m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05595
|
[1] | |||
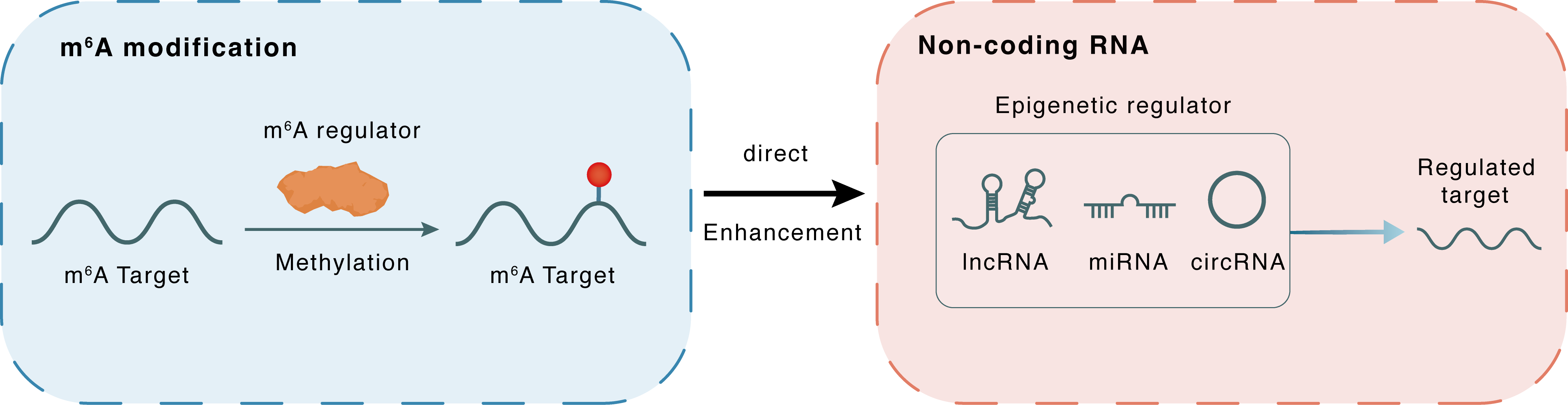 m6A modification
MIR126
MIR126
METTL3
Methylation
m6A modification
MIR126
MIR126
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR126
Regulated Target
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR126
Regulated Target
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | microRNA 126 (MIR126) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | MicroRNA 126 (MIR126) | microRNA | View Details | ||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | The METTL3/m6A/microRNA 126 (MIR126) pathway, whose inhibition contributes to endometriosis development by enhancing cellular migration and invasion. | ||||
| Responsed Disease | Endometriosis | ICD-11: GA10 | |||
| Cell Process | Cell migration | ||||
| Cell invasion | |||||
In-vitro Model |
HESC (Human endometrial stromal cells) | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| GA10: Endometriosis | 32 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Elagolix sodium | Approved | [2] | ||
| Synonyms |
Elagolix sodium salt; UNII-5948VUI423; Elagolix sodium [USAN]; 5948VUI423; Elagolix sodium (USAN); SCHEMBL1641994; NBI 56418NA; NBI-56418-NA; MolPort-003-984-965; NBI-56418 NA; DQYGXRQUFSRDCH-UQIIZPHYSA-M; AKOS030524154; VA12044; KS-0000063K; KB-76766; HY-14369; AC-29671; CS-0003317; D09336
Click to Show/Hide
|
|||
| External Link | ||||
| Histrelin | Approved | [3] | ||
| Synonyms |
Supprelin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Relugolix | Phase 3 | [4] | ||
| Synonyms |
737789-87-6; TAK-385; TAK 385; UNII-P76B05O5V6; CHEMBL1800159; TAK-385/TAK385; P76B05O5V6; 1-(4-(1-(2,6-difluorobenzyl)-5-((dimethylamino)methyl)-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl)phenyl)-3-methoxyurea; Relugolix [USAN:INN]; TAK385; Relugolix (JAN/INN); SCHEMBL778416; GTPL5586; DTXSID40224167; MolPort-044-567-649; AOMXMOCNKJTRQP-UHFFFAOYSA-N; EX-A1083; BCP21587; ZINC43206033; BDBM50347982; AKOS027440398; SB16721; DB11853; CS-5917
Click to Show/Hide
|
|||
| External Link | ||||
| Naproxen | Approved | [5] | ||
| Synonyms |
22204-53-1; (S)-Naproxen; Naproxene; Naprosyn; (+)-Naproxen; Equiproxen; Laraflex; Naproxenum; Naproxeno; d-Naproxen; (S)-(+)-2-(6-Methoxy-2-naphthyl)propionic acid; (S)-(+)-Naproxen; Calosen; Nycopren; Naprosyne; Bonyl; Reuxen; Naixan; Axer; (+)-(S)-Naproxen; Ec-Naprosyn; (S)-2-(6-methoxynaphthalen-2-yl)propanoic acid; Flexipen; Clinosyn; Artrixen; Anexopen; Acusprain; Novonaprox; Arthrisil; Leniartil; Danaprox; Bipronyl; Artroxen; Napren; Naposin; Napflam; Genoxen; Daprox; Atiflan; Artagen; Apronax; Naprius; Nalyxan; Lefaine; Congex
Click to Show/Hide
|
|||
| External Link | ||||
| Nafarelin | Approved | [6] | ||
