m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03588
|
[1], [2] | |||
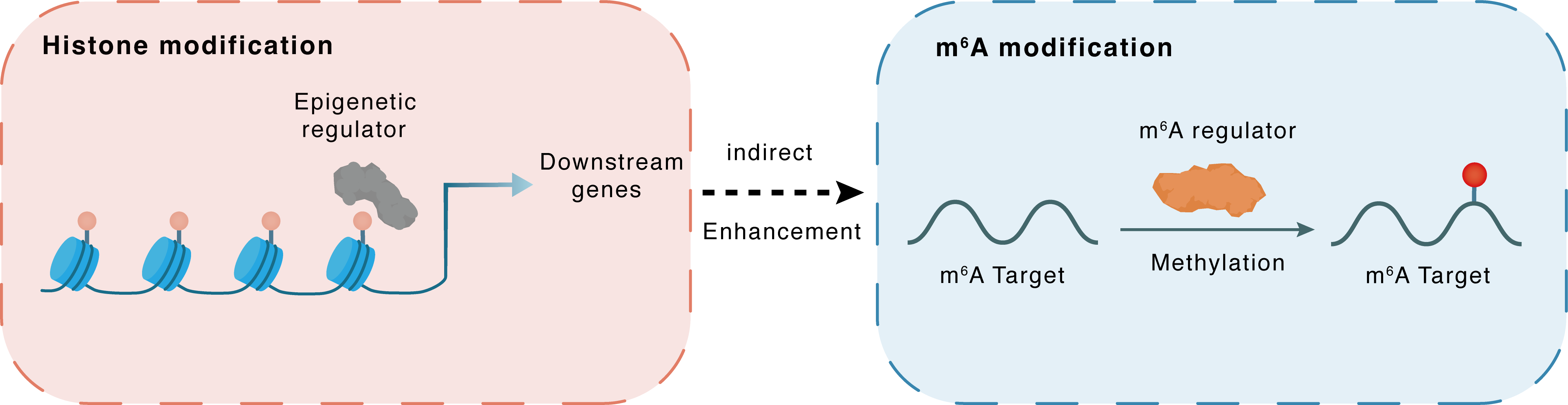 Histone modification
H3K4me3
SMYD2
LINC01605
Indirect
Enhancement
m6A modification
MAPK14
MAPK14
METTL3
Methylation
Histone modification
H3K4me3
SMYD2
LINC01605
Indirect
Enhancement
m6A modification
MAPK14
MAPK14
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Mitogen-activated protein kinase 14 (p38/MAPK14) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | N-lysine methyltransferase SMYD2 (SMYD2) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 4 trimethylation (H3K4me3) | View Details | |||
| Downstream Gene | LINC01605 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | LINC01605 was regulated by SMYD2-EP300-mediated modifications of histone Histone H3 lysine 4 trimethylation (H3K4me3) as well as H3K27ac. LINC01605 was found to bind to METTL3 and promote the m6A modification of SPTBN2 mRNA, thereby facilitating the translation of SPTBN2. METTL3 played a tumor-suppressive role in Colorectal cancer cell proliferation, migration and invasion through Mitogen-activated protein kinase 14 (p38/MAPK14)/ERK pathways, which indicated that METTL3 was a novel marker for CRC carcinogenesis, progression and survival. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell proliferation | ||||
| Cell migration | |||||
| Cell invasion | |||||
In-vitro Model |
HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| DLD-1 | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| KM12 | Colon carcinoma | Homo sapiens | CVCL_1331 | ||
| HCT 8 | Colon adenocarcinoma | Homo sapiens | CVCL_2478 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Mitogen-activated protein kinase 14 (p38/MAPK14) | 75 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Ozagrel | Phase 4 | [3] | ||
| Synonyms |
Ozagrel (ophthalmic, eye disorders); Ozagrel (ophthalmic, eye disorders), Kissei/ Teika
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Losmapimod | Phase 3 | [3] | ||
| Synonyms |
585543-15-3; GSK-AHAB; GW856553; GW856553X; UNII-F2DQF16BXE; F2DQF16BXE; GW-856553; 6-[5-(cyclopropylcarbamoyl)-3-fluoro-2-methylphenyl]-N-(2,2-dimethylpropyl)pyridine-3-carboxamide; Losmapimod [USAN:INN]; Losmapimod (GW856553X); GSKAHAB; GW 856553X; SB 856553; Losmapimod (USAN/INN); SCHEMBL1070401; GTPL7835; CHEMBL1088752; EX-A486; CHEBI:131167; KKYABQBFGDZVNQ-UHFFFAOYSA-N; MolPort-009-194-138; losmapimod pound> HMS3653G19; BCP09909; AOB87105; ZINC35793138; s7215; BDBM50418610; 2523AH; AKOS015994587
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 7.943 nM | |||
| External Link | ||||
| VX-702 | Phase 2a | [4] | ||
| Synonyms |
ST51054128; I14-1965; EC-000.2363; 6-[carbamoyl-(2,6-difluorophenyl)amino]-2-(2,4-difluorophenyl)pyridine-3-carboxamide
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| Dilmapimod | Phase 2 | [3] | ||
| Synonyms |
