HY-14831; D09579; UNII-K9TZK4MNO6 component BJBCSGQLZQGGIQ-QGZV
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 > 500 nM | |||
| External Link | ||||
| FK-614 | Phase 2 | [18] | ||
| Synonyms |
ATx08-001; ATx08-001); PPAR gamma agonist (oral, neuropathic pain), Aestus
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 163 nM | |||
| External Link | ||||
| Netoglitazone | Phase 2 | [19] | ||
| Synonyms |
Isaglitazone; Netoglitazone [USAN]; MCC 555; MCC-555; RWJ-241947; Netoglitazone (USAN/INN); 5-((6-((2-fluorophenyl)methoxy)-2-naphthalenyl)methyl)-2,4-thiazolidinedione; 5-({6-[(2-fluorobenzyl)oxy]naphthalen-2-yl}methyl)-1,3-thiazolidine-2,4-dione; 5-[[6-[(2-fluorophenyl)methoxy]naphthalen-2-yl]methyl]-1,3-thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| MBX-2044 | Phase 2 | [17] | ||
| Synonyms |
MBX-102 analogs, Metabolex
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| GED-0507-34-Levo | Phase 2 | [20] | ||
| Synonyms |
GED-0507; GED-0507-34; GED-0507-34E2
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| CHS-131 | Phase 2 | [21] | ||
| MOA | Modulator | |||
| External Link | ||||
| OMS405 | Phase 2 | [22] | ||
| MOA | Agonist | |||
| External Link | ||||
| Naveglitazar | Phase 2 | [23] | ||
| Synonyms |
LY 519818; LY-818; PPAR alpha/gamma co-agonists, Lilly/Ligand
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 33.96 nM | |||
| External Link | ||||
| ONO-5129 | Phase 2 | [24] | ||
| Synonyms |
Dual PPAR alpha/gamma agonist (metabolic disorder), Ono
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| T3D-959 | Phase 2 | [25] | ||
| MOA | Agonist | |||
| External Link | ||||
| T3D-959 | Phase 1/2 | [26] | ||
| MOA | Modulator | |||
| External Link | ||||
| CLX-0921 | Phase 1 | [27] | ||
| Synonyms |
CLX-090502; CLX-090503; CLX-090717; CLX-092402
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| DSP-8658 | Phase 1 | [28] | ||
| MOA | Modulator | |||
| External Link | ||||
| Englitazone sodium | Phase 1 | [29] | ||
| Synonyms |
Englitazone sodium < Rec INNM; CP-68722 (racemate); CP-72467-02; CP-72467-2; (-)-5-[2(R)-Benzyl-3,4-dihydro-2H-benzopyran-6-ylmethyl]thiazolidine-2,4-dione sodium salt
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Oxeglitazar | Phase 1 | [30] | ||
| Synonyms |
EMD-336340; EML-16156; EML-4156; LM-4156
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| GW-409544 | Phase 1 | [31] | ||
| Synonyms |
GW-409544X; GW-544; GW-6471; Lipid regulators, Ligand/Glaxo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 0.2 nM | |||
| External Link | ||||
| Spirolaxine derivative 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-L
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Phenylpropionic acid derivative 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-D
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| A-substituted phenylpropionic acid derivative 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-c
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| PMID25416646-Compound-Figure2-K | Patented | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| PMID25416646-Compound-Figure2-N | Patented | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| MRL24 | Patented | [32] | ||
| Synonyms |
MRL 24
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 1 nM | |||
| External Link | ||||
| Sulfonamide derivative 18 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-M
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Crystalline anhydrous toluene derivative 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-H
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| A-substituted phenylpropionic acid derivative 2 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-E
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Biaromatic compound 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-A
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| MRL20 | Patented | [32] | ||
| Synonyms |