| Synonyms |
Nafarelina; Nafareline; Nafarelinum; Synarel; NAFARELIN ACETATE; Nafarelina [Spanish]; Nafareline [French]; Nafarelinum [Latin]; HS-2018; Nafarelin (INN); Nafarelin Acetate, Hydrate; Nafarelin [INN:BAN]; Synarel (TN); RS-94991-298; 6-(3-(2-Naphthalenyl)-D-alanine)luteinizing hormone-releasing factor (pig)
Click to Show/Hide
|
|||
| External Link | ||||
| Oestradiol valerate and dienogest | Approved | [7] | ||
| Synonyms |
Dienogest; 65928-58-7; Dienogestrel; Dienogestum; Dienogestril; Dinagest; Natazia; Endometrion; Visanne; STS-557; STS 557; Dienogestum [Latin]; UNII-46M3EV8HHE; MJR-35; 17alpha-17-Hydroxy-3-oxo-19-norpregna-4,9-diene-21-nitrile; BAY86-5258; BAY 86-5258; 46M3EV8HHE; CHEBI:70708; 17-alpha-Cyanomethyl-17-beta-hydroxy-estra-4,9(10)-dien-3-one; 2-[(8S,13S,14S,17R)-17-hydroxy-13-methyl-3-oxo-1,2,6,7,8,11,12,14,15,16-decahydrocyclopenta[a]phenanthren-17-yl]acetonitrile; 17-alpha-Cyanomethyl-17-beta-hydroxyestra-4,9(10)-diene-3-one; Natazia (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| TZTX-001 | Phase 3 | [8] | ||
| Synonyms |
Teverelix LA; TX 12-001-HR
Click to Show/Hide
|
|||
| External Link | ||||
| Nestorone | Phase 3 | [9] | ||
| Synonyms |
Nestorone MDTS; Nestorone metered dose transdermal system; Nestorone transdermal spray, Acrux; ST-1435; Nestorone transdermal spray, Population Council/Acrux
Click to Show/Hide
|
|||
| External Link | ||||
| Telapristone | Phase 2 | [10] | ||
| Synonyms |
Telapristone acetate; Proellex; 198414-31-2; UNII-1K9EYK92PQ; CDB-4124; 1K9EYK92PQ; Telapristone acetate [USAN]; Telapristone acetate (USAN); CCRIS 9331; CDB 4124; SCHEMBL374762; CHEMBL2105694; DTXSID60173587; RU-44675; 17alpha-Acetoxy-11beta-(4-(dimethylamino)phenyl)-21-methoxy-19-norpregna-4,9-dien-3,20-dione; D09972; 19-Norpregna-4,9-diene-3,20-dione, 17-(acetyloxy)-11-(4-(dimethylamino)phenyl)-21-methoxy-, (11beta)-; A-Acetoxy-21-methoxy-11
Click to Show/Hide
|
|||
| External Link | ||||
| F-8-IL-10 fusion protein | Phase 2 | [11] | ||
| Synonyms |
Dekavil; F-8-IL-10; F-8-IL-10 fusion protein (rheumatoid arthritis/endometriosis); F-8-IL-10 fusion protein (rheumatoid arthritis/endometriosis), Philogen
Click to Show/Hide
|
|||
| External Link | ||||
| PGL-2 | Phase 2 | [12] | ||
| External Link | ||||
| BAY 98-7196 | Phase 2 | [13] | ||
| External Link | ||||
| KLH-2109 | Phase 2 | [14] | ||
| External Link | ||||
| Vilaprisan | Phase 2 | [15] | ||
| Synonyms |
UNII-IN59K53GI9; BAY1002670; IN59K53GI9; 1262108-14-4; Vilaprisan [INN]; BAY 1002670; Vilaprisan [USAN:INN]; Vilaprisan (USAN/INN); SCHEMBL2121854; CHEMBL3989936; BCP24069; DB11971; 20,20,21,21,21-Pentafluoro-17-hydroxy-11beta-(4-(methanesulfonyl)phenyl)-19-nor-17alpha-pregna-4,9-dien-3-one; 19-Norpregna-4,9-dien-3-one, 20,20,21,21,21-pentafluoro-17-hydroxy-11-(4-(methylsulfonyl)phenyl)-, (11beta,17alpha)-
