m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05003
|
[1] | |||
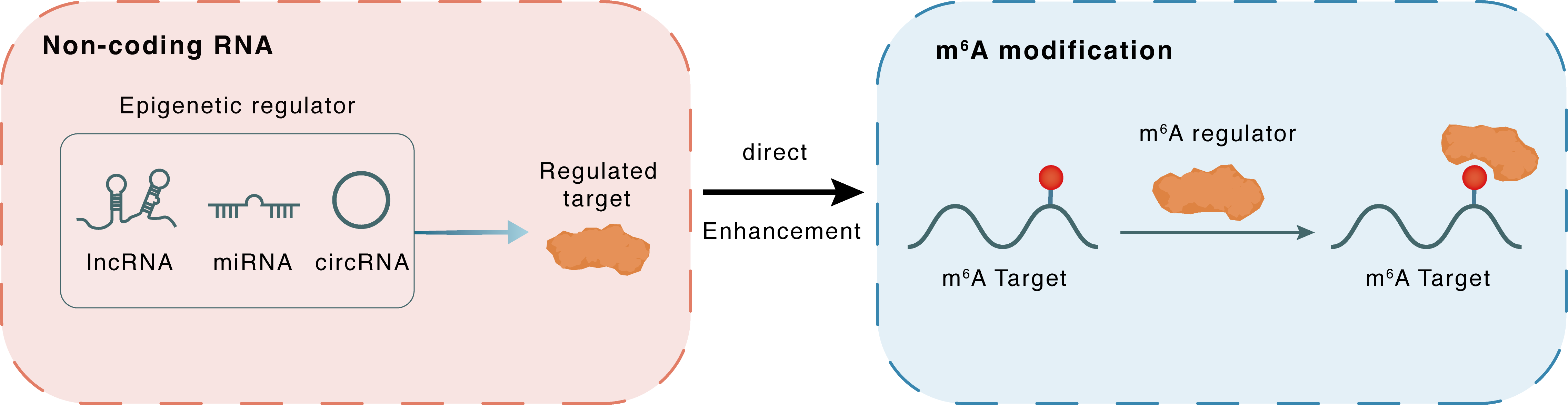 Non-coding RNA
VPS9D1-AS1
ELAVL1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
CDK4
CDK4
ELAVL1
Non-coding RNA
VPS9D1-AS1
ELAVL1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
CDK4
CDK4
ELAVL1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | ELAV-like protein 1 (ELAVL1) | READER | |||
| m6A Target | Cyclin-dependent kinase 4 (CDK4) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | VPS9D1 antisense RNA 1 (VPS9D1-AS1) | LncRNA | View Details | ||
| Regulated Target | ELAV-like protein 1 (HuR/ELAVL1) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | VPS9D1-AS1 silencing suppressed HCC tumor growth in vivo. Mechanistically, VPS9D1-AS1 was able to bind to ELAVL1 and thereby influence the stability and expression of the Cyclin-dependent kinase 4 (CDK4) mRNA, thus impacting HCC cell proliferation. The VPS9D1-AS1/HuR/CDK4 signaling axis regulates HCC tumor cell oncogenic activity, highlighting this pathway as a promising therapeutic target. | ||||
| Responsed Disease | Liver cancer | ICD-11: 2C12 | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Cyclin-dependent kinase 4 (CDK4) | 62 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Palbociclib | Approved | [2] | ||
| Synonyms |
571190-30-2; PD0332991; PD-0332991; Ibrance; PD 0332991; UNII-G9ZF61LE7G; Palbociclib(PD0332991); 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)pyridin-2-yl]amino]-8H-pyrido[2,3-d]pyrimidin-7-one; 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)pyrido[2,3-d]pyrimidin-7(8H)-one; G9ZF61LE7G; PD 332991; 6-ACETYL-8-CYCLOPENTYL-5-METHYL-2-[(5-PIPERAZIN-1-YLPYRIDIN-2-YL)AMINO]PYRIDO[2,3-D]PYRIMIDIN-7(8H)-ONE; LQQ; PD 332991, PD 0332991, PD0332991; 6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-ylpyridin-2-ylamino)-8H-pyrido(2,3-d)pyrimidin-7-one; 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one; HMR-2934
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 9 nM | |||
| External Link | ||||
| Ribociclib Succinate | Approved | [3] | ||
| Synonyms |
1374639-75-4; LEE011 succinate; LEE011 (succinate); UNII-BG7HLX2919; LEE011-BBA; Ribociclib succinate [USAN]; BG7HLX2919; Kisqali (TN); Ribociclib succinate (USAN); LEE-011 succinate; SCHEMBL2684999; EX-A1586; HY-15777B; 1374639-75-4 (succinate); AKOS030526460; CS-2277; ACN-040739
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| LY2835219 | Approved | [4] | ||
| Synonyms |
Abemaciclib; 1231929-97-7; Verzenio; LY-2835219; UNII-60UAB198HK; LY2835219 (free base);
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| Trilaciclib | Approved | [5] | ||
| Synonyms |
G1T28; 1374743-00-6; Trilaciclib [USAN]; G1T28(Trilaciclib); GTPL9626; CHEMBL3894860; SCHEMBL10082028; BDBM253928; US9464092, T; HY-101467; CS-0021431; 2'-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)amino)-7',8'-dihydro-6'H-spiro(cyclohexane-1,9'-pyrazino(1',2':1,5)pyrrolo(2,3-d)pyrimidin)-6'-one; Spiro(cyclohexane-1,9'(6'H)-pyrazino(1',2':1,5)pyrrolo(2,3-d)pyrimidin)-6'-one, 7',8'-dihydro-2'-((5-(4-methyl-1-piperazinyl)-2-pyridinyl)amino)-; 2-[[5-(4-methylpiperazin-1-yl)pyridin-2-yl]amino]spiro[7,8-dihydropyra
