m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05307
|
[1] | |||
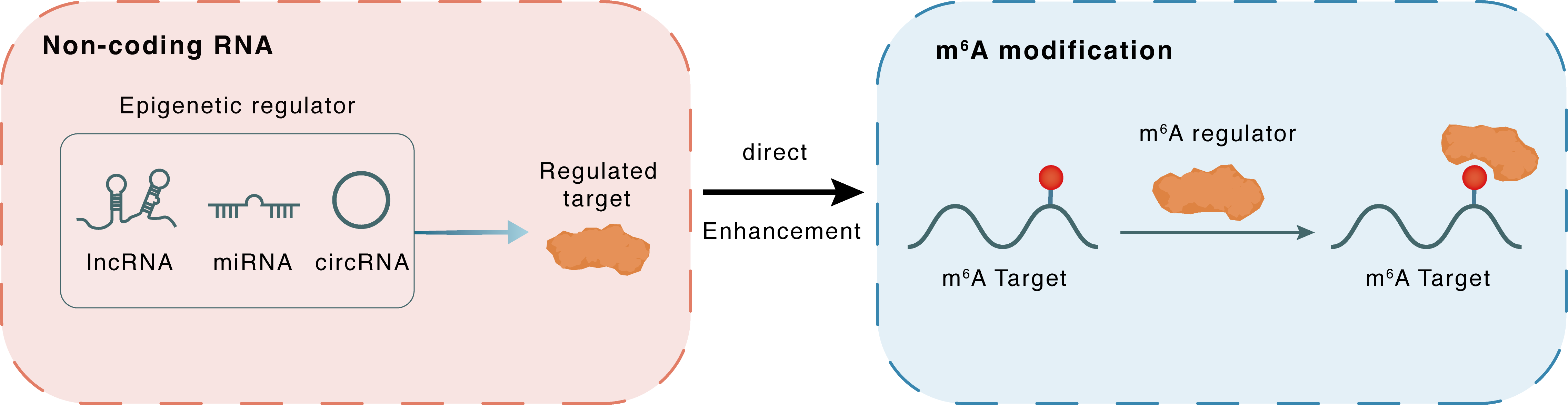 Non-coding RNA
A1BG-AS1
IGF2BP2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
ABCB1
ABCB1
IGF2BP2
Non-coding RNA
A1BG-AS1
IGF2BP2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
ABCB1
ABCB1
IGF2BP2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) | READER | |||
| m6A Target | ATP-dependent translocase ABCB1 (ABCB1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | A1BG antisense RNA 1 (A1BG-AS1) | LncRNA | View Details | ||
| Regulated Target | Insulin like growth factor 2 mRNA binding protein 2 (IGF2BP2) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | A1BG-AS1 promotes adriamycin resistance of breast cancer by recruiting IGF2BP2 to upregulate ATP-dependent translocase ABCB1 (ABCB1) in an m6A-dependent manner | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Responsed Drug | Adriamycin | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| ATP-dependent translocase ABCB1 (ABCB1) | 18 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| EDP-322 | Phase 1 | [2] | ||
| Synonyms |
EP-16322; Antibacterial (oral, Gram-positive infections), Enanta; EDP-MRSA-1 (oral), Enanta
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| W-198 | Phase 1 | [3] | ||
| Synonyms |
5-Bromotetrandrine
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| CBT-1 | Application submitted | [4] | ||
| MOA | Modulator | |||
| External Link | ||||
| LY335979 | Discontinued in Phase 3 | [5] | ||
| Synonyms |
Zosuquidar HCl; Zosuquidar Trihydrochloride; LY 335979; LY-335979; Zosuquidar (TN); Zosuquidar trihydrochloride (USAN); RS-33295-198; Zosuquidar trihydrochloride, RS-33295-198, LY335979; (R)-4-((1aR,6R,10bS)-1,2-Difluoro-1,1a,6,10b-tetrahydrodibenzo(a,e)cyclopropa(c)cycloheptan-6-yl)-alpha-((5-quinoloyloxy)methyl)-1-piperazineethanol, trihydrochloride
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 3490 nM | |||
| External Link | ||||
| Biricodar | Discontinued in Phase 2 | [6] | ||
| Synonyms |
Incel; Biricodar [INN]; Vx 710; Incel (TN); 1,7-dipyridin-3-ylheptan-4-yl (2S)-1-[2-oxo-2-(3,4,5-trimethoxyphenyl)acetyl]piperidine-2-carboxylate
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Tariquidar | Discontinued in Phase 2 | [7] | ||
| Synonyms |
Tariquidarth; XR 9576; XR9576; Tariquidar (USAN/INN); N-[2-[[4-[2-(6,7-dimethoxy-3,4-dihydro-1H-isoquinolin-2-yl)ethyl]phenyl]carbamoyl]-4,5-dimethoxyphenyl]quinoline-3-carboxamide; N-(2-((4-(2-(6,7-dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)ethyl)phenyl)carbamoyl)-4,5-dimethoxyphenyl)quinoline-3-carboxamide
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 0.66 nM | |||
| External Link | ||||
| S-9788 | Discontinued in Phase 2 | [8] | ||
| Synonyms |
S9788; S 9788; CHEMBL85156; 140945-01-3; 6-(4-(2,2-di(4-Fluorophenyl)ethylamino)-1-piperidinyl)-N,N'-di-2-propenyl-1,3,5-triazine-2,4-diamine; 1,3,5-Triazine-2,4-diamine, 6-(4-((2,2-bis(4-fluorophenyl)ethyl)amino)-1-piperidinyl)-N,N'-di-2-propenyl-; AC1L32YH; SCHEMBL1527824; DTXSID80161502; GERNFWKTMKWULM-UHFFFAOYSA-N; BDBM50053888; LS-187603; LS-186957; N,N''-Diallyl-6-{4-[2,2-bis-(4-fluoro-phenyl)-ethylamino]-piperidin-1-yl}-[1,3,5]triazine-2,4-diamine; H2O