SB-681323; 444606-18-2; UNII-Q3238VQW0N; GW 681323; SB 681323; Q3238VQW0N; GW681323; SB681323; Dilmapimod [USAN:INN]; GSK 681323; Dilmapimod (USAN/INN); GTPL7815; SCHEMBL1065268; CHEMBL2103838; ORVNHOYNEHYKJG-UHFFFAOYSA-N; BCP23819; ZINC34997404; CS-6731; DB12140; SB16737; 8-(2,6-difluorophenyl)-4-(4-fluoro-2-methylphenyl)-2-[[2-hydroxy-1-(hydroxymethyl)ethyl]amino]-Pyrido[2,3-d]pyrimidin-7(8H)-one; HY-10404; GW-681323; DB-070558; FT-0718750; J3.498.915J; D09602
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| VX-745 | Phase 2 | [5] | ||
| Synonyms |
5-(2,6-Dichlorophenyl)-2-((2,4-difluorophenyl)thio)-6H-pyrimido[1,6-b]pyridazin-6-one; 209410-46-8; Neflamapimod; VX 745; VX745; VRT-031745; UNII-TYL52QM320; TYL52QM320; CHEBI:90528; 5-(2,6-dichlorophenyl)-2-(2,4-difluorophenylthio)-6H-pyrimido[1,6-b]pyridazin-6-one; 5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfanylpyrimido[1,6-b]pyridazin-6-one; Neflamapimod (USAN); Neflamapimod [USAN]; AK-44905; C19H9Cl2F2N3OS; 5-(2,6-Dichlorophenyl)-2-[(2,4-Difluorophenyl)sulfanyl]-6h-Pyrimido[1,6-B]pyridazin-6-One; VX745, VX-745; 5-(2,6-Dichlorophenyl)-2-((2,4-difluorophenyl)thio)-6H-pyrimido(1,6-b)pyridazin-6-one; Vertex 745 (VX745)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 70 nM | |||
| External Link | ||||
| PMID25991433-Compound-O2 | Patented | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID25991433-Compound-L2 | Patented | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 5000 nM | |||
| External Link | ||||
| PMID25991433-Compound-L3 | Patented | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 10000 nM | |||
| External Link | ||||
| PMID25991433-Compound-L1 | Patented | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 190 nM | |||
| External Link | ||||
| PMID25991433-Compound-F2 | Patented | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3400 nM | |||
| External Link | ||||
| PMID25991433-Compound-A1 | Patented | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10000 nM | |||
| External Link | ||||
| PAMAPIMOD | Discontinued in Phase 2 | [7] | ||
| Synonyms |
449811-01-2; RO 4402257; UNII-8S2C9V11K4; Pamapimod (R-1503, Ro4402257); CHEMBL1090089; CHEBI:90685; 8S2C9V11K4; Ro4402257; Ro-4402257; R1503; R 1503; R-1503; 6-(2,4-Difluorophenoxy)-2-{[3-Hydroxy-1-(2-Hydroxyethyl)propyl]amino}-8-Methylpyrido[2,3-D]pyrimidin-7(8h)-One; Pamapimod [USAN:INN]; 6-(2,4-Difluorophenoxy)-2-((3-hydroxy-1-(2-hydroxyethyl)propyl)amino)-8-methylpyrido(2,3-d)pyrimidin-7(8H)-one; 6-(2,4-Difluorophenoxy)-2-[[3-hydroxy-1-(2-hydroxyethyl)propyl]amino]-8-methylpyrido[2,3-d]pyrimidin-7(8H)-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 14 nM | |||
| External Link | ||||
| R-1487 | Discontinued in Phase 1 | [8] | ||
| Synonyms |
449811-92-1; 6-(2,4-difluorophenoxy)-8-methyl-2-((tetrahydro-2H-pyran-4-yl)amino)pyrido[2,3-d]pyrimidin-7(8H)-one; UNII-IO0DCY55NQ; IO0DCY55NQ; CHEMBL1230122; 6-(2,4-difluorophenoxy)-8-methyl-2-[(tetrahydro-2H-pyran-4-yl)amino]-Pyrido[2,3-d]pyrimidin-7(8H)-one; 6-(2,4-difluorophenoxy)-8-methyl-2-(tetrahydro-2h-pyran-4-ylamino)pyrido[2,3-d]pyrimidin-7(8h)-one; Kinome_3762; SCHEMBL5120612; KKKRKRMVJRHDMG-UHFFFAOYSA-N; HMS3401C13; BDBM50341342; ZINC58633224; AKOS027420928; NCGC00262195-02; KB-80224; ACM449811921
