m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03618
|
[1], [2] | |||
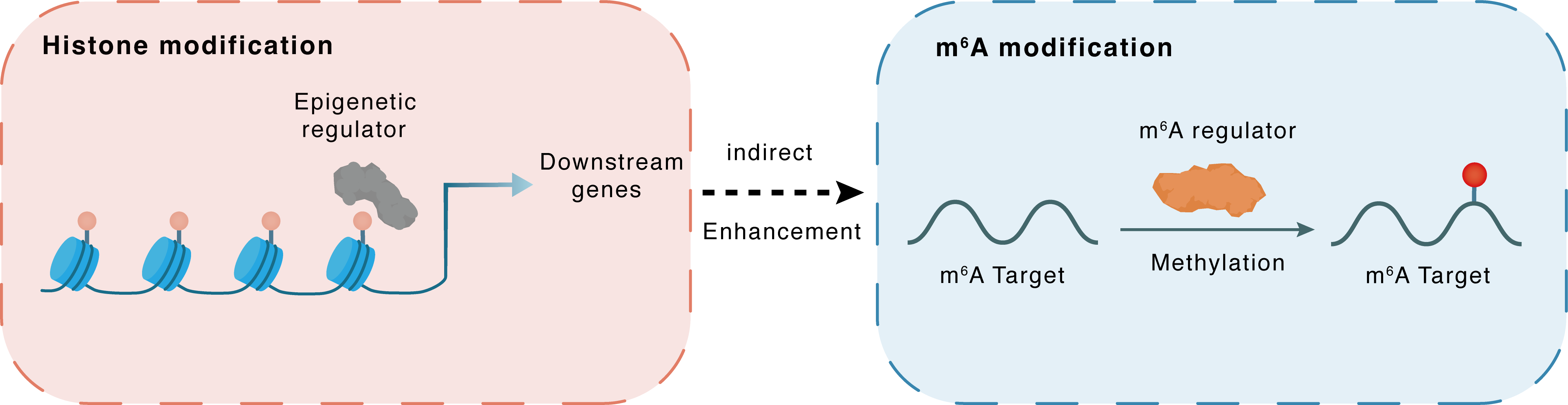 Histone modification
H3K4me3
SMYD2
LINC01605
Indirect
Enhancement
m6A modification
PLAU
PLAU
METTL3
Methylation
Histone modification
H3K4me3
SMYD2
LINC01605
Indirect
Enhancement
m6A modification
PLAU
PLAU
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Urokinase-type plasminogen activator (PLAU) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | N-lysine methyltransferase SMYD2 (SMYD2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | LINC01605 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | LINC01605 was regulated by SMYD2-EP300-mediated modifications of histone Histone H3 lysine 4 trimethylation (H3K4me3) as well as H3K27ac. LINC01605 was found to bind to METTL3 and promote the m6A modification of SPTBN2 mRNA, thereby facilitating the translation of SPTBN2. METTL3 upregulated Urokinase-type plasminogen activator (PLAU) mRNA in an m6A-dependent manner, and then participated in MAPK/ERK pathway to promote angiogenesis and metastasis in CRC. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell proliferation and metastasis | ||||
| Cell apoptosis | |||||
In-vitro Model |
HT29 | Colon cancer | Mus musculus | CVCL_A8EZ | |
| LoVo | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| In-vivo Model | 1 × 106 cells in 100 uL PBS (shMETTL3-1 or shNC) were respectively injected into each mouse through the tail vein. Pulmonary metastases were monitored after fourteen days using the imaging system (IVIS) Spectrum (PerkinElmer, USA). | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| N-lysine methyltransferase SMYD2 (SMYD2) | 6 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| AZ505 | Preclinical | [3] | ||
| Synonyms |
AZ 505; AZ-505
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 120 nM | |||
| External Link | ||||
| LLY-507 | Preclinical | [4] | ||
| Synonyms |
1793053-37-8; LLY507; CHEMBL3414623; 5-Cyano-2'-{4-[2-(3-Methyl-1h-Indol-1-Yl)ethyl]piperazin-1-Yl}-N-[3-(Pyrrolidin-1-Yl)propyl]biphenyl-3-Carboxamide; 3-cyano-5-(2-{4-[2-(3-methyl-1H-indol-1-yl)ethyl]piperazin-1-yl}phenyl)-N-[3-(pyrrolidin-1-yl)propyl]benzamide; 3-Cyano-5-[2-[4-[2-(3-methyl-1H-indol-1-yl)ethyl]piperazin-1-yl]phenyl]-N-[3-(pyrrolidin-1-yl)propyl]benzamide; GTPL8239; SCHEMBL19760400; EX-A899; LLY 507; MolPort-042-624-530; BCP17114; s7575; BDBM50075102; ZINC231558920; AKOS027470175; CS-5126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 15 nM | |||
| External Link | ||||
| EPZ032597 | Preclinical | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| EPZ033294 | Preclinical | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| A-893 | Preclinical | [6] | ||
| Synonyms |
1868232-32-9; (R)-N-cyclohexyl-3-((3,4-dichlorophenethyl)amino)-N-(2-((2-hydroxy-2-(3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-8-yl)ethyl)amino)ethyl)propanamide; CHEMBL3590526; N-Cyclohexyl-N~3~-[2-(3,4-Dichlorophenyl)ethyl]-N-(2-{[(2r)-2-Hydroxy-2-(3-Oxo-3,4-Dihydro-2h-1,4-Benzoxazin-8-Yl)ethyl]amino}ethyl)-Beta-Alaninamide; SCHEMBL17476248; EX-A2769; BDBM50095537; AKOS030235552; ZINC230499113; ACN-037539; AC-29886; HY-19563; CS-0015655; Q27454706; 4GQ; N-Cyclohexyl-3-[2-(3,4-dichlorophenyl)ethylamino]-N-[2-[[(2R)-2-hydroxy-2-(3-oxo-4H-1,4-benzoxazin-8-yl)ethyl]amino]ethyl]propanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BAY 598 | Preclinical | [7] | ||