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Apremilast | Approved | [6] | ||
| Synonyms |
Apremilast (USAN); CC-10004; N-[2-[1-(3-ethoxy-4-methoxy-phenyl)-2-methylsulfonyl-ethyl]-1,3-dioxo-isoindol-4-yl]acetamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LEE011 | Phase 3 | [7] | ||
| Synonyms |
Ribociclib; 1211441-98-3; LEE-011; Kisqali; Ribociclib(LEE011); UNII-TK8ERE8P56; LEE 011; 7-cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide; TK8ERE8P56; Ribociclib (LEE011); AK174906; 7-cyclopentyl-N,N-dimethyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide; 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo [2,3-d]pyrimidine-6-carboxylic acid dimethylamide; Ribociclib [USAN:INN]; LEE011A; Tube013
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| P-276 | Phase 2 | [8] | ||
| Synonyms |
CDK4 inhibitor (cancer), Nicholas Piramal; P-664-02; CDK4 inhibitor (iv, cancer), Piramal Life Sciences; CDK4 inhibitor(iv, cancer), NPIL Research & Development
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| G1T38 | Phase 2 | [9] | ||
| Synonyms |
YPJRHEKCFKOVRT-UHFFFAOYSA-N; SCHEMBL16036885; CHEMBL3904602; BDBM253941; US9464092, GG; 1628256-23-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro 31-7453 | Phase 2 | [10] | ||
| Synonyms |
Bisindolylmaleimide deriv. 44; MKC-1; Ro-31-7453; 3-(1-methylindol-3-yl)-4-(1-methyl-6-nitroindol-3-yl)pyrrole-2,5-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| P276-00 | Phase 2 | [11] | ||
| Synonyms |
CHEMBL2312181; SCHEMBL1180418
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GLR2007 | Phase 1/2 | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| FCN-437 | Phase 1/2 | [13] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| NUV-422 | Phase 1/2 | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| P1446A-05 | Phase 1 | [11] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| PF-07220060 | Phase 1 | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| G1T28-1 | Phase 1 | [4] | ||
| MOA | Modulator | |||
| External Link | ||||
| AG-024322 | Phase 1 | [11] | ||
| Synonyms |
AG-24322; N-[[5-[(3E)-3-(4,6-difluorobenzimidazol-2-ylidene)-1,2-dihydroindazol-5-yl]-4-methylpyridin-3-yl]methyl]ethanamine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PHA-793887 | Phase 1 | [8] | ||
| Synonyms |
Cyclin-dependent kinase inhibitor (cancer), Nerviano
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RGT-419B | Phase 1 | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| FN-1501 | Phase 1 | [17] | ||
| Synonyms |
1429515-59-2; CHEMBL4077071; UNII-6MC966B505; TQR1001; BDBM50270304; NSC781143; 6MC966B505; NSC-781143; HY-111361; CS-0039834; 4((7HPyrrolo[2,3d]pyrimidin-4-yl)amino)N(4-((4-methylpiperazin-1-yl)methyl)phenyl)1Hpyrazole-3-carboxamide; 4-((7H-Pyrrolo (2,3-d)pyrimidin-4-yl)amino)-N-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-1H-pyrazole-3-carboxamide; 4-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-N-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-1H-pyrazole-5-carboxamide; 4a?(7Ha'Pyrrolo[2,3a'd]pyrimidin-4-yl)amino)a'Na?4-((4-methylpiperazin-1-yl)methyl)phenyl)a?Ha'pyrazole-3-carboxamide; N-[4-[(4-methylpiperazin-1-yl)methyl]phenyl]-4-(7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-1H-pyrazole-5-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RGB-286638 | Phase 1 | [8] | ||