Click to Show/Hide
|
|||
| External Link | ||||
| PGL-2001 | Phase 2 | [16] | ||
| Synonyms |
Steroid sulfatase inhibitor (endometriosis), PregLem
Click to Show/Hide
|
|||
| External Link | ||||
| BGS-649 | Phase 2 | [17] | ||
| External Link | ||||
| ASP-1707 | Phase 2 | [18] | ||
| External Link | ||||
| BAY 1817080 | Phase 1 | [19] | ||
| Synonyms |
Eliapixant
Click to Show/Hide
|
|||
| External Link | ||||
| Alfa-interferon | Phase 1 | [20] | ||
| External Link | ||||
| PF-4418948 | Phase 1 | [21] | ||
| Synonyms |
PF-04418948; Prostaglandin E2 EP2 subtype antagonist (endometriosis), Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-1817080 | Phase 1 | [22] | ||
| External Link | ||||
| PF-2413873 | Phase 1 | [23] | ||
| Synonyms |
PF-02413873
Click to Show/Hide
|
|||
| External Link | ||||
| BAY 1026153 | Phase 1 | [24] | ||
| External Link | ||||
| PMID27215781-Compound-28 | Patented | [25] | ||
| External Link | ||||
| Asoprisnil | Discontinued in Phase 3 | [26] | ||
| Synonyms |
J867; J-867; Asoprisnil (USAN/INN); 11beta-(4-((E)-(Hydroxyimino)methyl)phenyl)-17beta-methoxy-17-(methoxymethyl)estra-4,9-dien-3-one
Click to Show/Hide
|
|||
| External Link | ||||
| FP-1096 | Discontinued in Phase 3 | [27] | ||
| External Link | ||||
| ASP-0265 | Terminated | [28] | ||
| External Link | ||||
| NS398 | Terminated | [29] | ||
| Synonyms |
ns-398; 123653-11-2; NS 398; N-(2-Cyclohexyloxy-4-nitrophenyl)methanesulfonamide; N-[2-(Cyclohexyloxy)-4-nitrophenyl]methanesulfonamide; CHEMBL7162; CHEBI:73458; Methanesulfonamide, N-(2-(cyclohexyloxy)-4-nitrophenyl)-; n-(2-cyclohexyloxy-4-nitrophenyl)methane sulfonamide; N-(2-Cyclohexyloxy-4-nitro-phenyl)-methanesulfonamide; Taisho NS 398; SR-01000597479; N-[2-Cyclohexyloxy-4-nitrophenyl]methanesulfonamide; N-(2-(cyclohexyloxy)-4-nitrophenyl)methanesulfonamide; CCRIS 8523; KTDZCOWXCWUPEO-UHFFFAOYSA-N; Tocris-0942
Click to Show/Hide
|
|||
| External Link | ||||
| LHRH | Investigative | [30] | ||
| Synonyms |
SCHEMBL7378993
Click to Show/Hide
|
|||
| External Link | ||||
| PF-02367982 | Investigative | [31] | ||
| Synonyms |
CHEMBL1083754; BDBM50318934
Click to Show/Hide
|
|||
| External Link | ||||
| Recombinant human CC10 | Investigative | [32] | ||
| Synonyms |
Claragen-WH; Recombinant human CC10 (intravaginal, endometriosis/infertility); Recombinant human CC10 (intravaginal, endometriosis/infertility), Clarassance; Uteroglobin (intravaginal, endometriosis/infertility), Clarassance
Click to Show/Hide
|
|||
| External Link | ||||
| DXL-1215 | Investigative | [32] | ||
| Synonyms |
Monoclonal antibody (endometriosis), InNexus
Click to Show/Hide
|
|||
| External Link | ||||
References