m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05258
|
[1], [2] | |||
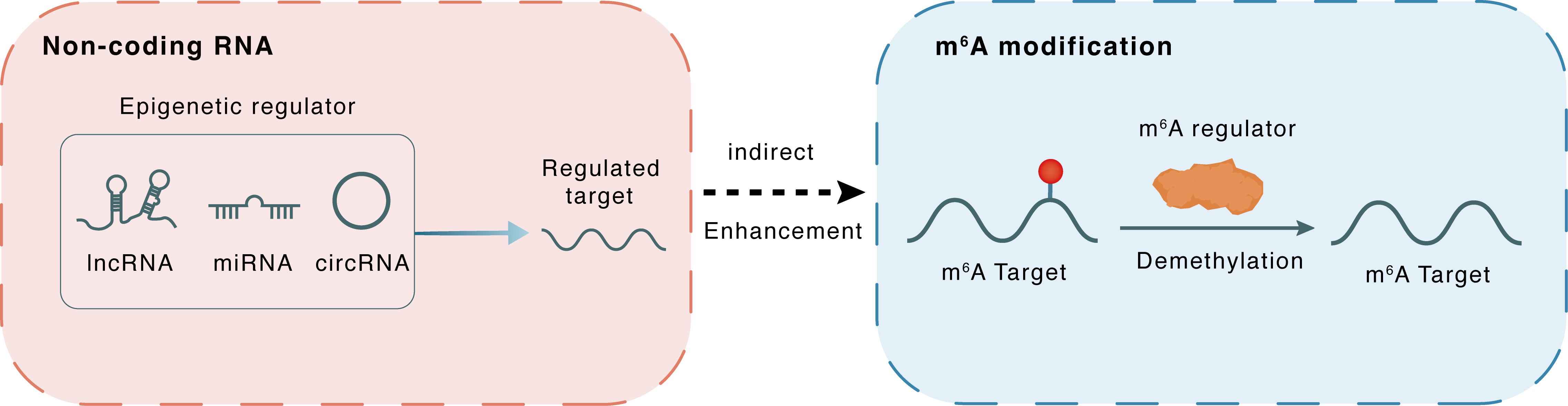 Non-coding RNA
hsa_Circ_0072309
miR-607
lncRNA miRNA circRNA
Indirect
Enhancement
m6A modification
FAP
FAP
FTO
Demethylation
Non-coding RNA
hsa_Circ_0072309
miR-607
lncRNA miRNA circRNA
Indirect
Enhancement
m6A modification
FAP
FAP
FTO
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Prolyl endopeptidase FAP (FAP) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa_circ_0072309 (Circ_LIFR) | circRNA | View Details | ||
| Regulated Target | hsa-miR-607 | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs indirectly impacts m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Circ_LIFR interacted with hsa-miR-607 via its miRNA response element to upregulate the expression of FTO, an m6A demethylase and downstream target of miR-607, thus promoting tumorigenesis of NSCLC.FTO facilitated NSCLC metastasis by modifying the m6A level of Prolyl endopeptidase FAP (FAP) in a YTHDF2-dependent manner.FTO promoted cell migration and invasion in NSCLC, and the FAK inhibitor defactinib (VS6063) suppressed NSCLC metastasis induced by overexpression of FTO. | ||||
| Responsed Disease | Non-small cell lung cancer | ICD-11: 2C25.Y | |||
| Responsed Drug | VS-6063 | ||||
In-vitro Model |
NCI-H1299 | Lung large cell carcinoma | Homo sapiens | CVCL_0060 | |
| A-549 | Lung adenocarcinoma | Homo sapiens | CVCL_0023 | ||
| NCI-H1650 | Minimally invasive lung adenocarcinoma | Homo sapiens | CVCL_1483 | ||
| NCI-H460 | Lung large cell carcinoma | Homo sapiens | CVCL_0459 | ||
| HCC827 | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| PC-9 | Lung adenocarcinoma | Homo sapiens | CVCL_B260 | ||
| SK-MES-1 | Lung squamous cell carcinoma | Homo sapiens | CVCL_0630 | ||
| BEAS-2B | Normal | Homo sapiens | CVCL_0168 | ||
| In-vivo Model | The Laboratory Animal Center of Soochow University provided female BALB/c nude mice (5 weeks old). Mice were kept under specific pathogen-free conditions. They were injected intravenously (i.v.) with FTO-overexpressing and control A549 cells (1.8 × 106 cells/mouse) to establish the in vivo model of NSCLC metastasis, and were then gavaged with dimethyl sulfoxide (DMSO) (25 mg/kg, daily) or the FAK inhibitor defactinib (VS6063) (Cat#S7654, Selleck, USA) (25 mg/kg, daily) beginning in the fifth week after injection. The mice were euthanized eight weeks after being inoculated, and their lungs were taken out and preserved in Bouin's solution for macroscopic investigation of metastatic nodules. Hematoxylin and eosin (H&E) staining of lung tissues was used to look for micrometastatic foci. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Prolyl endopeptidase FAP (FAP) | 12 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Talabostat | Phase 3 | [3] | ||
| Synonyms |
Talabostat mesylate; Boroproline compounds, DARA BioSciences; Boroproline compounds, Point Therapeutics; PT-100; Val-boro-Pro
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 66 nM | |||
| External Link | ||||
