m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05647
|
[1] | |||
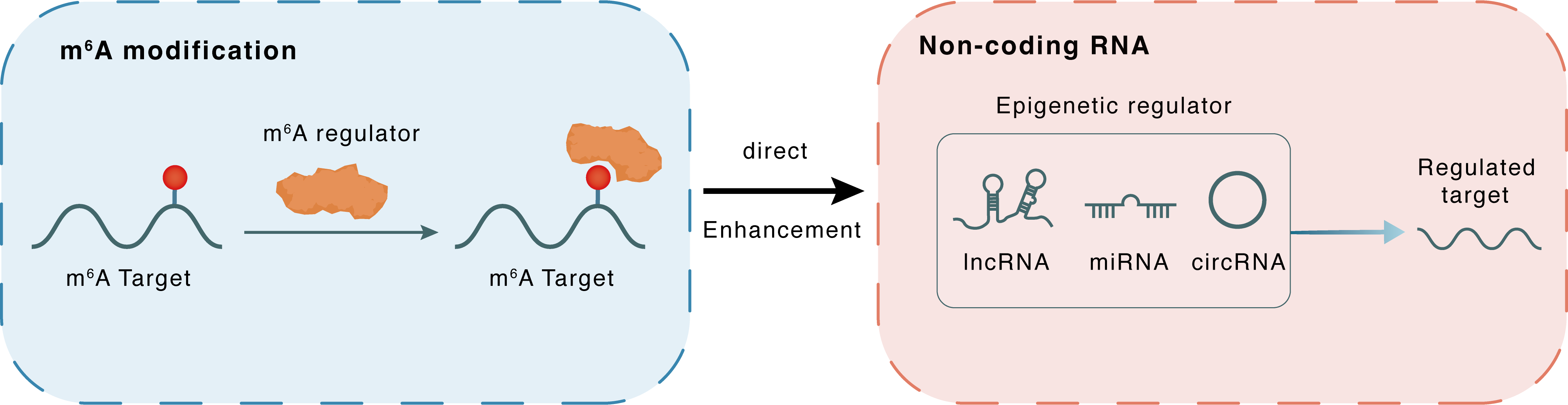 m6A modification
PVT1
PVT1
YTHDC1
m6A modification
PVT1
PVT1
YTHDC1
 : m6A sites
Direct
Enhancement
Non-coding RNA
PVT1
IL33
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
PVT1
IL33
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing protein 1 (YTHDC1) | READER | |||
| m6A Target | Pvt1 oncogene (PVT1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Pvt1 oncogene (PVT1) | LncRNA | View Details | ||
| Regulated Target | Interleukin-33 (IL33) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | Pvt1 oncogene (PVT1) positively regulated Interleukin-33 (IL33) expression by recruiting YTHDC1 to mediate m6A modification of IL-33. In conclusion, silencing PVT1 demonstrated beneficial effects in alleviating BPD by facilitating YTHDC1-mediated m6A modification of IL-33. | ||||
| Responsed Disease | Chronic respiratory disease originating in the perinatal period | ICD-11: KB29.0 | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Cell Process | Cell apoptosis | ||||
In-vitro Model |
MLE-12
|
N.A. | Mus musculus | CVCL_3751 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| In-vivo Model | Before hyperoxia treatment, mice in the BPD/PVT1 KO group were intratracheally instilled with 5 μL adenovirus vector expressing sh-PVT1 at a titer of 1 × 109 pfu/100 μL. Mice in the BPD/PVT1 KO + IL-33 group were intratracheally instilled with 5 μL adenovirus vector expressing sh-PVT1 and 5 μL adenovirus vector expressing IL-33. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Interleukin-33 (IL33) | 6 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Tozorakimab | Phase 3 | [2] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| SAR440340 | Phase 3 | [3] | ||
| Synonyms |
Itepekimab
Click to Show/Hide
|
|||
| External Link | ||||
| MEDI3506 | Phase 3 | [4] | ||
| Synonyms |
Tozorakimab
Click to Show/Hide
|
|||
| External Link | ||||
| Itepekimab | Phase 3 | [3] | ||
| External Link | ||||
| AMG 282 | Phase 1 | [5] | ||
| External Link | ||||
| PF-07264660 | Phase 1 | [6] | ||
| External Link | ||||
References