CHEMBL181937; (2s)-2-(2-{[1-(4-Methoxybenzoyl)-2-Methyl-5-(Trifluoromethoxy)-1h-Indol-3-Yl]methyl}phenoxy)propanoic Acid; SCHEMBL5172443; GTPL6742; BDBM50157936; (2S)-2-[2-[[1-(4-methoxybenzoyl)-2-methyl-5-(trifluoromethoxy)indol-3-yl]methyl]phenoxy]propanoic acid; (2S)-2-[2-({1-[(4-methoxyphenyl)carbonyl]-2-methyl-5-(trifluoromethoxy)-1H-indol-3-yl}methyl)phenoxy]propanoic acid; (S)-2-{2-[1-(4-Methoxy-benzoyl)-2-methyl-5-trifluoromethoxy-1H-indol-3-ylmethyl]-phenoxy}-propionic acid
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 2 nM | |||
| External Link | ||||
| Fused ring compound 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-G
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| 3-phenyl acrylic acid derivative 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-B
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Thiazolidine dione crystalline derivative 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-I
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Fused aromatic compound 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure2-F
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| PMID25416646-Compound-Figure2-J | Patented | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| Cannabinoid quinone derivative 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure3-A
Click to Show/Hide
|
|||
| MOA | Ligand | |||
| External Link | ||||
| PMID25416646-Compound-Figure5-H | Patented | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| Flavonoid derivative 8 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure5-B
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| PMID25416646-Compound-Figure5-E | Patented | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| Phenylpyridine derivative 3 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure5-G
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| PMID25416646-Compound-Figure5-A | Patented | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| 1-(biphenyl-4-yl-methyl)-1H-imidazole derivative 1 | Patented | [32] | ||
| Synonyms |
PMID25416646-Compound-Figure5-F
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| PMID25416646-Compound-Figure5-C | Patented | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| YM-440 | Discontinued in Phase 2 | [33] | ||
| Synonyms |
2,2'-[2(Z)-Butene-1,4-diyl]dioxybis(1,4-phenylene)bis(methylene)bis[1,2,4-oxadiazole-3,5(2H,4H)-dione]
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| KRP-297 | Discontinued in Phase 2 | [34] | ||
| Synonyms |
MK 767; MK-767; KRP297; KRP 297; 213252-19-8; L410198; L 410198; 5-((2,4-Dioxo-5-thiazolidinyl)methyl)-2-methoxy-N-((4-(trifluoromethyl)phenyl)methyl)benzamide; Benzamide, 5-((2,4-dioxo-5-thiazolidinyl)methyl)-2-methoxy-N-((4-(trifluoromethyl)phenyl)methyl)-; 5-[(2,4-dioxo-1,3-thiazolidin-5-yl)methyl]-2-methoxy-N-[[4-(trifluoromethyl)phenyl]methyl]benzamide; NFFXEUUOMTXWCX-UHFFFAOYSA-N; SCHEMBL3922; AC1L45TL; MLS006010319; GTPL2677; CTK4E6495; MolPort-018-657-358; AKOS005067111; NCGC00263123-01; SMR004701384
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Reglixane | Discontinued in Phase 2 | [24] | ||
| MOA | Modulator | |||
| External Link | ||||
| AVE-0847 | Discontinued in Phase 2 | [35] | ||
| Synonyms |
PPAR alpha/gamma agonists (diabetes/dyslipidemia), Genfit; PPAR alpha/gamma agonists (diabetes/dyslipidemia), aventis; PPAR alpha/gamma agonists (diabetes/dyslipidemia), sanofi-aventis
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Sodelglitazar | Discontinued in Phase 2 | [36] | ||
| Synonyms |
447406-78-2; UNII-6G973E04VI; 6G973E04VI; GW677954; Sodelglitazar [USAN:INN]; 2-[4-[[[2-[2-Fluoro-4-(trifluoromethyl)phenyl]-4-methyl-1,3-thiazol-5-yl]methyl]sulfanyl]-2-methylphenoxy]-2-methylpropanoic acid; 2-(4-(((2-(2-Fluoro-4-(trifluoromethyl)phenyl)-4-methyl-1,3-thiazol-5-yl)methyl)sulfanyl)-2-methylphenoxy)-2-methylpropanoic acid; Sodelglitazar (USAN); SCHEMBL4822839; CHEMBL2104984; DTXSID90196289; ZUGQWAYOWCBWGM-UHFFFAOYSA-N; ZINC1553281; AN-28238; ACM447406782; FT-0743554; D06647; 406S782; Propanoic ac