| BIBH 1 | Phase 2 | [4] | ||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [4] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| RG7461 | Phase 1 | [5] | ||
| MOA | Antagonist | |||
| External Link | ||||
| AMG 506 | Phase 1 | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| NG-641 | Phase 1 | [7] | ||
| External Link | ||||
| FAP5-DM1 | Preclinical | [8] | ||
| External Link | ||||
| PT630 | Preclinical | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| MIP-1231 | Investigative | [10] | ||
| Synonyms |
MIP-1232; MIP-1233; MIP-1259; MIP-1260; MIP-1271; MIP-1272; Seprase inhibitors (cancer, imaging); Iodine-131-MIP-1232; Seprase inhibitors (cancer, imaging), Molecular Insight; FAP-alpha antagonists (cancer, imaging), Molecular Insight; Fibroblast-activating protein antagonists (cancer, imaging), Molecular Insight
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| FE-999040 | Investigative | [10] | ||
| Synonyms |
FAP inhibitors, Vantia; Fibroblast activation protein inhibitors (sc/oral, cancer/pulmonary fibrosis/IBD), Vantia
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| VA-119930 | Investigative | [10] | ||
| Synonyms |
Fibroblast activation protein inhibitors (IBD), Vantia; Seprase inhibitor (IBD), Vantia
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ARI-3099 | Investigative | [11] | ||
| Synonyms |
compound 6 [PMID: 23594271]; compound 226 [PMID: 24997602]; Py(D)AlaboroPro
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 9 nM | |||
| External Link | ||||
| 2C25: Lung cancer | 52 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Adagrasib | Approved | [12] | ||
| Synonyms |
2326521-71-3; MRTX-849; UNII-8EOO6HQF8Y; 8EOO6HQF8Y; 2-((S)-4-(7-(8-Chloronaphthalen-1-yl)-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile; CHEMBL4594350; SCHEMBL20974691; GTPL10888; Kras G12C inhibitor MRTX849; BCP31538; EX-A3258; MRTX 849; MFCD32263433; s8884; compound 20 [PMID: 32250617]; BS-16211; HY-130149; CS-0105265; 2-Piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2S)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2S)-
Click to Show/Hide
|
|||
| External Link | ||||
| Amivantamab | Approved | [13] | ||
| External Link | ||||
| Mobocertinib | Approved | [14] | ||
| Synonyms |
1847461-43-1; TAK-788; TAK788; AP32788; UNII-39HBQ4A67L; 39HBQ4A67L; propan-2-yl 2-[4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxy-5-(prop-2-enamido)anilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Mobocertinib [INN]; Mobocertinib [USAN]; AP-32788; Mobocertinib (TAK788); Mobocertinib(TAK-788); SCHEMBL17373133; GTPL10468; BDBM368374; BCP31045; EX-A3392; US10227342, Example 10; MFCD32669806; NSC825519; s6813; TAK-788;AP32788; WHO 11183; NSC-825519; example 94 [WO2015195228A1]; HY-135815; CS-0114256; TAK-788;TAK 788; AP 32788; 5-Pyrimidinecarboxylic acid, 2-((4-((2-(dimethylamino)ethyl)methylamino)-2-methoxy-5-((1-oxo-2-propen-1-yl)amino)phenyl)amino)-4-(1-methyl-1H-indol-3-yl)-, 1-methylethyl ester; C(C=C)(=O)NC=1C(=CC(=C(C=1)NC1=NC=C(C(=N1)C1=CN(C2=CC=CC=C12)C)C(=O)OC(C)C)OC)N(C)CCN(C)C; Isopropyl 2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Isopropyl 2-(5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenylamino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Propan-2-yl 2-(5-(acryloylamino)-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyanilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Sugemalimab | Approved in China | [15] | ||
