m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05434
|
[1] | |||
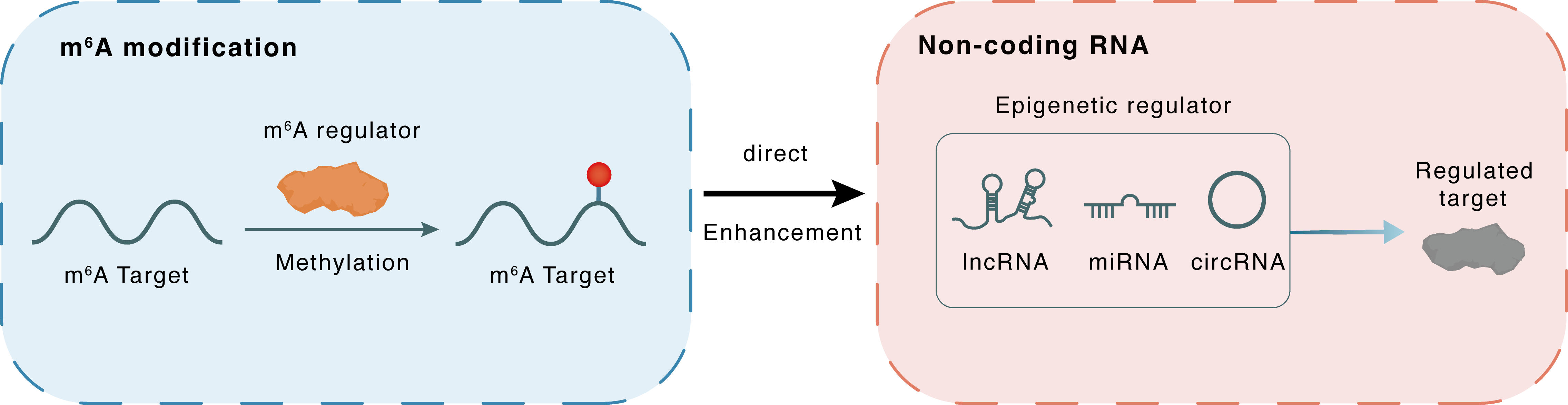 m6A modification
MIR335
MIR335
METTL3
Methylation
m6A modification
MIR335
MIR335
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR-335
ZFP36L1
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR-335
ZFP36L1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | microRNA 335 (MIR335) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | MicroRNA 335 (MIR335) | microRNA | View Details | ||
| Regulated Target | ZFP36 ring finger protein like 1 (ZFP36L1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | METTL3-mediated m6A methylation increases the maturation of microRNA 335 (MIR335), which promotes SG formation and reduces the apoptosis level of injury neurons and cells, and provides a potential therapeutic strategy for acute ischemic stroke. miR-335 enhanced SG formation through degradation of the mRNA of the ZFP36 ring finger protein like 1 (ZFP36L1). | ||||
| Responsed Disease | Acute ischemic stroke | ICD-11: 8B11 | |||
| Pathway Response | Apoptosis | hsa04210 | |||
| Cell Process | RNA maturation | ||||
| Cell apoptosis | |||||
In-vitro Model |
PC12 | Rat adrenal gland pheochromocytoma | Rattus norvegicus | CVCL_0481 | |
| In-vivo Model | After a median incision on the neck, the left common carotid artery (CCA), internal carotid artery (ICA), and external carotid artery (ECA) were isolated. The left CCA and the ECA were ligated. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 8B11: Acute ischemic stroke | 7 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| PEG-bHb-CO | Phase 2 | [2] | ||
| Synonyms |
Sanguinate; Oxygen transfer agent (trauma/cardiovascular disease), Prolong Pharmaceuticals; PEG-bHb-CO (trauma/cardiovascular disease); PEGylated bovine hemoglobin-carbon monoxide (trauma/cardiovascular disease), Prolong Pharmaceuticals; PEG-bHb-CO (trauma/cardiovascular disease), Prolong Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| DM199 | Phase 2 | [3] | ||
| External Link | ||||
| BIIB131 | Phase 2 | [4] | ||
| Synonyms |
(2S)-2,5-bis[(2S,3S)-2-[(3E)-4,8-dimethylnona-3,7-dienyl]-3,5-dihydroxy-2-methyl-7-oxo-4,9-dihydro-3H-pyrano[2,3-e]isoindol-8-yl]pentanoic acid; 733805-92-0; BCP33210; BIIB131; CS-0083560; GTPL12300; HY-122311; Orniplabin; SCHEMBL2624852; SMTP 7; SMTP7; SMTP7; SMTP-7; Stachybotrys microspora triprenyl phenol 7; TMS-007
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986177 | Phase 2 | [5] | ||
| Synonyms |
Milvexian; UNII-0W79NDQ608; Milvexian (USAN); Milvexian [USAN]; BMS 986177; JNJ-70033093; 1802425-99-5; CHEMBL4112929; SCHEMBL16982989; WHO 11401; D11802; (5R,9S)-9-(4-(5-Chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl)-6-oxopyrimidin-1(6H)-yl)-21-(difluoromethyl)-5-methyl-21H-3-aza-1(4,2)-pyridina-2(5,4)-pyrazolacyclonaphan-4-one; (9R,13S)-13-{4-[5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl]-6-oxo-1,6-dihydropyrimidin-1-yl}-3-(difluoromethyl)-9-methyl-3,4,7,15-tetraazatricyclo[12.3.1.02,6]octadeca-1(18),2(6),4,14,16-pentaen-8-one; 11,15-Metheno-15H-pyrazolo(4,3-b)(1,7)diazacyclotetradecin-5(6H)-one, 10-(4-(5-chloro-2-(4-chloro-1H-1,2,3-triazol-1-yl)phenyl)-6-oxo-1(6H)-pyrimidinyl)-1-(difluoromethyl)-1,4,7,8,9,10-hexahydro-6-methyl-, (6R,10S)-2
Click to Show/Hide
|
|||
| External Link | ||||
| SonoLysis Prolyse | Phase 2 | [6] | ||
| External Link | ||||
| ACT017 | Phase 1/2 | [7] | ||
| External Link | ||||
| Dimethoxybenzylidene-2-thio-imidazole-4-one derivative 1 | Patented | [8] | ||
| Synonyms |
PMID27998201-Compound-16
Click to Show/Hide
|
|||
| External Link | ||||
References