| Synonyms |
GPC-286199; RGB-286199; RGB-344064; Non-selective CDK inhibitors, Agennix; Non-selective CDK inhibitors, GPC Biotech; Non-selective cyclin dependent kinase inhibitors, Agennix; Non-selective cyclin dependent kinase inhibitors, GPC Biotech
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Imidazo pyridine derivative 3 | Patented | [18] | ||
| Synonyms |
PMID26161698-Compound-22
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| Palbociclib/ribociclib analog 1 | Patented | [18] | ||
| Synonyms |
PMID26161698-Compound-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrrolo[2,3-d]pyrimidine derivative 10 | Patented | [18] | ||
| Synonyms |
PMID26161698-Compound-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| PMID26161698-Compound-17 | Patented | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrrolo[2,3-d]pyrimidine derivative 9 | Patented | [18] | ||
| Synonyms |
PMID26161698-Compound-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-49 | Patented | [19] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID25726713-Compound-51 | Patented | [19] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| Indole-based analog 13 | Patented | [18] | ||
| Synonyms |
PMID26161698-Compound-20
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Isoquinoline 1,3-dione derivative 1 | Patented | [18] | ||
| Synonyms |
PMID26161698-Compound-49
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| PMID25726713-Compound-48 | Patented | [19] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID25726713-Compound-47 | Patented | [19] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID25726713-Compound-50 | Patented | [19] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID25991433-Compound-A1 | Patented | [20] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 910 nM | |||
| External Link | ||||
| Oxazolyl methylthiothiazole derivative 1 | Patented | [18] | ||
| Synonyms |
PMID26161698-Compound-52
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 100 nM | |||
| External Link | ||||
| BAY 10-00394 | Discontinued in Phase 2 | [8] | ||
| Synonyms |
roniciclib; BAY 1000394; KB-145902; 1223498-69-8; Tube010; SCHEMBL875845; GTPL7874
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| R547 | Discontinued in Phase 1 | [11] | ||
| Synonyms |
LIA; R-547; [4-amino-2-[(1-methylsulfonylpiperidin-4-yl)amino]pyrimidin-5-yl]-(2,3-difluoro-6-methoxyphenyl)methanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ZK 304709 | Discontinued in Phase 1 | [11] | ||
| Synonyms |
1010440-84-2; ZK CDK; UNII-87GI98VT0I; SCHEMBL955299; 87GI98VT0I; DTXSID20143701
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| INOC-005 | Preclinical | [8] | ||
| Synonyms |
Capridine beta (prostate cancer), Prostagenics
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CYC-103 | Terminated | [8] | ||
| Synonyms |
Cyclin groove inhibitors, Cyclacel; CYC-103 (Pimetics series); CYC-103 cyclin groove inhibitors, Cyclacel; CYC-103 program, Cyclacel
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PD-0183812 | Terminated | [21] | ||
| Synonyms |