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| FR167653 | Discontinued in Phase 1 | [9] | ||
| Synonyms |
HMPQTEPEMQZWQH-ROUUACIJSA-N; 2-(5-AMINO-6-OXO-2-PHENYL-6H-PYRIMIDIN-1-YL)-N-[2-(5-TERT-BUTYL-1,3,4-OXADIAZOL-2-YL)-1-(METHYLETHYL)-2-HYDROXYETHYL]ACETAMIDE; AC1NR9VL; SCHEMBL15034365; 2-(5-amino-6-oxo-2-phenylpyrimidin-1-yl)-N-[(1S,2S)-1-(5-tert-butyl-1,3,4-oxadiazol-2-yl)-1-hydroxy-3-methylbutan-2-yl]acetamide; ONO-6818
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-242235 | Discontinued in Phase 1 | [10] | ||
| Synonyms |
193746-75-7; SB242235; SB 242235; 4-(4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl)-2-methoxypyrimidine; CHEMBL95692; 4-[5-(4-fluorophenyl)-3-piperidin-4-ylimidazol-4-yl]-2-methoxypyrimidine; 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]-2-methoxypyrimidine; Kinome_3169; SCHEMBL2267209; BDBM15458; SYN1076; PDTYLGXVBIWRIM-UHFFFAOYSA-N; MolPort-028-720-427; HMS3244I18; HMS3244J17; HMS3244I17; EX-A1881; BCP05992; ZINC1487129; 3254AH; RS0056; AKOS027323444; CS-2097; NCGC00345831-01; NCGC00345831-03
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19 nM | |||
| External Link | ||||
| SB 235699 | Discontinued in Phase 1 | [5] | ||
| Synonyms |
4-(4-(4-Fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl)pyridine; VK-19911; CHEMBL279416; UNII-NP7J08ZRYY; NP7J08ZRYY; 180869-32-3; SCHEMBL140202; HEP 689; sb235699; BDBM50099331; SB-235699; 4-(5-(4-Fluorophenyl)-3-(4-piperidyl)imidazol-4-yl)pyridine; Pyridine, 4-(4-(4-fluorophenyl)-1-(4-piperidinyl)-1H-imidazol-5-yl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 38 nM | |||
| External Link | ||||
| SC-102 | Terminated | [11] | ||
| Synonyms |
4-[3-(4-Fluorophenyl)-1h-Pyrazol-4-Yl]pyridine; 3hvc; GG5; SCHEMBL1554633; CHEMBL1233024; BDBM19429; BILJSHVAAVZERY-UHFFFAOYSA-N; ZINC16052350; DB07829; 5-(4-Fluorophenyl)-4-(pyridin-4-yl)pyrazole; 4-[3-(4-Fluorophenyl)-1H-pyrazole-4-yl]pyridine; 4-(3-(4-fluorophenyl)-1h-pyrazol-4-yl); SPC-0038B; SPC-0172; Temoporfin derivatives (cancer); Temoporfin derivatives (cancer), Scotia Holdings
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 67 nM | |||
| External Link | ||||
| SB220025 | Terminated | [12] | ||
| Synonyms |
3erk; sb 220025; SB-220025; CHEMBL274064; 165806-53-1; CHEBI:82713; 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]pyrimidin-2-amine; 4-(4-FLUOROPHENYL)-1-(4-PIPERIDINYL)-5-(2-AMINO-4-PYRIMIDINYL)-IMIDAZOLE; SB4; 5-(2-Amino-4-pyrimidinyl)-4-(4-fluorophenyl)-1-(4-piperidinlyl)imidazole; 4-[5-(4-fluorophenyl)-3-(4-piperidyl)imidazol-4-yl]pyrimidin-2-amine; SB-220025-A; 1-(4-piperidinyl)-4-(4-fluorophenyl)-5-(2-(amino)-4-pyrimidinyl)imidazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19 nM | |||
| External Link | ||||
| SB 203580 | Terminated | [13] | ||
| Synonyms |