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Indeglitazar | Discontinued in Phase 2 | [37] | ||
| Synonyms |
PLX-204
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| GSK-677954 | Discontinued in Phase 2 | [38] | ||
| Synonyms |
SCHEMBL2065429
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| DS-6930 | Discontinued in Phase 1 | [39] | ||
| MOA | Modulator | |||
| External Link | ||||
| E-3030 | Discontinued in Phase 1 | [40] | ||
| Synonyms |
Dual PPAR alpha/gamma agonists, Eisai
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| LY-929 | Discontinued in Phase 1 | [41] | ||
| Synonyms |
LY-510929; Dual PPAR-alpha/PPAR-gamma agonists
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 3.981 nM | |||
| External Link | ||||
| Ciglitazone | Preclinical | [42] | ||
| Synonyms |
74772-77-3; Ciglitizone; ADD-3878; Ciglitazonum; Ciglitazona; Ciglitazonum [Latin]; Ciglitazona [Spanish]; Ciglitazone [USAN:INN]; ADD 3878; CHEBI:64227; U-63287; (+-)-5-(p-((1-Methylcyclohexyl)methoxy)benzyl)-2,4-thiazolidinedione; C18H23NO3S; 5-(4-((1-methylcyclohexyl)methoxy)benzyl)thiazolidine-2,4-dione; YZFWTZACSRHJQD-UHFFFAOYSA-N; (+/-)-5-[4-(1-Methylcyclohexylmethoxy)benzyl]thiazolidine-2,4-dione; U 63287; 5-{4-[(1-methylcyclohexyl)methoxy]benzyl}-1,3-thiazolidine-2,4-dione; NCGC00164446-01
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| MC-3002 | Preclinical | [24] | ||
| Synonyms |
PPAR dual agonists, MaxoCore
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| SB-219994 | Terminated | [43] | ||
| Synonyms |
SB-219993
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 1 nM | |||
| External Link | ||||
| AKP-320 | Terminated | [44] | ||
| Synonyms |
Bis(ethylmaltolato)oxovanadium (IV) + rosiglitazone (type 2 diabetes), Akesis
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Edaglitazone | Terminated | [45] | ||
| Synonyms |
Edaglitazone sodium; BM-131258; BM-152054; R-483; Ro-205; BM-13.258; BM-15.2054; Ro-205-2349; Ro-2052349-602
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| DARGLITAZONE | Terminated | [46] | ||
| Synonyms |
CP-86325; Darglitazone < Rec INN; Rac-5-[4-[3-(5-Methyl-2-phenyloxazol-4-yl)propionyl]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| BVT-142 | Terminated | [47] | ||
| Synonyms |
BVT-13; BVT-142 analogs, Biovitrum; Dual PPAR alpha/gamma agonists (type II diabetes); Dual PPAR alpha/gamma agonists (type II diabetes), Biovitrum
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| AD-5075 | Terminated | [48] | ||
| Synonyms |
2,4-Thiazolidinedione, 5-((4-(2-hydroxy-2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy)phenyl)methyl)-; AD 5075; 103788-05-2; 5-[[4-[2-hydroxy-2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 2,4-Thiazolidinedione,5-[[4-[2-hydroxy-2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]-; YVQKIDLSVHRBGZ-UHFFFAOYSA-N; [3H]AD5075; [3H]-AD5075; ACMC-20m6lh; AC1L2TSO; CHEMBL88496; SCHEMBL131888; GTPL2702; GTPL2701; CTK4A2384; AD5075; 5-(4-(2-(5-Methyl-2-phenyl-4-oxazolyl)-2-hydroxyethoxy)benzyl)-2,4-thiazolidinedione; [3H]AD-5075
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Sipoglitazar | Terminated | [15] | ||
| Synonyms |
TAK-654
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| CS-204 | Terminated | [49] | ||
| Synonyms |
CS-00088; PPAR alpha/gamma/delta agonist (type 2 diabetes), Chipscreen Bioscience
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| 15-deoxy-Delta(12, 14)-prostaglandin J(2) | Investigative | [3] | ||
| Synonyms |
15d-PGJ2; 15-Deoxy-PGJ2; 15-Deoxy-delta-12,14-prostaglandin J2; 15-deoxy-delta12,14-prostaglandin J2; delta12,14-PGJ 2; UNII-ALI977775J; 87893-55-8; CHEMBL482477; CHEBI:34159; (5Z,12E,14E)-11-oxoprosta-5,9,12,14-tetraen-1-oic acid; ALI977775J; delta-12,14-15-Deoxy-PGJ2; 15-Deoxy-delta12,14-prostaglandin; 15-deoxy-Delta(12,14)-prostaglandin J2; Prostaglandin J2, 15-Deoxy-Delta12,14; 15-Deoxy-delta 12, 14-Prostaglandin J2
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| (2S)-2-(biphenyl-4-yloxy)-3-phenylpropanoic acid | Investigative | [50] | ||