| External Link | ||||
| Sacituzumab govitecan | Approved | [16] | ||
| Synonyms |
1491917-83-9; 1535963-91-7; 1796566-95-4; CYSTEINYL CL2A-SN-38; DA64T2C2IO; DTXSID401335985; EX-A4354; F82944; GOVITECAN CYSTEINYL CONJUGATE; hRS 7SN38; hRS7-SN38; IMMU 132; IMMU-132; M9BYU8XDQ6; Sacituzumab govitecan; Sacituzumab govitecan [USAN]; sacituzumab-govitecan; Satralizumab linker; SN-38 CYSTEINYL CONJUGATE; UNII-M9BYU8XDQ6
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [17] | ||
| External Link | ||||
| Tepotinib | Approved | [18] | ||
| Synonyms |
1100598-32-0; EMD 1214063; UNII-1IJV77EI07; Tepotinib (EMD 1214063); EMD1214063; 1IJV77EI07; MSC-2156119J; Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]-; C29H28N6O2; Tepotinib [INN]; Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]; Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo
Click to Show/Hide
|
|||
| External Link | ||||
| Sotorasib | Approved | [19] | ||
| Synonyms |
AMG-510; AMG510; AMG-510 racemate; 2252403-56-6; AMG 510; Kras G12C inhibitor 9; 2296729-00-3; UNII-2B2VM6UC8G; 2B2VM6UC8G; CHEMBL4535757; 2296729-00-3 (racemate); 4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one; Sotorasib [INN]; 6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-((2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl)pyrido(2,3-d)pyrimidin-2-one; AMG510 racemate; Sotorasib [USAN]; AMG-510(racemate); Kras mutant-targeting AMG 510; SCHEMBL20560375; GTPL10678; AMG 510 pound>>AMG-510; AMY16918; BCP30452; BCP33368; EX-A3538; BDBM50514402; NSC818433; s8830; WHO 11370; DB15569; NSC-818433; BS-16684; HY-114277; CS-0081316; compound (R)-38 [PMID: 31820981]; (1m)-6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)-4-((2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl)pyrido(2,3-d)pyrimidin-2(1H)-one; (1S)-4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 2296729-66-1; Pyrido(2,3-d)pyrimidin-2(1H)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(1-methylethyl)-3-pyridinyl)-4-((2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Entrectinib | Approved | [20] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Repotrectinib | Approved | [21] | ||
| Synonyms |
FIKPXCOQUIZNHB-RRKGBCIJSA-N; SCHEMBL20438940; TPX 0005; BCP19778
Click to Show/Hide
|
|||
| External Link | ||||
| MYL-1402O | Phase 3 | [22] | ||
| Synonyms |
bevacizumab biosimilar
Click to Show/Hide
|
|||
| External Link | ||||
| AB154 | Phase 3 | [23] | ||
| Synonyms |
Domvanalimab
Click to Show/Hide
|
|||
| External Link | ||||
| Datopotamab deruxtecan | Phase 3 | [24] | ||
| External Link | ||||
| CS1001 | Phase 3 | [25] | ||
| External Link | ||||
| JDQ443 | Phase 3 | [26] | ||
| Synonyms |