152121-47-6; SB-203580; SB203580; 4-(4-(4-fluorophenyl)-2-(4-(methylsulfinyl)phenyl)-1H-imidazol-5-yl)pyridine; 4-(4-Fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-5-(4-pyridyl)-1H-imidazole; CHEBI:90705; 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)-1H-imidazole; CHEMBL10; RWJ 64809; 4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole; 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine; PB 203580; UNII-WS78QR6DSV
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| 9-Benzyl-6-phenylsulfanyl-9H-purine | Investigative | [14] | ||
| Synonyms |
SCHEMBL6662063
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(3-(trifluoromethyl)benzyl)-4-phenoxybenzamide | Investigative | [15] | ||
| Synonyms |
CHEMBL198563
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 450 nM | |||
| External Link | ||||
| UCB-1277763 | Investigative | [16] | ||
| Synonyms |
P38 kinase inhibitors (inflammation), UCB
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Small molecule 34 | Investigative | [12] | ||
| Synonyms |
3-(2-(4-pyridyl)ethyl)indole; 3-(2-PYRIDIN-4-YLETHYL)-1H-INDOLE; INHIBITOR OF P38 KINASE; 3-(2-(Pyridin-4-yl)ethyl)-1H-indole; 3-[2-(pyridin-4-yl)ethyl]-1H-indole; 3-(2-Pyridin-4-yl-ethyl)-1H-indole; L12; Maybridge1_000718; Cambridge id 5373101; AC1L1E9J; Oprea1_574113; Oprea1_420786; SCHEMBL735580; CHEMBL193156; HMS543I14; ZINC94256; BDBM13346; UUEYCHLWAOBOHG-UHFFFAOYSA-N; 1w84; MolPort-001-886-488; HMS3604M06; 3-[2-(4-pyridyl)-ethyl]-indole; STL328644; CCG-45397; 3-[2-(4-Pyridyl)ethyl]-1H-indole; AKOS000545583; MCULE-4657429599
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-218655 | Investigative | [17] | ||
| Synonyms |
1bmk; 4-[1-(cyclopropylmethyl)-4-(4-fluorophenyl)-1h-imidazol-5-yl]pyrimidin-2-amine; 4-(FLUOROPHENYL)-1-CYCLOPROPYLMETHYL-5-(2-AMINO-4-PYRIMIDINYL)IMIDAZOLE; 165806-51-9; SB 218655; C14316; 4-[3-(cyclopropylmethyl)-5-(4-fluorophenyl)imidazol-4-yl]pyrimidin-2-amine; SB5; AC1L1JRC; AC1Q4ON9; SCHEMBL4323457; CHEMBL590753; BDBM15238; CTK4D2173; DTXSID50274456; sb218655; AKOS030547879
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 25 nM | |||
| External Link | ||||
| 9-Benzyl-6-(4-fluoro-phenylsulfanyl)-9H-purine | Investigative | [14] | ||
| Synonyms |
SCHEMBL6659707
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Phenylsulfanyl-9H-purine | Investigative | [14] | ||
| Synonyms |
Purine, 6-(phenylthio)-; 6-(Phenylthio)purine; 1H-Purine, 6-(phenylthio)-; 5450-35-1; NSC 15746; UNII-3KH11DU8DY; 6-phenylsulfanyl-7H-purine; BRN 0013668; 3KH11DU8DY; AI3-52068; AC1Q4XUH; AC1L3TI0; 4-26-00-01985 (Beilstein Handbook Reference); 9H-Purine,6-(phenylthio)-; SCHEMBL796397; 6-(phenylsulfanyl)-7H-purine; CHEMBL175600; 9H-Purine, 6-(phenylthio)-; CTK5A1336; DTXSID20202908; NSC15746; ZINC13608565; NSC-15746; AKOS030562402
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Talmapimod | Investigative | [18] | ||
| Synonyms |