| Synonyms |
LT175; 862901-87-9; CHEMBL191275; LRG; (2S)-3-phenyl-2-(4-phenylphenoxy)propanoic Acid; SCHEMBL20553479; BDBM28759; SYN5198; AOB2972; MolPort-039-138-772; ZINC13671695; LT 175; DB08121; LT175 (S-1); NCGC00402292-03
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 480 nM | |||
| External Link | ||||
| 3-(5-methoxy-1H-indol-3-yl)propanoic acid | Investigative | [50] | ||
| Synonyms |
39547-16-5; 3-(5-Methoxy-1H-indol-3-yl)-propionic acid; 1H-Indole-3-propanoic acid, 5-methoxy-; 3-(5-methoxyindol-3-yl)propanoic acid; CBMicro_030931; ChemDiv2_000435; AC1L48GT; Oprea1_046476; SCHEMBL3024961; CTK1C3093; 5-methoxyindole-3-propionic acid; ZLSZCJIWILJKMR-UHFFFAOYSA-N; MolPort-002-085-835; ZINC195148; HMS3604K06; HMS1370D17; 2429AE; STK984200; SBB028111; AKOS000300418; MCULE-1797370196; DB07723; CCG-108176; DS-3311; AK403360; SC-43672; BIM-0030775.P001; TR-055403; AX8188175
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (2S)-2-(4-ethylphenoxy)-3-phenylpropanoic acid | Investigative | [50] | ||
| Synonyms |
CHEMBL191060; ZINC13671697; BDBM50171895; DB07842; (S)-2-(4-Ethyl-phenoxy)-3-phenyl-propionic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 3600 nM | |||
| External Link | ||||
| (2S)-2-(4-chlorophenoxy)-3-phenylpropanoic acid | Investigative | [50] | ||
| Synonyms |
CHEMBL364748; SCHEMBL20553473; ZINC13671687; BDBM50171897; DB08760; (S)-2-(4-chlorophenoxy)-3-phenylpropanoic acid; (S)-2-(4-Chloro-phenoxy)-3-phenyl-propionic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 2691.53 nM | |||
| External Link | ||||
| GW7845 | Investigative | [51] | ||
| Synonyms |
GW-7845; GW 7845; CHEMBL106666; 196809-22-0; SCHEMBL614392; GTPL2704; BDBM50085046; ZINC49748569; GW 845; GW 347845; UNII-23Y783RURX component KEGOAFNIGUBYHZ-SANMLTNESA-N; N-(2-(Methoxycarbonyl)phenyl]-O-[2-(5-methyl-2-phenyl-4-oxazolyl)ethyl)-L-tyrosine; 2-((S)-1-Carboxy-2-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-ethoxy]-phenyl}-ethylamino)-benzoic acid methyl ester; 720711-63-7; 2-(1-Carboxy-2-{4-[2-(5-methyl-2-phenyl-oxazol-4-yl)-ethoxy]-phenyl}-ethylamino)-benzoic acid methyl ester(GW 7845)
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 0.6166 nM | |||
| External Link | ||||
| (9Z,11E,13S)-13-hydroxyoctadeca-9,11-dienoic acid | Investigative | [50] | ||
| Synonyms |
13(S)-Hydroxyoctadeca-9Z,11E-dienoic acid; 29623-28-7; 13(S)-HODE; (13S)-Hydroxyoctadecadienoic acid; 13S-HODE; 13-Hydroxyoctadecadienoic acid; CHEMBL451721; CHEBI:34154; 13S-Hydroxy-9Z,11E-octadecadienoic acid; 13(S)-Hydroxy-9(Z),11(E)-octadecadienoic acid; (9Z, 11E)-(13S)-13-Hydroxyoctadeca-9,11-dienoic acid; (9Z,11E)-(13S)-13-Hydroxyoctadeca-9,11-dienoic acid; 9,11-Octadecadienoic acid, 13-hydroxy-, (R-(E,Z))-; 5204-88-6; 10219-69-9; (S)-Coriolic acid; AC1O5YAH
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GW-2331 | Investigative | [52] | ||
| Synonyms |
190844-95-2; PPAR ligand, Glaxo Wellcome
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | EC50 = 243 nM | |||
| External Link | ||||
| 9-hydroxyoctadecadienoic acid | Investigative | [50] | ||
| Synonyms |
(9S,10E,12Z)-9-Hydroxyoctadeca-10,12-dienoic acid; 9(S)-HODE; alpha-dimorphecolic acid; 9S-HODE; (9S)-Hydroxyoctadecadienoic acid; UNII-42KE04U9BM; 9S-hydroxy-10E,12Z-octadecadienoic acid; 42KE04U9BM; CHEBI:34496; (9S)-Hydroxyoctadecadinoiec acid; 73543-67-6; (10E,12Z)-(9S)-9-Hydroxyoctadeca-10,12-dienoic acid; (9s,10e,12z)-9-hydroxy-10,12-octadecadienoic acid; (9S,10E,12Z)-9-hydroxyoctadeca-10,12-dienoate; 9(S)-hydroxyoctadecadienoic acid; Alpha-dimorphecolic; AC1NSNNN; (9S)-Hydroxyoctadecadienoate; (+)-alpha-Dimorphecolic
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ZY H2 | Investigative | [53] | ||
| Synonyms |
ZYH2
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 2-chloro-5-nitro-N-phenylbenzamide | Investigative | [50] | ||
| Synonyms |