| Synonyms |
BAY-598; 1906919-67-2; CHEMBL3818617; 1906919-67-2 (S-isomer); (S,E)-N-(1-(N'-cyano-N-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; BAY 598 - Bio-X; BAY598; GTPL8953; EX-A1835; BDBM50180955; ZINC504786915; AC-31567; BS-16389; HY-19546; CS-0015642; J3.601.000B; Q27074893; (S)-N-(1-(N'-Cyano-N-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; (S,E)-N-(1-(N-cyano-N'-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; (S,Z)-N-(1-(N-cyano-N'-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; N-[(4S)-1-[(Cyanoamino)[[3-(difluoromethoxy)phenyl]imino]methyl]-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl]-N-ethyl-2-hydroxyacetamide; N-[(4S)-1-[(Z)-N'-cyano-N-[3-(difluoromethoxy)phenyl]carbamimidoyl]-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl]-N-ethyl-2-hydroxyacetamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Urokinase-type plasminogen activator (PLAU) | 42 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Pro-urokinase | Approved | [8] | ||
| Synonyms |
Thrombolyse (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Urokinase | Approved | [9] | ||
| MOA | Modulator | |||
| External Link | ||||
| PAI-1 | Phase 4 | [10] | ||
| Synonyms |
PA Autoinducer; Pseudomonas aeruginosa autoinducer; N-(3-Oxododecanoyl)homoserine lactone; 3-Oxo-N-(tetrahydro-2-oxo-3-furanyl)dodecanamide; 152833-54-0; CHEBI:29639; 3-oxo-N-(2-oxotetrahydrofuran-3-yl)dodecanamide; N-(3-ketododecanoyl)homoserine lactone; 3-oxo-C12-AHL; 3-oxo-N-(2-oxooxolan-3-yl)dodecanamide; AC1L2SRN; CHEMBL482476; SCHEMBL10076544; BCP19350; AN-30880; RT-014202; 3-(3-Oxododecanoylamino)tetrahydrofuran-2-one; C11840; Dodecanamide, 3-oxo-N-(tetrahydro-2-oxo-3-furanyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Amediplase | Phase 3 | [11] | ||
| Synonyms |
Amediplase [INN]; Trans,trans-3,4:12,13-Tetrahydroxy-3,4,12,13-tetrahydro-dibenz(a,h)anthracene; (3S,4S,12S,13S)-3,4,12,13-tetrahydronaphtho[1,2-b]phenanthrene-3,4,12,13-tetrol
Click to Show/Hide
|
|||
| MOA | Activator | |||
| External Link | ||||
| Upamostat | Phase 2 | [12] | ||
| Synonyms |
WX-671
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 410 nM | |||
| External Link | ||||
| Saruplase | Phase 2 | [13] | ||
| Synonyms |
Rescupase; CG-4509; PUK, Grunenthal; Pro-urokinase, Grunenthal; Rscu-PA, Grunenthal
Click to Show/Hide
|
|||
| MOA | Activator | |||
| External Link | ||||
| HTU-PA | Phase 1/2 | [14] | ||
| Synonyms |
Human tissue urokinase type plasminogen activator, Global Biotech
Click to Show/Hide
|
|||
| MOA | Activator | |||
| External Link | ||||
| PMID18163548C4 | Clinical trial | [15] | ||
| Synonyms |
7IN; 1vja; AC1L9MNX; GTPL6545; BDBM50231520; US8476306, 6.12
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 20 nM | |||
| External Link | ||||
| UK-356202 | Clinical trial | [16] | ||
| Synonyms |
compound 13j [PMID: 15149680]; UK-356,202
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 37 nM | |||
| External Link | ||||
| PAI-2 | Discontinued in Phase 2 | [17] | ||
| Synonyms |