SCIO-469; 309913-83-5; Scios 469; UNII-B1E00KQ6NT; SCIO 469; B1E00KQ6NT; CHEMBL514201; SCIO 469 hydrochloride; CHEBI:90683; Talmapimod hydrochloride; 2-(6-Chloro-5-{[(2r,5s)-4-(4-Fluorobenzyl)-2,5-Dimethylpiperazin-1-Yl]carbonyl}-1-Methyl-1h-Indol-3-Yl)-N,N-Dimethyl-2-Oxoacetamide; 2-[6-chloro-5-[(2R,5S)-4-[(4-fluorophenyl)methyl]-2,5-dimethylpiperazine-1-carbonyl]-1-methylindol-3-yl]-N,N-dimethyl-2-oxoacetamide; 750646-72-1; Talmapimod [USAN]; Talmapimod [USAN:INN]
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 96 nM | |||
| External Link | ||||
| N-(4-fluorobenzyl)-N-(pyridin-4-yl)-2-naphthamide | Investigative | [19] | ||
| Synonyms |
CHEMBL1091737
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1260 nM | |||
| External Link | ||||
| Dihydro-quinolinone | Investigative | [17] | ||
| Synonyms |
553-03-7; 3,4-Dihydro-2(1H)-quinolinone; 3,4-Dihydroquinolin-2(1H)-one; Hydrocarbostyril; 1,2,3,4-tetrahydroquinolin-2-one; 3,4-Dihydrocarbostyril; 3,4-dihydro-1H-quinolin-2-one; 2-Oxo-1,2,3,4-tetrahydroquinoline; 3,4-Dihydro-2-quinolinol; o-Aminohydrocinnamic acid lactam; UNII-2CKG6TX32F; 3,4-Dihydro-2(1H)quinolinone; 2(1H)-Quinolinone, 3,4-dihydro-; NSC 49170; 2CKG6TX32F; CHEMBL388582; TZOYXRMEFDYWDQ-UHFFFAOYSA-N; 3,4-DIHYDRO-2-QUINOLONE; MFCD00016722; 3,4-Dihydro-2(1H)-quinolinone, 98%
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.74 nM | |||
| External Link | ||||
| (5-amino-1-phenyl-1H-pyrazol-4-yl)phenylmethanone | Investigative | [20] | ||
| Synonyms |
54606-37-0; (5-Amino-1-phenyl-1H-pyrazol-4-yl)phenyl methanone; (5-AMINO-1-PHENYL-1H-PYRAZOL-4-YL)(PHENYL)METHANONE; 4-benzoyl-5-aminopyrazole 1; CHEMBL203333; (5-amino-1-phenyl-1H-pyrazol-4-yl)-phenyl-methanone; SCHEMBL13662120; BDBM15714; 5-amino-4-benzoyl-1-phenylpyrazole; ZINC12365464; 1-Phenyl-4-benzoyl-1H-pyrazol-5-amine; 4-benzoyl-1-phenyl-1H-pyrazol-5-amine; KB-208557; FT-0728731; (5-Amino-1-phenyl-1H-pyrazol-4-yl)(phenyl)methanone, AldrichCPR
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 570 nM | |||
| External Link | ||||
| 3-(1-NAPHTHYLMETHOXY)PYRIDIN-2-AMINE | Investigative | [11] | ||
| Synonyms |
3-(naphthalen-1-ylmethoxy)pyridin-2-amine; 2-amino-3-(1-naphthylmethyloxy)pyridine; LI4; 1wbw; CHEMBL195393; AC1NS185; SCHEMBL6080116; BDBM13340; AKOS009255557; DB08093; Pyridine derived fragment based inhibitor 5
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID22521646C12 | Investigative | [21] | ||
| Synonyms |
GTPL8125; BDBM50420761
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(4-methyl-benzyl)-4-phenoxy-benzamide | Investigative | [15] | ||
| Synonyms |
CHEMBL199174; AC1OVMY0; BDBM50174096
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1600 nM | |||
| External Link | ||||
| 2-Chlorophenol | Investigative | [12] | ||
| Synonyms |
o-Chlorophenol; 95-57-8; Phenol, 2-chloro-; 2-Hydroxychlorobenzene; o-Chlorphenol; o-Chlorophenic acid; Phenol, o-chloro-; 2-Chloro-1-hydroxybenzene; CHLOROPHENOL; Septi-Kleen; 2-chloro-phenol; RCRA waste number U048; Pine-O Disinfectant; Caswell No. 203; o-Chlorphenol [German]; UNII-K9KAV4K6BN; Phenol, chloro-; NSC 2870; CCRIS 640; HSDB 1415; EINECS 202-433-2; K9KAV4K6BN; RCRA waste no. U048; 1-Chloro-2-hydroxybenzene; EPA Pesticide Chemical Code 062204; AI3-09060; CHEBI:47083; ISPYQTSUDJAMAB-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK-280 | Investigative | [16] | ||