GW9662; 22978-25-2; GW 9662; 2-Chloro-5-nitrobenzanilide; GW-9662; MLS001056751; CHEBI:79993; 2-Chloro-5-nitro-N-phenyl-benzamide; 2-Chloro-5-nitro-N-4-phenylbenzamide; benzamide, 2-chloro-5-nitro-N-phenyl-; SMR000326735; (2-chloro-5-nitrophenyl)-N-benzamide; SR-01000075999; Tocris-1508; Spectrum5_001989; Lopac-M-6191; AC1LD8S0; DSSTox_RID_79570; DSSTox_CID_20723; DSSTox_GSID_40723; Lopac0_000798; KBioGR_000361; KBioSS_000361; BSPBio_001021; SCHEMBL420231; CHEMBL375270; GTPL3442; cid_644213
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 2 nM | |||
| External Link | ||||
| DB-900 | Investigative | [54] | ||
| MOA | Agonist | |||
| External Link | ||||
| Bardoxolone methyl | Phase 3 | [55] | ||
| Synonyms |
BARD; WPTTVJLTNAWYAO-OYWPANLISA-N; 218600-53-4
Click to Show/Hide
|
|||
| External Link | ||||
| AD-5075 | Terminated | [48] | ||
| Synonyms |
2,4-Thiazolidinedione, 5-((4-(2-hydroxy-2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy)phenyl)methyl)-; AD 5075; 103788-05-2; 5-[[4-[2-hydroxy-2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]phenyl]methyl]-1,3-thiazolidine-2,4-dione; 2,4-Thiazolidinedione,5-[[4-[2-hydroxy-2-(5-methyl-2-phenyl-4-oxazolyl)ethoxy]phenyl]methyl]-; YVQKIDLSVHRBGZ-UHFFFAOYSA-N; [3H]AD5075; [3H]-AD5075; ACMC-20m6lh; AC1L2TSO; CHEMBL88496; SCHEMBL131888; GTPL2702; GTPL2701; CTK4A2384; AD5075; 5-(4-(2-(5-Methyl-2-phenyl-4-oxazolyl)-2-hydroxyethoxy)benzyl)-2,4-thiazolidinedione; [3H]AD-5075
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| (E)-9-nitrooctadec-9-enoic acid | Investigative | [55] | ||
| Synonyms |
9-nitro-9E-octadecenoic acid; 9-Nitro Oleic Acid; 9-Nitrooleic acid; Nitroleic Acid; 875685-44-2; 9-Nitrooleate; CHEMBL550762; (9E)-9-nitrooctadecenoic acid; CHEBI:86329; 9-nitro-9E-Octadecenoate; (9E)-9-Nitrooctadecenoate; 9-Nitro-9-octadecenoic acid; (e)-9-Nitrooctadec-9-enoate; GTPL6296; SCHEMBL1015108; MolPort-027-641-228; CQOAKBVRRVHWKV-SAPNQHFASA-N; (e)-9-nitro-octadec-9-enoic acid; (9E)-9-nitrooctadec-9-enoic acid; (9E)-9-Nitro-9-octadecenoic Acid; BDBM50295048; 1573AH; LMFA01120004; nitrooleic acid
Click to Show/Hide
|
|||
| Activity | IC50 = 980 nM | |||
| External Link | ||||
| tagitinin A | Investigative | [55] | ||
| Synonyms |
59979-61-2; GTPL8701; CHEMBL518700; AC1L48P2; MEGxp0_000075; ACon0_000437; ACon1_000272; BDBM50394802; NCGC00180721-01
Click to Show/Hide
|
|||
| Activity | IC50 = 55000 nM | |||
| External Link | ||||
| T0070907 | Investigative | [55] | ||
| Synonyms |
2-Chloro-5-nitro-N-4-pyridinylbenzamide; 313516-66-4; 2-chloro-5-nitro-N-(pyridin-4-yl)benzamide; T 0070907; T-0070907; 2-chloro-5-nitro-N-pyridin-4-ylbenzamide; 2-Chloro-5-nitro-N-(4-pyridyl)benzamide; CHEMBL510698; Benzamide, 2-chloro-5-nitro-N-4-pyridinyl-; 2-chloro-5-nitro-n-4-pyridinyl-benzamide; SR-01000392700; AC1MCROG; Oprea1_586106; ZINC3381; GTPL3444; SCHEMBL2128178; CTK6H1028; KS-00000MYU; CHEBI:92553; DTXSID30380504; MolPort-001-763-336; FRPJSHKMZHWJBE-UHFFFAOYSA-N; HMS3268J16; HMS3651P21; HMS3262J21
Click to Show/Hide
|
|||
| Activity | IC50 = 1000 nM | |||
| External Link | ||||
| GW1929 | Investigative | [55] | ||
| Synonyms |
GW 1929; GW-1929
Click to Show/Hide
|
|||
| Activity | Ki = 0.0497 nM | |||
| External Link | ||||
| (E)-9-Nitrohexadec-9-enoicAcid | Investigative | [55] | ||
| Synonyms |
CHEMBL560513; 9-nitropalmitoleic acid; E-9-nitropalmitoleic acid; SCHEMBL15633556; YZNSJIAWRWPQGX-NTCAYCPXSA-N; (E)-9-Nitro-9-hexadecenoic acid; BDBM50295047
Click to Show/Hide
|
|||
| Activity | IC50 = 830 nM | |||
| External Link | ||||
| (E)-10-nitrooctadec-9-enoic acid | Investigative | [55] | ||
| Synonyms |
10-nitrooleic acid; 10-nitro-9E-octadecenoic acid; 10-Nitro Oleic Acid; 10-nitroelaidic acid; 875685-46-4; UNII-1N19AGY57Y; CHEMBL561371; 1N19AGY57Y; CHEBI:86285; (9E)-10-nitrooctadecenoic acid; 10-Nitrooleate; 10-nitro-oleic acid; 10-Nitroelaidate; 88127-53-1; 9-Octadecenoic acid, 10-nitro-, (9E)-; E-10-nitrooleic acid; Oa-NO2; 10-nitro-9E-Octadecenoate; (9E)-10-Nitrooctadecenoate; 10-Nitro-9-octadecenoic acid; SCHEMBL1018141; CXA-10; (e)-10-Nitrooctadec-9-enoate; WRADPCFZZWXOTI-BMRADRMJSA-N
Click to Show/Hide
|
|||
| Activity | IC50 = 1610 nM | |||