N-Butyryl-DL-homoserine lactone; N-butanoyl-lhomoserine lactone; N-(2-oxooxolan-3-yl)butanamide; N-(2-oxotetrahydrofuran-3-yl)butanamide; N-Butyrylhomoserine lactone; 98426-48-3; N-(2-Oxotetrahydro-3-furanyl)butanamide; AC1L9ENE; SCHEMBL787006; Homoserine lactone, N-butanoyl-; N-Butanoyl-DL-homoserine lactone; VFFNZZXXTGXBOG-UHFFFAOYSA-N; LMFA08030002; AN-38291; N-Butyryl-DL-homoserine lactone, >
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| WX-UK1 | Discontinued in Phase 1/2 | [18] | ||
| Synonyms |
UKI-1; UNII-00LOF6890B; CHEMBL107955; 00LOF6890B; 220355-63-5; Wx-uk1 free base; compound 2r-L; GTPL6498; BDBM23891; 3-amidinophenylalanine deriv., 35; ZINC4426028; AKOS030526723; CS-5726; HY-100415; 1-Piperazinecarboxylic acid, 4-((2S)-3-(3-(aminoiminomethyl)phenyl)-1-oxo-2-(((2,4,6-tris(1-methylethyl)phenyl)sulfonyl)amino)propyl)-, ethyl ester; ethyl 4-[(2S)-3-(3-carbamimidoylphenyl)-2-[[2,4,6-tri(propan-2-yl)phenyl]sulfonylamino]propanoyl]piperazine-1-carboxylate; ethyl 4-[(2S)-3-(3-carbamimidoylphenyl)-2-{[2,4,6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 960 nM | |||
| External Link | ||||
| B-428 | Terminated | [19] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 79.43 nM | |||
| External Link | ||||
| UPA-targeted oncolytic Sendai virus | Investigative | [8] | ||
| Synonyms |
BioKnife; RSeV/Fct14(uPA2)dM; UPA-targeted oncolytic Sendai virus, DNAVEC
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CRA_10655 | Investigative | [20] | ||
| Synonyms |
AC1O4QGF
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 220 nM | |||
| External Link | ||||
| 5-Methylsulfanyl-thiophene-2-carboxamidine | Investigative | [21] | ||
| Synonyms |
CHEMBL28890; 2-Thiophenecarboximidamide, 5-(methylthio)-; SCHEMBL5982145; AXSQTCBARFBKPH-UHFFFAOYSA-N; BDBM50099912; 5-methylthiothiophene-2-carboxamidine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 6000 nM | |||
| External Link | ||||
| ATN-658 | Investigative | [8] | ||
| Synonyms |
ATN-291; ATN-292; HuATN-658; UPA mAbs, Attenuon; MAb (urokinase plasminogen activator system), Attenuon/Kyowa Hakko Kirin
Click to Show/Hide
|
|||
| MOA | Activator | |||
| External Link | ||||
| (2R)-1-(2,6-dimethylphenoxy)propan-2-amine | Investigative | [20] | ||
| Synonyms |
94991-73-8; (R)-1-(2,6-dimethylphenoxy)propan-2-amine; 2-Propanamine, 1-(2,6-dimethylphenoxy)-, (2R)-; CHEMBL147507; zlchem 1301; (R)-MEXILETINE; (R)-(-)-Mexiletine; AC1L47IL; SCHEMBL16082; BIDD:GT0498; ZINC20257; ZLE0076; DTXSID50241709; BDBM50135883; AKOS017529564; DB07129; AJ-08428; KB-209407; (2R)-1-(2,6-dimethylphenoxy)-2-propanamine; A821017; (R)-2-(2,6-Dimethyl-phenoxy)-1-methyl-ethylamine; UNII-1U511HHV4Z component VLPIATFUUWWMKC-SNVBAGLBSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 > 1000000 nM | |||
| External Link | ||||
| B-623 | Investigative | [22] | ||
| Synonyms |
149732-37-6
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 530 nM | |||
| External Link | ||||
| 4-chloro-1-guanidino-7-isoquinolinesulphonamide | Investigative | [23] | ||
| Synonyms |
223671-02-1; 1-GUANIDINO-4-CHLORO-7-SULFAMOYL-ISOQUINOLINE; 2-(4-chloro-7-sulfamoylisoquinolin-1-yl)guanidine; CHEMBL227782; SCHEMBL6437735; CTK4E9305; BDBM16132; DTXSID90586545; FXVHAOFNNRNCRJ-UHFFFAOYSA-N; AKOS015966420; 1-guanidino-7-sulfonamidoisoquinoline 6; ACM223671021; 4-chloro-1-guanidino-7-sulphamoylisoquinoline
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 140 nM | |||
| External Link | ||||