(S)-JDQ-443; 1-(6-((4S)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl)-2-azaspiro[3.3]heptan-2-yl)prop-2-en-1-one; 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-inda zol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]- 2-Propen-1-one; 1-[6-[(4R)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-2-propen-1-one; 1-[6-[4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methylindazol-5-yl)pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl]prop-2-en-1-one; 1-{6-[(4M)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5- methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2- azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 1-{6-[(4M)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 2653994-08-0; 2653994-10-4; 2-Propen-1-one, 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-; AKOS040757949; AT36708; BDBM50579985; CHEMBL5077861; compound 5 [PMID: 35404998]; CS-0226220; CS-0311034; EX-A5693; example 1a [WO2021120890A1]; GLXC-25533; GTPL11715; HY-139612A; JDQ 443; JDQ 443 [WHO-DD]; JDQ443; JDQ-443; MS-29737; NSC846146; NSC-846146; NVP-JDQ443; NVP-JDQ-443; Opnurasib; opnurasib [INN]; -PROPEN-1-ONE, 1-(6-((4R)-4-(5-CHLORO-6-METHYL-1H-INDAZOL-4-YL)-5-METHYL-3-(1-METHYL-1H-INDAZOL-5-YL)-1H-PYRAZOL-1-YL)-2-AZASPIRO(3.3)HEPT-2-YL)-; Q3W0H3V1LQ; SCHEMBL23533580
Click to Show/Hide
|
|||
| External Link | ||||
| TRS003 | Phase 3 | [27] | ||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [28] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| RG6058 | Phase 3 | [29] | ||
| Synonyms |
Tiragolumab
Click to Show/Hide
|
|||
| External Link | ||||
| GSK4069889 | Phase 2 | [30] | ||
| Synonyms |
TSR-022
Click to Show/Hide
|
|||
| External Link | ||||
| APL-101 | Phase 2 | [31] | ||
| Synonyms |
Bozitinib; PLB-1001; 1440964-89-5; Vebreltinib; Vebreltinib [USAN]; UNII-2WZP8A9VFN; 2WZP8A9VFN; Bozitinib (PLB-1001); SCHEMBL15594471; BDBM107096; CBI-3103; s6762; WHO 11677; HY-125017; CS-0088607; US9695175, 44; 1,2,4-Triazolo(4,3-b)pyridazine, 6-(1-cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6- fluoro-2-methyl-2H-indazol-5-yl)methyl)-; 6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5- yl)methyl)-1,2,4-triazolo(4,3-b)pyridazine
Click to Show/Hide
|
|||
| External Link | ||||
| SGN-LIV1A | Phase 2 | [32] | ||
| Synonyms |
Ladiratuzumab Vedotin
Click to Show/Hide
|
|||
| External Link | ||||
| BNT116 | Phase 2 | [33] | ||
| External Link | ||||
| AZD7789 | Phase 2 | [34] | ||
| External Link | ||||
| L-DOS47 | Phase 1/2 | [35] | ||
| External Link | ||||
| NC318 | Phase 2 | [36] | ||
| External Link | ||||
| Vorolanib | Phase 2 | [37] | ||
| Synonyms |
UNII-YP8G3I74EL; YP8G3I74EL; 1013920-15-4; (S,Z)-N-(1-(Dimethylcarbamoyl)pyrrolidin-3-yl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; Vorolanib [INN]; SCHEMBL2439528; CHEMBL4297587; N-((3S)-1-(dimethylcarbamoyl)pyrrolidin-3-yl)-5-((Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; s6843; DB15247; HY-109019; CS-0030517; Q27294638; 1H-Pyrrole-3-carboxamide, N-((3S)-1-((dimethylamino)carbonyl)-3-pyrrolidinyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| Xofigo | Phase 2 | [38] | ||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [39] | ||