| External Link | ||||
| SB-213068 | Investigative | [55] | ||
| Synonyms |
CHEMBL306229; SCHEMBL7822831; FJENKDXEWRLENI-UHFFFAOYSA-N; BDBM50085043; 3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid; alpha-Ethoxy-4-[2-[methyl(benzoxazole-2-yl)amino]ethoxy]benzenepropanoic acid; 3-[4-[2-[N-(2-Benzoxazolyl)-N-methylamino]ethoxy]phenyl]-2-ethoxypropanoic acid; 3-{4-[2-(Benzooxazol-2-yl-methyl-amino)-ethoxy]-phenyl}-2-ethoxy-propionic acid(SB-213068)
Click to Show/Hide
|
|||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| tirotundin | Investigative | [55] | ||
| Synonyms |
56377-67-4; MEGxp0_000078; GTPL8700; CHEMBL464453; MolPort-039-052-526; ZINC40393738; BDBM50394803; AKOS032948657
Click to Show/Hide
|
|||
| Activity | IC50 = 27000 nM | |||
| External Link | ||||
| L-Tryptophan-L-aspartic acid | Investigative | [55] | ||
| Synonyms |
H-TRP-ASP-OH; 71835-78-4; CHEMBL476008; CHEBI:74868; L-TRYPTOPHYL-L-ASPARTIC ACID; (S)-2-((S)-2-Amino-3-(1H-indol-3-yl)Propanamido)succinic acid; Tryptophyl-Aspartate; tryptophylaspartic acid; L-Trp-L-Asp; SCHEMBL10434701; CTK7I5235; WD; ZINC2556664; BDBM50266632
Click to Show/Hide
|
|||
| Activity | IC50 = 5200 nM | |||
| External Link | ||||
| BRL-48482 | Investigative | [55] | ||
| Synonyms |
SCHEMBL1850299; ZYKPNHFCNSVFNF-UHFFFAOYSA-N; 5-[4-[2-[N-(2-benzoxazolyl)-N-methylamino]ethoxy]benzyl]-2,4-thiazolidinedione; 5-[4-[2-(benzoxazol-2-yl-methylamino)ethoxy] benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| nTzDpa | Investigative | [55] | ||
| Synonyms |
118414-59-8; 5-Chloro-1-(4-Chlorobenzyl)-3-(Phenylthio)-1h-Indole-2-Carboxylic Acid; 5-CHLORO-1-[(4-CHLOROPHENYL)METHYL]-3-(PHENYLTHIO)-1H-INDOLE-2-CARBOXYLIC ACID; CHEMBL370152; 1H-Indole-2-carboxylicacid, 5-chloro-1-[(4-chlorophenyl)methyl]-3-(phenylthio)-; NZA; ACMC-1CBOZ; SCHEMBL6709652; GTPL2699; CTK0H3131; DTXSID40433292; MolPort-003-983-790; HMS3268N03; ZINC1492396; BDBM50173365; BS0133; AKOS024456957; NCGC00092313-01; RT-014770; KB-245430; B6955; J-003750; BRD-K54708045-001-01-3
Click to Show/Hide
|
|||
| Activity | IC50 = 26 nM | |||
| External Link | ||||
| COOH | Investigative | [55] | ||
| Synonyms |
LS-191838
Click to Show/Hide
|
|||
| External Link | ||||
| ISIS 105990 | Investigative | [55] | ||
| External Link | ||||
| ISIS 106008 | Investigative | [55] | ||
| External Link | ||||
| CHLOROCYCLINONE A | Investigative | [55] | ||
| Synonyms |
CHEMBL253120; BDBM50228358; methyl 2-chloro-9-ethyl-6,8-dihydroxy-1-methoxy-3-methyl-7,12-dioxo-7,12-dihydrotetraphene-10-carboxylate
Click to Show/Hide
|
|||
| Activity | IC50 = 160 nM | |||
| External Link | ||||
| ISIS 105987 | Investigative | [55] | ||
| External Link | ||||
| L-Tryptophan-L-glutamine | Investigative | [55] | ||
| Synonyms |
CHEMBL476173; SCHEMBL5970454
Click to Show/Hide
|
|||
| Activity | IC50 = 18700 nM | |||
| External Link | ||||
| PAT5A | Investigative | [55] | ||
| Synonyms |
GTPL2709
Click to Show/Hide
|
|||
| External Link | ||||
| GNF-PF-2893 | Investigative | [55] | ||
| Synonyms |
MLS001098045; SMR000657694; ({[4-(dimethylamino)anilino]carbonyl}amino)(4-methylphenyl)dioxo-lambda~6~-sulfane; AC1MDRKI; CHEMBL312032; cid_2815701; BDBM71503; MolPort-002-902-750; ZINC158170; HMS2998H18; CCG-43375; AKOS024379092; MCULE-5924445847; 1-[4-(dimethylamino)phenyl]-3-tosyl-urea; ST51023936; SR-01000633302-1; 1-[4-Dimethylaminophenyl]-3-(4-methylphenylsulfonyl)urea; 1-[4-(dimethylamino)phenyl]-3-(4-methylphenyl)sulfonylurea; 1-(4-dimethylaminophenyl)-3-(4-methylphenyl)sulfonylurea
Click to Show/Hide
|
|||
| External Link | ||||
| L-Tryptophan-L-asparagine | Investigative | [55] | ||
| Synonyms |
CHEMBL513911; 175027-11-9; WN dipeptide; H-Trp-Asn-OH; W-N Dipeptide; L-Tryptophyl-L-Asparagine; Tryptophan Asparagine dipeptide; CHEBI:141447; WN; ZINC2561118; BDBM50266679; (2S)-4-amino-2-{[(2S)-2-amino-3-(1H-indol-3-yl)propanoyl]amino}-4-oxobutanoic acid
Click to Show/Hide
|
|||
| Activity | IC50 = 18400 nM | |||
| External Link | ||||
| L-Tryptophan-L-2-aminoadipic acid | Investigative | [55] | ||