PETCVZZPKYJZAU-UHFFFAOYSA-N; PD183812; AC1NS8PJ; CHEMBL139653; SCHEMBL5268115; BDBM6280; PD 0183812; N8 Pyrido[2,3-d]pyrimidin-7-one deriv 72; 8-{bicyclo[221]heptan-2-yl}-2-({4-[4-(3-hydroxypropyl)piperidin-1-yl]phenyl}amino)-7H,8H-pyrido[2,3-d]pyrimidin-7-one; 8-(3-bicyclo[221]heptanyl)-2-[4-[4-(3-hydroxypropyl)piperidin-1-yl]anilino]pyrido[2,3-d]pyrimidin-7-one; PD0183813
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 20 nM | |||
| External Link | ||||
| N-(2-(1H-Indol-3-yl)ethyl)biphenyl-4-carboxamide | Investigative | [22] | ||
| Synonyms |
CHEMBL521733; N-[2-(1H-indol-3-yl)ethyl]biphenyl-4-carboxamide; AC1LG1FS; Oprea1_829316; Oprea1_192683; MolPort-001-987-537; ZINC285233; STK129364; BDBM50272836; AKOS000554028; MCULE-1062167425; NCGC00304458-01; BAS 03049800; ST45172084; AB01300868-01; N-[2-(1H-indol-3-yl)ethyl]-4-phenylbenzamide; N-(2-indol-3-ylethyl)(4-phenylphenyl)carboxamide; Biphenyl-4-carboxylic acid [2-(1H-indol-3-yl)-ethyl]-amide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-(1H-Indazol-6-yl)-3-pyridin-2-yl-urea | Investigative | [23] | ||
| Synonyms |
Diarylurea deriv. 14a; AC1NS9HT; BDBM6656; CHEMBL143759; ZINC13471116; AKOS027814075; 3-1H-indazol-6-yl-1-pyridin-2-ylurea; 1-(1H-indazol-6-yl)-3-pyridin-2-ylurea; N-(1H-Indazol-6-yl)-N -pyridin-2-ylurea
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-(7-Hydroxy-naphthalen-1-yl)-3-pyridin-2-yl-urea | Investigative | [23] | ||
| Synonyms |
Diarylurea deriv. 14; AC1NS9HN; CHEMBL140624; BDBM6654; ZINC13471112; 1-(7-Hydroxy-1-naphthyl)-3-(2-pyridyl)urea; N-(7-Hydroxy-1-naphthyl)-N -pyridin-2-ylurea; 1-(7-hydroxynaphthalen-1-yl)-3-pyridin-2-ylurea
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-Pyridin-2-yl-3-quinolin-5-yl-urea | Investigative | [23] | ||
| Synonyms |
Diarylurea deriv. 14b; AC1NS9HW; BDBM6657; CHEMBL143704; ZINC13471117; 1-pyridin-2-yl-3-quinolin-5-ylurea; 3-pyridin-2-yl-1-quinolin-5-ylurea; 1-(5-Quinolyl)-3-(2-pyridyl)urea; N-Pyridin-2-yl-N -quinolin-5-ylurea
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-(9-Oxo-9H-fluoren-4-yl)-3-pyridin-2-yl-urea | Investigative | [23] | ||
| Synonyms |
Diarylurea deriv. 15; AC1NS9HQ; BDBM6655; CHEMBL140589; SCHEMBL12856374; ZINC13471114; 1-(9-oxofluoren-4-yl)-3-pyridin-2-ylurea; 3-(9-oxo-9H-fluoren-4-yl)-1-pyridin-2-ylurea; 1-(9-Oxo-9H-fluorene-4-yl)-3-(2-pyridyl)urea; N-(9-Oxo-9H-fluoren-4-yl)-N -pyridin-2-ylurea
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cdk4 inhibitor III | Investigative | [24] | ||
| Synonyms |
Ryuvidine; 265312-55-8; CHEMBL290904; 2-Methyl-5-[(4-methylphenyl)amino]benzothiazole-4,7-dione; 5-(N-(4-Methylphenyl)amino)-2-methyl-4,7-dioxobenzothiazole; 2-methyl-5-(4-methylanilino)-1,3-benzothiazole-4,7-dione; AC1Q6BBC; AC1LA59T; 2-Methyl-5-p-tolylamino-benzothiazole-4,7-dione; SCHEMBL2169284; GTPL5952; CTK4F8075; CHEBI:92119; AOB6479; MolPort-023-276-509; HMS3269F11; HMS3229E08; ZINC5930916; BDBM50086655; 2-METHYL-5-[(4-METHYLPHENYL)AMINO]-4,7-BENZOTHIAZOLEDIONE; AKOS024457195; CCG-206830
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6000 nM | |||
| External Link | ||||
| Fascaplysin | Investigative | [21] | ||
| Synonyms |