| External Link | ||||
| Voyager-V1 | Phase 2 | [40] | ||
| Synonyms |
VSV-IFNBeta-NIS
Click to Show/Hide
|
|||
| External Link | ||||
| AB-106 | Phase 2 | [41] | ||
| Synonyms |
DS6051b; GTPL11198; AB106
Click to Show/Hide
|
|||
| External Link | ||||
| RO-5126766 | Phase 2 | [42] | ||
| Synonyms |
VS-6766; CH-5126766; Dual Raf/MEK protein kinase inhibitor (cancer), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| TC-210 | Phase 1/2 | [43] | ||
| External Link | ||||
| EMB-01 | Phase 1/2 | [44] | ||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [45] | ||
| External Link | ||||
| MRx0518 | Phase 1/2 | [46] | ||
| External Link | ||||
| DZD9008 | Phase 1/2 | [47] | ||
| External Link | ||||
| BGB-A425 | Phase 1/2 | [48] | ||
| External Link | ||||
| Rilvegostomig | Phase 1/2 | [49] | ||
| Synonyms |
AZD2936
Click to Show/Hide
|
|||
| External Link | ||||
| IK-007 | Phase 1/2 | [50] | ||
| Synonyms |
grapiprant
Click to Show/Hide
|
|||
| External Link | ||||
| IBI318 | Phase 1 | [51] | ||
| External Link | ||||
| GEN-011 | Phase 1 | [52] | ||
| External Link | ||||
| ENV-105 | Phase 1 | [53] | ||
| Synonyms |
Carotuximab
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 160 | Phase 1 | [54] | ||
| External Link | ||||
| ADP-A2M10 | Phase 1 | [55] | ||
| External Link | ||||
| MEDI5752 | Phase 1 | [56] | ||
| External Link | ||||
| PF-07104091 | Phase 1 | [57] | ||
| External Link | ||||
| PF-06936308 | Phase 1 | [58] | ||
| External Link | ||||
| GEM3PSCA | Phase 1 | [59] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [60] | ||
| External Link | ||||
| Cosibelimab | Phase 1 | [61] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| Gemcitabine | Approved | [62] | ||
| External Link | ||||
| SMI-4a | Investigative | [63] | ||
| Synonyms |
438190-29-5; SMI 4a; TCS PIM-1 4a; (Z)-SMI-4a; (Z)-5-(3-(trifluoromethyl)benzylidene)thiazolidine-2,4-dione; (5Z)-5-[3-(trifluoromethyl)benzylidene]-1,3-thiazolidine-2,4-dione; CHEMBL183906; (5Z)-5-[[3-(TRIFLUOROMETHYL)PHENYL]METHYLENE]-2,4-THIAZOLIDINEDIONE; (5Z)-5-[[3-(trifluoromethyl)phenyl]methylidene]-1,3-thiazolidine-2,4-dione; 327033-36-3; C11H6F3NO2S; (Z)-5-(3-(Trifluoromethyl)benzylidene)-thiazolidine-2,4-dione; (5Z)-5-{[3-(trifluoromethyl)phenyl]methylidene}-1,3-thiazolidine-2,4-dione; Pim inhibitor 4a; 3vc4; SMI-4q; TCS PIM-1-4a; 5-(3-(Trifluoromethyl)benzylidene)thiazolidine-2,4-dione; 5-[3-(Trifluoromethyl)benzylidene]thiazolidine-2,4-dione; cc-717; thiazolidine-2,4-dione, 4a; SCHEMBL2541382; SCHEMBL2541388; BDBM26626; AOB6260; EX-A111; SYN1113; BDBM138364; HMS3229J21; 2720AH; HY-16576A; MFCD01152003; s8005; ZINC12576047; AKOS001314163; SMI-4a, >=98% (HPLC); CCG-265027; NCGC00345836-02; NCGC00345836-14; AC-32861; HY-15474; AB0165836; EC-000.2291; J3.561.866J; A11945; W-5256; US8877795, 12; Q27451064; 5-[[3-(trifluoromethyl)phenyl]methylene]-2,4-thiazolidinedione
Click to Show/Hide
|
|||
| External Link | ||||
References