| Synonyms |
CHEMBL515773
Click to Show/Hide
|
|||
| Activity | IC50 = 15100 nM | |||
| External Link | ||||
| L-764406 | Investigative | [55] | ||
| Synonyms |
L 764406; L764406
Click to Show/Hide
|
|||
| External Link | ||||
| L-Tryptophan-L-arginine | Investigative | [55] | ||
| Synonyms |
CHEMBL477417; CHEBI:74866; 88831-09-8; Tryptophyl-Arginine; L-tryptophyl-L-arginine; L-Arginine, L-tryptophyl-; tryptophanyl-arginine; L-Trp-L-Arg; SCHEMBL4947728; CTK3A5775; WR; ZINC2561117; BDBM50266680; AKOS030606594; H-Trp-Arg-OH inverted exclamation mark currency 2 HCl
Click to Show/Hide
|
|||
| Activity | IC50 = 15400 nM | |||
| External Link | ||||
| ISIS 105989 | Investigative | [55] | ||
| External Link | ||||
| [125I]SB-236636 | Investigative | [55] | ||
| Synonyms |
GTPL2710; (2S)-3-(4-{2-[(1,3-benzoxazol-2-yl)(methyl)amino]ethoxy}-3-(125I)iodophenyl)-2-ethoxypropanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| BADGE | Investigative | [55] | ||
| Synonyms |
SCHEMBL7915436; GTPL2708; 2,2-Bis[4-(oxiranyloxy)phenyl]propane
Click to Show/Hide
|
|||
| Activity | IC50 = 100000 nM | |||
| External Link | ||||
| (E)-5-Nitrooctadec-5-enoic Acid | Investigative | [55] | ||
| Synonyms |
CHEMBL561776; (E)-5-Nitro-5-octadecenoic acid; BDBM50295050
Click to Show/Hide
|
|||
| Activity | IC50 = 1680 nM | |||
| External Link | ||||
| CHLOROCYCLINONE C | Investigative | [55] | ||
| Synonyms |
CHEMBL254815; BDBM50228359; methyl 2-chloro-6,8-dihydroxy-9-{1-[(hydroxyacetyl)oxy]ethyl}-1-methoxy-3-methyl-7,12-dioxo-7,12-dihydrotetraphene-10-carboxylate
Click to Show/Hide
|
|||
| Activity | EC50 = 130 nM | |||
| External Link | ||||
| CHLOROCYCLINONE B | Investigative | [55] | ||
| Synonyms |
CHEMBL254814; BDBM50228360; methyl 9-[1-(acetyloxyethyl)]-2-chloro-6,8-dihydroxy-1-methoxy-3-methyl-7,12-dioxo-7,12-dihydrotetraphene-10-carboxylate
Click to Show/Hide
|
|||
| Activity | EC50 = 160 nM | |||
| External Link | ||||
| GW0072 | Investigative | [55] | ||
| Synonyms |
GW 0072
Click to Show/Hide
|
|||
| External Link | ||||
| [3H]GW2331 | Investigative | [55] | ||
| Synonyms |
GTPL2706; 2-[4-[2-[(2,4-difluoro-6-tritiophenyl)carbamoyl-heptylamino]ethyl]phenoxy]-2-methylbutanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| CHLOROCYCLINONE D | Investigative | [55] | ||
| Synonyms |
CHEMBL400132; BDBM50228361
Click to Show/Hide
|
|||
| Activity | EC50 = 260 nM | |||
| External Link | ||||
| (E)-10-Nitrohexadec-9-enoic Acid | Investigative | [55] | ||
| Synonyms |
CHEMBL564368; 10-nitropalmitoleic acid; E-10-nitropalmitoleic acid; SCHEMBL15633718; WZWPBDQYCAJWSX-FYWRMAATSA-N
Click to Show/Hide
|
|||
| Activity | IC50 = 630 nM | |||
| External Link | ||||
| L-Tryptophan-L-leucine | Investigative | [55] | ||
| Synonyms |
H-Trp-Leu-OH; 13123-35-8; L-tryptophyl-L-leucine; CHEMBL477627; CHEBI:74871; Tryptophyl-Leucine; L-Trp-L-Leu; L-Trp-L-Leu-OH; L-Leucine,L-tryptophyl-; AC1OE28P; SCHEMBL7622341; (S)-2-((S)-2-Amino-3-(1H-indol-3-yl)propanamido)-4-methylpentanoic acid; CTK4B7168; ZINC1865984; WL; BDBM50266681; AKOS022180848; AJ-32151; FT-0772989; C-48470; (2S)-2-[[(2S)-2-amino-3-(1H-indol-3-yl)propanoyl]amino]-4-methylpentanoic acid
Click to Show/Hide
|
|||
| Activity | IC50 = 25800 nM | |||
| External Link | ||||
| PD-068235 | Investigative | [55] | ||
| Synonyms |
CHEMBL455856; BDBM50266362; PD-068253
Click to Show/Hide
|
|||
| Activity | IC50 = 820 nM | |||
| External Link | ||||
| Ploglitazone | Investigative | [55] | ||
| External Link | ||||
| (E)-6-Nitrooctadec-5-enoic Acid | Investigative | [55] | ||
| Synonyms |
CHEMBL569372
Click to Show/Hide
|
|||
| Activity | IC50 = 1720 nM | |||
| External Link | ||||
| AD-5061 | Investigative | [55] | ||
| Synonyms |
AD-7057; AD7057; AD 7057; AD5061; AD 5061
Click to Show/Hide
|
|||
| External Link | ||||
| TZD18 | Investigative | [55] | ||
| Synonyms |
TZD 18; TZD-18
Click to Show/Hide
|
|||
| External Link | ||||
| (9Z,12E)-12-nitrooctadeca-9,12-dienoic acid | Investigative | [55] | ||
| Synonyms |