Pyrido[1,2-a:3,4-b']diindol-5-ium,12,13-dihydro-13-oxo-, chloride; GNF-PF-1458; ACMC-20bu3v; AC1L2JLY; AC1Q6JA3; SCHEMBL1728912; CHEMBL602937; GTPL5969; BDBM59087; CTK4A8872; CHEBI:93765; ZINC1616841; pyrido[1,2-a:3,4-b']diindol-5-ium, 12,13-dihydro-13-oxo-; HSCI1_000331; NCGC00346951-01; CJ-26101; BRD-K13287209-003-03-2; BRD-K13287209-311-02-1; BRD-K13287209-311-01-3; BRD-K13287209-003-02-4; BRD-K13287209-003-01-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 350 nM | |||
| External Link | ||||
| Ro-0505124 | Investigative | [25] | ||
| Synonyms |
RO0505124
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-625987 | Investigative | [26] | ||
| Synonyms |
NSC 625987
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 10-hydroxy-18-methoxybetaenone | Investigative | [27] | ||
| Synonyms |
CHEMBL498247; BDBM50269144
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PYRAZOLOPYRIDAZINE 1 | Investigative | [28] | ||
| Synonyms |
551920-54-8; GW810576X; n-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-yl-2-pyrimidinamine; pyrazolo[1,5-b]pyridazine deriv. 19; AC1O6ZIQ; CHEMBL187081; BDBM8128; SCHEMBL4489357; CTK1F7320; DTXSID60424889; HMS3305F24; HMS3303K24; ZINC13582569; NCGC00242229-01; DA-42106; FT-0707969; AB01092291-01; 2-Pyrimidinamine, N-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-yl-; N-(3-methoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 60 nM | |||
| External Link | ||||
| K00024 | Investigative | [29] | ||
| Synonyms |
indolocarbazole deriv. 4(d)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PYRAZOLOPYRIDAZINE 2 | Investigative | [28] | ||
| Synonyms |
pyrazolo[1,5-b]pyridazine deriv. 25; AC1O6ZJ2; BDBM8134; CHEMBL186054; N-(3,4-dimethoxyphenyl)-4-pyrazolo[1,5-b]pyridazin-3-ylpyrimidin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 190 nM | |||
| External Link | ||||
| PMID18986805C9b | Investigative | [30] | ||
| Synonyms |
GTPL8160; BDBM50246396; ZINC38224644
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 50 nM | |||
| External Link | ||||
| NU-6102 | Investigative | [31] | ||
| Synonyms |
nu6102; 444722-95-6; NU 6102; O6-CYCLOHEXYLMETHOXY-2-(4'-SULPHAMOYLANILINO) PURINE; Cdk1/2 Inhibitor II, NU6102; 6-Cyclohexylmethoxy-2-(4& -sulfamoylanilino)purine; 4-{[6-(cyclohexylmethoxy)-9H-purin-2-yl]amino}benzenesulfonamide; 4SP; 1h1s; 4-[[6-(cyclohexylmethoxy)-7H-purin-2-yl]amino]benzenesulfonamide; 4-{[6-(cyclohexylmethoxy)-7h-purin-2-yl]amino}benzenesulfonamide; 4-[[6-(cyclohexylmethoxy)-9h-purin-2-yl]amino]benzenesulfonamide; 4eor; 4eok; 2iw9; 2c6o; 2iw8; AC1L1IGA; SCHEMBL2170816; CHEMBL319467
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1600 nM | |||
| External Link | ||||
| NU6140 | Investigative | [32] | ||
| Synonyms |
Cdk2 Inhibitor IV, NU6140; 444723-13-1; NU 6140; CHEMBL1802728; 4-(6-Cyclohexylmethoxy-9H-purin-2-ylamino)-N,N-diethylbenzamide; 4-{[6-(cyclohexylmethoxy)-7H-purin-2-yl]amino}-N,N-diethylbenzamide; Cdk2 inhibitor IV; SCHEMBL2169233; GTPL5949; CTK8E7940; DTXSID30436732; MolPort-023-276-742; MolPort-044-561-419; HMS3229E18; IN1369; ZINC22309248; BDBM50347924; AKOS024457537; CCG-206836; NCGC00370819-01; NU6140, > RT-011957; 4-(6-cyclohexylmethoxy-9hpurin-2-ylamino)-N,N-diethyl-benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(indole-3-yl)-4-phenyl-1H-pyrrole-2,5-dione | Investigative | [33] | ||