| 2-Amino-5-Hydroxy-Benzimidazole | Investigative | [24] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 200000 nM | |||
| External Link | ||||
| 6-(N-Phenylcarbamyl)-2-Naphthalenecarboxamidine | Investigative | [24] | ||
| Synonyms |
CHEMBL104937; 6-[(Z)-AMINO(IMINO)METHYL]-N-PHENYL-2-NAPHTHAMIDE; 6-carbamimidoyl-N-phenylnaphthalene-2-carboxamide; 1owe; uPa_7; AC1L9L6A; SCHEMBL4324160; ZINC2047486; BDBM50138670; 6-carbamimidoyl-N-phenyl-2-naphthamide; DB01977; 6-Carbamimidoyl-naphthalene-2-carboxylic acid phenylamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 630 nM | |||
| External Link | ||||
| Thieno[2,3-B]Pyridine-2-Carboxamidine | Investigative | [20] | ||
| Synonyms |
amino(thieno[2,3-b]pyridin-2-yl)methaniminium; [amino(thieno[2,3-b]pyridin-2-yl)methylidene]azanium; AC1L1KCT; BDBM14171; CTK7D1810; APC-7538
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Recombinant human pro-urokinase | Investigative | [8] | ||
| Synonyms |
Recombinant human pro-urokinase (myocardial infarction)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(1-Adamantyl)-N'-(4-Guanidinobenzyl)Urea | Investigative | [24] | ||
| Synonyms |
AGB; 1ejn; AC1L1HTN; SCHEMBL4328331; WX293T; SCHEMBL14524522; CTK7G4253; BDBM16176; WX-293T; DB03782; 3-adamantan-1-yl-1-[(4-carbamimidamidophenyl)methyl]urea; 3-(1-adamantyl)-1-[(4-carbamimidamidophenyl)methyl]urea; 3-(adamantan-1-yl)-1-[(4-carbamimidamidophenyl)methyl]urea; 1-(1-adamantyl)-3-[[4-(diaminomethylideneamino)phenyl]methyl]urea; 1-{4-[(diaminomethylidene)amino]benzyl}-3-tricyclo[3.3.1.13,7]dec-1-ylurea
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (4-guanidino-benzyl)-carbamic acid benzyl ester | Investigative | [25] | ||
| Synonyms |
CHEMBL391969
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 16000 nM | |||
| External Link | ||||
| 1-[4-(2-oxo-2-phenylethyl)phenyl]guanidine | Investigative | [20] | ||
| Synonyms |
SCHEMBL20553177; DB07122
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-methoxy-N'-(2-phenylacetyl)benzohydrazide | Investigative | [26] | ||
| Synonyms |
4-methoxy-N'-(phenylacetyl)benzohydrazide; benzohydrazide, 15; AC1LEZHC; CHEMBL244921; ARONIS002261; BDBM23731; ZINC68809; KS-00003VJE; MolPort-000-681-545; STK056698; AKOS000492246; MCULE-6943344531; ST040019; 4-methoxy-N -(phenylacetyl)benzohydrazide; KB-115318; 4-methoxy-N''-(2-phenylacetyl)benzohydrazide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2500 nM | |||
| External Link | ||||
| ATF-HI-8 | Investigative | [27] | ||
| Synonyms |
urokinase/urinary trypsin inhibitor chimera, Nissin/Hamamatsu University
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 1-guanidino-7-isoquinolinesulphonamide | Investigative | [23] | ||
| Synonyms |
SCHEMBL6436143; CHEMBL227583; 7-Isoquinolinesulfonamide, 1-[(aminoiminomethyl)amino]-; BDBM16130; NRVVFOKWKSWIIV-UHFFFAOYSA-N; 1-Guanidino-7-sulphamoylisoquinoline; 1-guanidino-7-sulfonamidoisoquinoline 4; 2-(7-sulfamoylisoquinolin-1-yl)guanidine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 280 nM | |||
| External Link | ||||
| CRA_8696 | Investigative | [20] | ||
| Synonyms |
AC1O4QGN
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 8 nM | |||
| External Link | ||||
| 1-guanidino-N-phenyl-7-isoquinolinesulphonamide | Investigative | [23] | ||
| Synonyms |