12-Nitrolinoleic acid; 12-Nitro-9Z,12Z-octadecadienoic acid; CHEMBL554608; CHEBI:34150; AC1NQZV1; C13958; SCHEMBL2371323; BDBM50295045; LMFA01120002; 12-Nitro-9-cis,12-cis-octadecadienoic acid; (9Z,12Z)-12-Nitrooctadeca-9,12-dienoic acid
Click to Show/Hide
|
|||
| Activity | EC50 = 45 nM | |||
| External Link | ||||
| reglitazar | Investigative | [55] | ||
| Synonyms |
JTT-501
Click to Show/Hide
|
|||
| External Link | ||||
| DRF 2519 | Investigative | [55] | ||
| Synonyms |
5-[[4-[2-(4-Oxo-2H-1,3-benzoxazin3(4H)-yl)ethoxy]phenyl]methyl2,4-thiazolidinedione; SCHEMBL6953746; GTPL2671; CHEMBL1491825; DTXSID5040754; NOCAS_40754; CTK8F9377; API0008459; NCGC00165785-01; NCGC00164420-01; SR-05000000444; SR-05000000444-2
Click to Show/Hide
|
|||
| External Link | ||||
| (E)-12-Nitrooctadec-12-enoic Acid | Investigative | [55] | ||
| Synonyms |
CHEMBL549351; BDBM50295043; (E)-12-Nitro-12-octadecenoic acid
Click to Show/Hide
|
|||
| Activity | IC50 = 39 nM | |||
| External Link | ||||
| (E)-13-Nitrooctadec-12-enoic Acid | Investigative | [55] | ||
| Synonyms |
CHEMBL540732
Click to Show/Hide
|
|||
| Activity | IC50 = 190 nM | |||
| External Link | ||||
| GNF-PF-3037 | Investigative | [55] | ||
| Synonyms |
SMR000116549; MLS000526075; Oprea1_099756; Oprea1_432483; Oprea1_261700; CHEMBL600336; cid_1160447; BDBM40315; MolPort-001-513-008; MolPort-001-991-899; C23H16N4O2S2; HMS2507A20; ZINC8671257; STK223484; AKOS003264843; AKOS000636670; MCULE-7239362503; BAS 03422558; MLS-0072644.0001; N-[4-[6-(2-thenoylamino)-1H-benzimidazol-2-yl]phenyl]thiophene-2-carboxamide; N-(4-{5-[(thiophen-2-ylcarbonyl)amino]-1H-benzimidazol-2-yl}phenyl)thiophene-2-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| LY-465608 | Investigative | [55] | ||
| Synonyms |
LY465608; LY 465608
Click to Show/Hide
|
|||
| Activity | IC50 = 548 nM | |||
| External Link | ||||
| 2-chloro-5-nitro-N-phenylbenzamide | Investigative | [50] | ||
| Synonyms |
GW9662; 22978-25-2; GW 9662; 2-Chloro-5-nitrobenzanilide; GW-9662; MLS001056751; CHEBI:79993; 2-Chloro-5-nitro-N-phenyl-benzamide; 2-Chloro-5-nitro-N-4-phenylbenzamide; benzamide, 2-chloro-5-nitro-N-phenyl-; SMR000326735; (2-chloro-5-nitrophenyl)-N-benzamide; SR-01000075999; Tocris-1508; Spectrum5_001989; Lopac-M-6191; AC1LD8S0; DSSTox_RID_79570; DSSTox_CID_20723; DSSTox_GSID_40723; Lopac0_000798; KBioGR_000361; KBioSS_000361; BSPBio_001021; SCHEMBL420231; CHEMBL375270; GTPL3442; cid_644213
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 2 nM | |||
| External Link | ||||
| L-796449 | Investigative | [55] | ||
| Synonyms |
UNII-O937X0Z5EM; CHEMBL278994; O937X0Z5EM; 194608-80-5; KAPDPGZDHUCILF-UHFFFAOYSA-N; GTPL2689; SCHEMBL4296303; BDBM50085040; Benzeneacetic acid, 3-chloro-4-((3-((3-phenyl-7-propyl-6-benzofuranyl)oxy)propyl)thio)-; L796449; L 796449; L-796,449; 3-chloro-4-(3-(3-phenyl-7-propylbenzofuran-6-yloxy)propylthio)-phenylacetic acid; {3-Chloro-4-[3-(3-phenyl-7-propyl-benzofuran-6-yloxy)-propylsulfanyl]-phenyl}-acetic acid; 2-[3-chloro-4-[3-[(3-phenyl-7-propyl-1-benzofuran-6-yl)oxy]propylsulfanyl]phenyl]acetic acid
Click to Show/Hide
|
|||
| Activity | EC50 = 5.2 nM | |||
| External Link | ||||
| L-165461 | Investigative | [55] | ||
| Synonyms |
CHEMBL279053; SCHEMBL6753428; GTPL2690; BDBM50126016; AKOS027321335; L165461; L 165461; 3-Chloro-4-[3-(3-ethyl-7-propyl-1,2-benzisoxazole-6-yloxy)propylthio]benzeneacetic acid; 2-(3-chloro-4-(3-(3-ethyl-7-propylbenzo[d]isoxazol-6-yloxy)propylthio)phenyl)acetic acid; {3-Chloro-4-[3-(3-ethyl-7-propyl-benzo[d]isoxazol-6-yloxy)-propylsulfanyl]-phenyl}-acetic acid; 2-[3-chloro-4-[3-[(3-ethyl-7-propyl-1,2-benzoxazol-6-yl)oxy]propylsulfanyl]phenyl]acetic acid
Click to Show/Hide
|
|||
| Activity | EC50 = 19 nM | |||
| External Link | ||||
| L-783483 | Investigative | [55] | ||
| Synonyms |
F3MethylAA
Click to Show/Hide
|
|||
| Activity | EC50 = 23 nM | |||
| External Link | ||||
| LG100754 | Investigative | [55] | ||
| Synonyms |
LG 100754; LG754
Click to Show/Hide
|
|||
| External Link | ||||
References