m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05449
|
[1] | |||
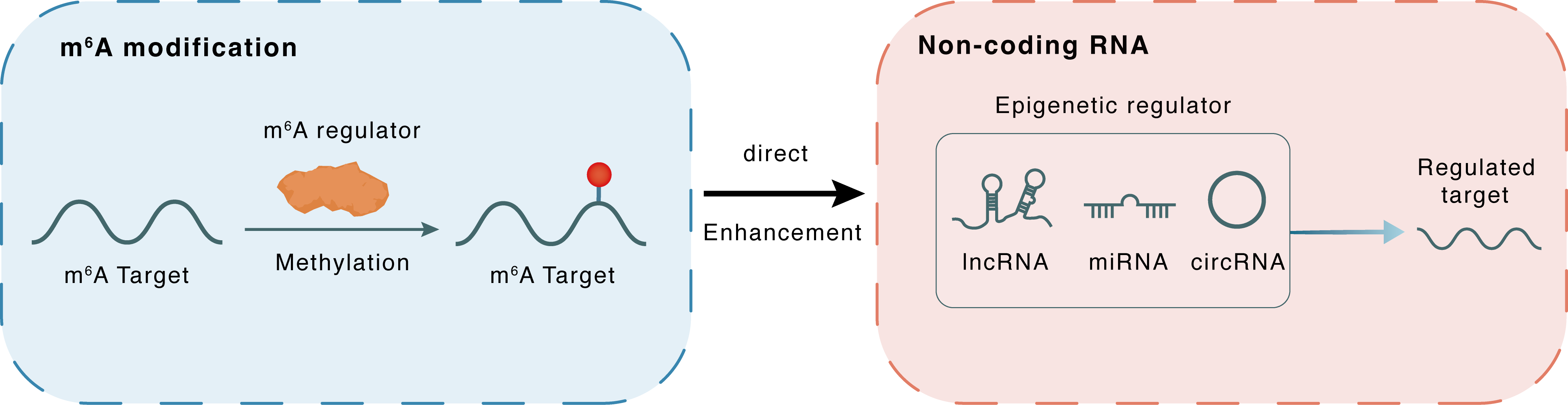 m6A modification
hsa-miR-126-5p
hsa-miR-126-5p
METTL3
Methylation
m6A modification
hsa-miR-126-5p
hsa-miR-126-5p
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR-126-5p
PIK3R2
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR-126-5p
PIK3R2
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | hsa-miR-126-5p | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-126-5p | microRNA | View Details | ||
| Regulated Target | Phosphatidylinositol 3-kinase regulatory subunit beta (PI3K-p85/PIK3R2) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | Interleukin 1-beta (IL-1-beta) is an important inducer of cartilage degeneration that can induce an inflammatory cascade reaction in chondrocytes and inhibit the normal biological function of cells. METTL3 could regulate hsa-miR-126-5p maturation, we first confirmed that METTL3 can bind the key protein underlying pri-miRNA processing, DGCR8. Additionally, when METTL3 expression was inhibited, the miR-126-5p maturation process was blocked. miR-126-5p can inhibit the PI3K/Akt signalling pathway by targeting Phosphatidylinositol 3-kinase regulatory subunit beta (PI3K-p85/PIK3R2) gene, leading to the disorder of cell vitality and functional metabolism. | ||||
| Responsed Disease | Chondropathies | ICD-11: FB82 | |||
| Cell Process | RNA mature | ||||
In-vitro Model |
Cartilage cells (From the cartilage tissue samples from patients) | ||||