SCHEMBL6435184; CHEMBL227781; BDBM16131; NNEJXIJKGKRBBF-UHFFFAOYSA-N; 1-guanidino-7-sulfonamidoisoquinoline 5; 1-guanidino-7-phenylsulphamoylisoquinoline
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 160 nM | |||
| External Link | ||||
| (4-nitro-1H-pyrazol-1-yl)(o-tolyl)methanone | Investigative | [26] | ||
| Synonyms |
BAS 02052986; AC1LDSC0; N-Benzoylpyrazole deriv., 20; CHEMBL244072; ZINC37170; BDBM23712; MolPort-001-971-782; AKOS000577347; 2-methylphenyl 4-nitropyrazolyl ketone; MCULE-4726161589; ST033205; (4-Nitro-pyrazol-1-yl)-o-tolyl-methanone; (2-methylphenyl)-(4-nitropyrazol-1-yl)methanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 nM | |||
| External Link | ||||
| 4-iodobenzo[b]thiophene 2-carboxamidine | Investigative | [28] | ||
| Synonyms |
amino(4-iodo-1-benzothiophen-2-yl)methaniminium; ESI; AC1L1C5C; CTK7C3490; BDBM14169; APC-6860; CRA-6860; DB03136; [amino-(4-iodo-1-benzothiophen-2-yl)methylidene]azanium
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (4-nitro-1H-pyrazol-1-yl)(phenyl)methanone | Investigative | [26] | ||
| Synonyms |
BAS 02052938; AC1LDSCA; N-Benzoylpyrazole deriv., 5; 4-nitropyrazolyl phenyl ketone; CHEMBL244908; 1-benzoyl-4-nitro-1H-pyrazole; ZINC37183; BDBM23703; AKOS000577075; (4-nitropyrazol-1-yl)-phenylmethanone; MCULE-3158742884; ST033219; (4-Nitro-pyrazol-1-yl)-phenyl-methanone; SR-01000312627
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 18200 nM | |||
| External Link | ||||
| 2-nas-phe(3-am)-4-(2-guanidinoethyl)piperidine | Investigative | [29] | ||
| Synonyms |
(S)-3-(3-(4-(2-GUANIDINOETHYL)PIPERIDIN-1-YL)-2-(NAPHTHALENE-2-SULFONAMIDO)-3-OXOPROPYL)BENZIMIDAMIDE; 2gv6; AC1OA9XW; CHEMBL210771; SCHEMBL12960819; BDBM23902; 3-amidinophenylalanine deriv., 8; 3-[(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-yl]-2-(naphthalene-2-sulfonamido)-3-oxopropyl]benzene-1-carboximidamide; 3-{(2S)-3-[4-(2-carbamimidamidoethyl)piperidin-1-yl]-2-[(naphthalen-2-ylsulfonyl)amino]-3-oxopropyl}benzenecarboximidamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1300 nM | |||
| External Link | ||||
| (4-bromo-1H-pyrazol-1-yl)(p-tolyl)methanone | Investigative | [26] | ||
| Synonyms |
AC1LDSCF; N-Benzoylpyrazole deriv., 7; CHEMBL244909; ZINC37188; BDBM23705; MolPort-002-174-815; AKOS000576906; 4-bromopyrazolyl 4-methylphenyl ketone; MCULE-8194486011; ST033225; AB00100488-01; (4-bromopyrazol-1-yl)-(4-methylphenyl)methanone; SR-01000521427; SR-01000521427-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1700 nM | |||
| External Link | ||||
| Benzamidine | Investigative | [24] | ||
| Synonyms |
Benzimidamide; Benzenecarboximidamide; 618-39-3; Phenylamidine; UNII-KUE3ZY3J1F; NSC 243704; CCRIS 2952; Benzamidine (Protonated); EINECS 210-546-3; KUE3ZY3J1F; CHEMBL20936; CHEBI:41033; PXXJHWLDUBFPOL-UHFFFAOYSA-N; BDN; 1oss; 2ast; 1bra; Benzenecarboxamidine; 1v2m; 1v2j; 1h4w; 1ce5; 1c5o; 2j9n; 1f5k; 1c5p; 1v2v; 1v2s; 1v2l; 1c5z; 1v2u; AC1L1DFX; Lopac-B-6506; ACMC-1B9LG; SCHEMBL9207; Lopac0_000203; MLS001066369; GTPL7566; AC1Q1U98; DTXSID8045012; 1670-14-0 (hydrochloride); ZINC36634; CTK2F5055; 1w80; 1j16; 1j15; MolPort-000-001-395
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 180000 nM | |||
| External Link | ||||
| (3-nitro-1H-pyrazol-1-yl)(p-tolyl)methanone | Investigative | [26] | ||
| Synonyms |