| Synonyms |
CHEMBL380598; SCHEMBL3148490; HVQJGNALTWNDMX-UHFFFAOYSA-N; BDBM50375058; 2-(1H-Indole-3-yl)-3-phenylmaleimide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3,4-di-(4-methoxyphenyl)-1H-pyrrole-2,5-dione | Investigative | [33] | ||
| Synonyms |
1H-Pyrrole-2,5-dione, 3,4-bis(4-methoxyphenyl)-; 108774-82-9; ACMC-20mbs9; CHEMBL381099; CTK0G2626; DTXSID90449388
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3,4-diphenyl-1H-pyrrole-2,5-dione | Investigative | [33] | ||
| Synonyms |
2,3-diphenylmaleimide; 1H-Pyrrole-2,5-dione, 3,4-diphenyl-; 31295-36-0; AC1MBL6S; SCHEMBL114611; CHEMBL201949; CTK1B9880; 3,4-diphenylpyrrole-2,5-dione; DTXSID70372903; ZINC3847556
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(4-methoxyphenyl)-4-phenyl-1H-pyrrole-2,5-dione | Investigative | [33] | ||
| Synonyms |
CHEMBL372076; SCHEMBL3822337
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [34] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C12: Liver cancer | 49 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| 90Y-loaded resin microspheres | Approved | [35] | ||
| External Link | ||||
| Thymalfasin | Phase 2 | [36] | ||
| Synonyms |
Zadaxin; 62304-98-7; Thymosin alpha1; Thymosin alpha 1; Thymosin alpha1 (ox); Thymosin alpha1 (human); Thymalfasin [USAN:INN]; UNII-W0B22ISQ1C; Thymosin-alpha-1; 69440-99-9; Thymosin alpha1 (cattle); C129H215N33O55; W0B22ISQ1C; Zadaxin (TN); Thymalfasin alfa 1
Click to Show/Hide
|
|||
| External Link | ||||
| Ferumoxides | Approved | [37] | ||
| Synonyms |
AMI-25; 119683-68-0; Feridex; Feridex IV; Superparamagnetic iron oxide; UNII-G6N3J05W84; Ferumoxides [USAN:USP:BAN]; CCRIS 6722; HSDB 8072; AC1O5DID; G6N3J05W84; iron(2+); iron(3+); Iron oxide crystal is inverse spinel (X-ray data); Fe(II) and Fe(III) are present (Mossbauer Spectroscopy; Physical form is a colloidal particle of nonstoichiometric
Click to Show/Hide
|
|||
| External Link | ||||
| DTI-015 | Approved | [38] | ||
| Synonyms |
Carmustine; 154-93-8; 1,3-Bis(2-chloroethyl)-1-nitrosourea; BCNU; Carmustin; Nitrumon; Carmubris; Gliadel; BiCNU; Bi CNU; Carmustinum; Bischlorethylnitrosurea; Bischlorethylnitrosourea; Carmustina; Becenun; Becenum; Bischloroethyl nitrosourea; N,N'-BIS(2-CHLOROETHYL)-N-NITROSOUREA; Bis(2-chloroethyl)nitrosourea; Urea, N,N'-bis(2-chloroethyl)-N-nitroso-; Gliadel Wafer; FDA 0345; Bischloroethylnitrosourea; SRI 1720; 1,3-Bis(2-chloroethyl)nitrosourea; BiCNU (TN); Carmustinum [INN-Latin]; Carmustina [INN-Spanish]; DTI 015; NCI-C04773; SK; Injectable carmustine, Direct Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Nofazinlimab | Phase 3 | [39] | ||
| Synonyms |
CS1003; EQ176
Click to Show/Hide
|
|||
| External Link | ||||
| PV-10 | Phase 3 | [9] | ||
| Synonyms |
632-69-9; Rose bengal sodium; Rose bengal disodium salt; R105 sodium; Rose-bengal (131 I) natrium; Food Red No 105, sodium salt; EINECS 211-183-3; Food Red Color No 105, sodium salt; Sel disodique de rose bengale iodee (131 I); Rose bengale (131 I) sodique [INN-French]; Rosa bengala sodica (131 I) [INN-Spanish]; Roseum bengalense (131 I) natricum [INN-Latin]; 2,4,5,7-Tetraido(m,p,o',m')tetrachlorofluorescein, disodium salt; Fluorescein, 4,5,6,7-tetrachloro-2',4',5',7'-tetraiodo-, disodium salt; Disodium