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| XR-9051 | Discontinued in Phase 1 | [9] | ||
| Synonyms |
Multidrug resistance inhibitor, Xenova
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Elacridar | Discontinued in Phase 1 | [10] | ||
| Synonyms |
Elacridar hydrochloride; 143851-98-3; Elacridar HCl; Elacridar (hydrochloride); gf 120918a; UNII-NX2BHH1A5B; NX2BHH1A5B; Elacridar hydrochloride [USAN]; GF-120918A; Elacridar hydrochloride (USAN); GF 120918; AC1Q3EOG; AC1L55DX; C34H34ClN3O5; SCHEMBL15847793; CHEMBL2074730; AOB5938; MolPort-023-332-877; BCP14056; 7066AA; AKOS016005297; CS-1113; ACN-041487; 4CA-0489; HY-50880; AC-30266; FT-0696337; W-5457; D03968; N-[4-[2-(3,4-Dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)ethyl]phenyl]-9,10-dihydro-5-methoxy-9-oxo-4-acridinecarboxamide hydro
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 2000 nM | |||
| External Link | ||||
| ONT-093 | Discontinued in Phase 1 | [11] | ||
| Synonyms |
MDR inhibitors, Ontogen; OC-144-093
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 31.62 nM | |||
| External Link | ||||
| 6-(3,5-dimethoxy-phenyl)-naphthalen-2-ol | Investigative | [12] | ||
| Synonyms |
CHEMBL362997; BDBM50186758
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 16000 nM | |||
| External Link | ||||
| [1,1':2',1'']-terphenyl-4,3'',5''-triol | Investigative | [12] | ||
| Synonyms |
CHEMBL208098; BDBM50186757; ZINC35856323
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 12000 nM | |||
| External Link | ||||
| XR-9456 | Investigative | [13] | ||
| Synonyms |
CHEMBL346292; SCHEMBL7170174; BDBM50375810
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 251.19 nM | |||
| External Link | ||||
| XR-9504 | Investigative | [13] | ||
| Synonyms |
CHEMBL258896; SCHEMBL7168501
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 524.81 nM | |||
| External Link | ||||
| XR-9544 | Investigative | [13] | ||
| Synonyms |
CHEMBL444075; SCHEMBL14290581; BDBM50375811
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 165.96 nM | |||
| External Link | ||||
| 4,3'',5''-trimethoxy-[1,1':2',1'']-terphenyl | Investigative | [12] | ||
| Synonyms |
CHEMBL379687; BDBM50186763
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 16000 nM | |||
| External Link | ||||
| XR-9577 | Investigative | [13] | ||
| Synonyms |
CHEMBL428402
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 354.81 nM | |||
| External Link | ||||
| TRISMETHOXYRESVERATROL | Investigative | [12] | ||
| Synonyms |
22255-22-7; trans-Trimethoxyresveratrol; (E)-1,3-Dimethoxy-5-(4-methoxystyryl)benzene; (E)-3,5,4'-Trimethoxystilbene; 3,4',5-trimethoxy-trans-stilbene; 3,4',5-trimethoxystilbene; 3,5,4'-trimethoxystilbene; TRIMETHOXYSTILBENE; E-Resveratrol trimethyl ether; CHEMBL296411; 1,3-dimethoxy-5-[(E)-2-(4-methoxyphenyl)ethenyl]benzene; trans-3,4',5-trimethoxystilbene; GDHNBPHYVRHYCC-SNAWJCMRSA-N; (E)-3,4',5-Trimethoxystilbene; 5-[2-(4-Methoxyphenyl)Ethenyl]-1,3-Dimethoxy Benzene
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8000 nM | |||
| External Link | ||||
| 2C60: Breast cancer | 2 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Entrectinib | Approved | [14] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Everolimus | Approved | [15] | ||
| External Link | ||||
References