(4-methylphenyl)(3-nitro-1H-pyrazol-1-yl)methanone; AC1LF99H; N-Benzoylpyrazole deriv., 18; CHEMBL244939; ZINC78678; BDBM23710; A2012/0084499; MolPort-002-705-255; STK760276; AKOS001746005; 4-methylphenyl 3-nitropyrazolyl ketone; MCULE-9242110239; ST073763; (4-methylphenyl)-(3-nitropyrazol-1-yl)methanone; SR-01000524341; 1-[(4-methylphenyl)carbonyl]-3-nitro-1H-pyrazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3100 nM | |||
| External Link | ||||
| 1-benzoyl-N-phenyl-1H-pyrazole-3-carboxamide | Investigative | [26] | ||
| Synonyms |
AC1LERN1; TimTec1_001906; Oprea1_174326; N-Benzoylpyrazole deriv., 6; CHEMBL388239; BDBM23704; MolPort-001-664-680; ZINC115253; HMS1539G14; STK398485; AKOS003748858; MCULE-1390939767; 1-benzoyl-N-phenylpyrazole-3-carboxamide; ST025783; N-phenyl[1-(phenylcarbonyl)pyrazol-3-yl]carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1500 nM | |||
| External Link | ||||
| (3,4-dichlorophenyl)(1H-pyrazol-1-yl)methanone | Investigative | [26] | ||
| Synonyms |
AC1LDSBN; BAS 02052919; N-Benzoylpyrazole deriv., 8; CHEMBL244917; ZINC37156; BDBM23706; MolPort-001-906-669; 3,4-dichlorophenyl pyrazolyl ketone; STK044137; AKOS000577014; MCULE-6190861509; ST033189; (3,4-dichlorophenyl)-pyrazol-1-ylmethanone; (3,4-Dichloro-phenyl)-pyrazol-1-yl-methanone; 1-[(3,4-dichlorophenyl)carbonyl]-1H-pyrazole; SR-01000521426; SR-01000521426-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2600 nM | |||
| External Link | ||||
| 2-(2-Hydroxy-phenyl)-1H-indole-5-carboxamidine | Investigative | [30] | ||
| Synonyms |
amino[2-(2-hydroxyphenyl)-1H-indol-5-yl]methaniminium; AC1L1BMI; BDBM13942; CTK8A0256; APC-8328; DB02463; [amino-[2-(2-hydroxyphenyl)-1H-indol-5-yl]methylidene]azanium
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Fucose | Investigative | [24] | ||
| Synonyms |
L-galactomethylose; 6-Desoxygalactose; SCHEMBL13092958; AKOS030212707
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [31] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [32] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [33] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [34] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [34] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [35] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [34] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [32] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [36] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [37] | ||
| External Link | ||||
| CV301 | Phase 2 | [38] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [39] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [40] | ||
| External Link | ||||
| RG7221 | Phase 2 | [41] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [42] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [43] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [44] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [45] | ||
| External Link | ||||
| MGD007 | Phase 1 | [41] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [46] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [34] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [47] | ||
| External Link | ||||
| Nimesulide | Terminated | [48] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [49] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [50] | ||
| External Link | ||||
References