Click to Show/Hide
|
|||
| External Link | ||||
| Brivanib | Phase 3 | [40] | ||
| Synonyms |
649735-46-6; BMS-540215; Brivanib (BMS-540215); BMS 540215; UNII-DDU33B674I; Brivanib [USAN]; BMS540215; DDU33B674I; CHEMBL377300; (2R)-1-[4-(4-Fluoro-2-methyl-1H-indol-5-yloxy)-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yloxy]propanol; Brivanib (USAN); (2R)-1-[4-[(4-FLUORO-2-METHYL-1H-INDOL-5-YL)OXY]-5-METHYL-PYRROLO[2,1-F][1,2,4]TRIAZIN-6-YL]OXYPROPAN-2-OL; (2R)-1-({4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-5-methylpyrrolo[2,1-f][1,2,4]triazin-6-yl}oxy)propan-2-ol
Click to Show/Hide
|
|||
| External Link | ||||
| JX-594 | Phase 3 | [41] | ||
| Synonyms |
Pexastimogene devacirepvec
Click to Show/Hide
|
|||
| External Link | ||||
| ABH001 | Phase 3 | [42] | ||
| External Link | ||||
| MTC-DOX | Phase 2/3 | [43] | ||
| Synonyms |
MTC-doxorubicin
Click to Show/Hide
|
|||
| External Link | ||||
| KD018 | Phase 2 | [44] | ||
| External Link | ||||
| Doxorubicin-eluting beads | Phase 2 | [45] | ||
| Synonyms |
DC Bead (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| 32-P BioSilicon | Phase 2 | [46] | ||
| Synonyms |
BrachySil
Click to Show/Hide
|
|||
| External Link | ||||
| Cixutumumab | Phase 2 | [47] | ||
| Synonyms |
LY3012217
Click to Show/Hide
|
|||
| External Link | ||||
| [131I]-Metuximab | Phase 2 | [48] | ||
| External Link | ||||
| Darinaparsin | Phase 2 | [49] | ||
| Synonyms |
ZIO-101
Click to Show/Hide
|
|||
| External Link | ||||
| Tigatuzumab | Phase 2 | [50] | ||
| External Link | ||||
| CMC-001 | Phase 2 | [51] | ||
| Synonyms |
Manganese-based MRI contrast agent (liver tumor imaging), CMC Contrast
Click to Show/Hide
|
|||
| External Link | ||||
| OBP-301 | Phase 1/2 | [52] | ||
| External Link | ||||
| MBO7133 | Phase 1/2 | [53] | ||
| External Link | ||||
| INCB62079 | Phase 1/2 | [9] | ||
| External Link | ||||
| NV-1020 | Phase 1/2 | [54] | ||
| External Link | ||||
| DCVax-Liver | Phase 1/2 | [55] | ||
| Synonyms |
Dendritic cell-based immunotherapy (liver cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| SRF388 | Phase 1 | [56] | ||
| External Link | ||||
| ET140202 | Phase 1 | [57] | ||
| External Link | ||||
| ADP-A2AFP | Phase 1 | [58] | ||
| External Link | ||||
| SM04755 | Phase 1 | [59] | ||
| External Link | ||||
| Anti-CEA CAR-T therapy | Phase 1 | [9] | ||
| External Link | ||||
| PI-166 | Phase 1 | [60] | ||
| External Link | ||||
| CRS-100 | Phase 1 | [61] | ||
| External Link | ||||
| Autologous ET1402L1-CART cells | Phase 1 | [62] | ||
| External Link | ||||
| Anti-CD133-CAR vector-transduced T cells | Phase 1 | [63] | ||
| External Link | ||||
| MRX34 | Phase 1 | [64] | ||
| External Link | ||||
| ALN-VSP | Phase 1 | [65] | ||
| External Link | ||||
| EPCAM-targeted CAR-T cells | Clinical trial | [66] | ||
| External Link | ||||
| ADI | Discontinued in Phase 3 | [67] | ||
| Synonyms |
Arginine deiminase
Click to Show/Hide
|
|||
| External Link | ||||
| GN-1140 | Discontinued in Phase 2 | [68] | ||
| External Link | ||||
| OGT-719 | Discontinued in Phase 2 | [69] | ||
| Synonyms |
OGS-719
Click to Show/Hide
|
|||
| External Link | ||||
| AFP-Scan | Discontinued in Phase 2 | [70] | ||
| External Link | ||||
| SR1078 | Preclinical | [71] | ||
| Synonyms |
1246525-60-9; SR 1078; SR-1078; N-[4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl]-4-(trifluoromethyl)benzamide; CHEMBL3094387; N-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)phenyl)-4-(trifluoromethyl)benzamide; N-[4-[2,2,2-Trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-4-(trifluoromethyl)benzamide; SCHEMBL4880524; C17H10F9NO2; DTXSID30591895; BCP09203; EX-A2215; 4063AH; BDBM50444350; s5775; ZINC98052696; AKOS024458390; CS-1045; NCGC00379222-02; AK547149; AS-55921; HY-14422; W-5797; SR-03000001078; SR-03000001078-1; SR-03000001078-2
Click to Show/Hide
|
|||
| External Link | ||||
| Occlusin | Preclinical | [72] | ||
| Synonyms |
Occlusin 50 Injection; Occlusin 500 injection
Click to Show/Hide
|
|||
| External Link | ||||
| HRC-201 | Terminated | [73] | ||
| Synonyms |
Hemoglobin-imaging conjugate (HepSelect), Hemosol
Click to Show/Hide
|
|||
| External Link | ||||
| 1,2,3,4,5,6-hexabromocyclohexane | Investigative | [74] | ||
| Synonyms |
1837-91-8; Benzene hexabromide; Cyclohexane, 1,2,3,4,5,6-hexabromo-; Hexabromocyclohexane; JAK2 Inhibitor II; ACMC-1BQJT; SCHEMBL459442; trans-alpha-Benzene hexabromide; CHEMBL444236; DTXSID4052687; CHEBI:93940; NSC7908; HMS3268H22; HMS3413C10; HMS3677C10; NSC-7908; ZINC1586309; ANW-23174; MFCD00059127; s5902; Cyclohexane,2,3,4,5,6-hexabromo-; AKOS015836040; 1,2,3,4,5,6-Hexabromo-cyclohexane; 1,2,3,4,5,6-Hexabromocyclohexane #; NCGC00092358-01; NCGC00092358-02; 1,2,3,4,5,6-hexakis(bromanyl)cyclohexane; A4510; FT-0633875; JAK2 Inhibitor II - CAS 1837-91-8; 1,2,3,4,5,6-Hexabromocyclohexane;NSC7908; A812818; 1,2,3,4,5,6-Hexabrom-cyclohexan (I(2)-Form); J-011778; 1,2,3,4,5,6-Hexabromocyclohexane, >=98% (HPLC); BRD-K06817181-001-01-5; Q27165694
Click to Show/Hide
|
|||
| External Link | ||||
| STP-322 | Investigative | [75] | ||
| Synonyms |
Multi-targeted siRNA therapeutic cocktail (nanoparticle, liver tumor), Sirnaomics
Click to Show/Hide
|
|||
| External Link | ||||
| AMB-8LK | Investigative | [75] | ||
| Synonyms |
Cancer therapy (monoclonal antibody), MAT Biopharma; Y90 anti-ferritin monoclonal antibody (cancer), MAT Biopharma; Yttrium-90 anti-ferritin mAb (cancer), MAT Biopharma; Yttrium-90 anti-ferritin mAb (Hodgkin's disease/pancreatic/liver cancer), MAT Biopharma; 90Y-AMB8LK mAb (cancer), MAT Biopharma; 90Y-AMB8LK monoclonal antibody (cancer), MAT Biopharma; 90Y-labelled anti-ferritin monoclonal antibody (cancer), MAT Biopharma
Click to Show/Hide
|
|||
| External Link | ||||
| MiR-34a mimics | Investigative | [75] | ||
| Synonyms |
MiR-34a mimics (liver cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| P53 fusion protein | Investigative | [75] | ||
| Synonyms |
P53 fusion protein (pancreatic/liver cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| OP-05 | Investigative | [75] | ||
| Synonyms |
OP-05 program (prodrug, liver tumor); OP-05 program (prodrug, liver tumor), Onco-Pharmakon
Click to Show/Hide
|
|||
| External Link | ||||
| GR-DD1 | Investigative | [75] | ||
| Synonyms |
Cytotoxin (hepatic metastasis), ERYtech
Click to Show/Hide
|
|||
| External Link | ||||
References