| Synonyms |
P38 kinase inhibitors (inflammation), GSK
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-216995 | Investigative | [17] | ||
| Synonyms |
PYRIDINYLIMIDAZOLE; CHEMBL96741; sb216995; 4-[1-(cyclopropylmethyl)-4-(4-fluorophenyl)-1h-imidazol-5-yl]pyridine; 4-(4-FLUOROPHENYL)-1-CYCLOROPROPYLMETHYL-5-(4-PYRIDYL)-IMIDAZOLE; SB6; 4-[3-(cyclopropylmethyl)-5-(4-fluorophenyl)imidazol-4-yl]pyridine; 1bl6; AC1L1JR9; SCHEMBL4915662; CTK7C0283; BDBM15237; ZINC2047872; DB08522
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 160 nM | |||
| External Link | ||||
| N-(4-(trifluoromethyl)benzyl)-4-phenoxybenzamide | Investigative | [15] | ||
| Synonyms |
CHEMBL199074
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1400 nM | |||
| External Link | ||||
| PHA-666859 | Investigative | [16] | ||
| Synonyms |
SC-409; SD-0006; P38-alpha kinase inhibitor (inflammation/diabetic retinopathy); P38-alpha kinase inhibitor (inflammation/diabetic retinopathy), Pfizer
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-(4-Fluoro-benzyl)-6-phenylsulfanyl-9H-purine | Investigative | [14] | ||
| Synonyms |
CHEMBL176245
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SB-227931 | Investigative | [22] | ||
| Synonyms |
CHEMBL371720; BDBM50173166
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 46 nM | |||
| External Link | ||||
| N-(3-(trifluoromethoxy)benzyl)-4-phenoxybenzamide | Investigative | [15] | ||
| Synonyms |
CHEMBL198832
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 600 nM | |||
| External Link | ||||
| 6-(4-Fluoro-phenylsulfanyl)-9H-purine | Investigative | [14] | ||
| Synonyms |
6-(4-fluorophenylsulfanyl)-9H-purine; 736142-69-1; CHEMBL175603
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-PHENOXY-N-(PYRIDIN-2-YLMETHYL)BENZAMIDE | Investigative | [11] | ||
| Synonyms |
CHEMBL199237; CHEBI:41135; 4-phenoxy-N-[(pyridin-2-yl)methyl]benzamide; BI5; 1zyj; AC1M5EGY; Oprea1_773674; MolPort-004-000-036; HMS3604L15; BDBM50174097; ZINC13284470; AKOS027682522; MCULE-5005773275; DB07459; Z30166389
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1500 nM | |||
| External Link | ||||
| DP-802 | Investigative | [16] | ||
| Synonyms |
P38-alpha inhibitors (inflammation); P38-alpha inhibitors (inflammation), Deciphera
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro-3201195 | Investigative | [23] | ||
| Synonyms |
[5-AMINO-1-(4-FLUOROPHENYL)-1H-PYRAZOL-4-YL](3-{[(2R)-2,3-DIHYDROXYPROPYL]OXY}PHENYL)METHANONE; CHEMBL203567; CHEBI:45116; Ro 320-1195; 2gfs; PQB; [5-amino-1-(4-fluorophenyl)-1H-pyrazol-4-yl](3-{[(2S)-2,3-dihydroxypropyl]oxy}phenyl)methanone; aminopyrazole 63; RO3201195; AC1NS1L4; SCHEMBL4108597; BDBM15754; IJDQETGUEUJVTB-HNNXBMFYSA-N; DB08424; Ro-320-1195; J3.504.577E; UNII-TE3ESF890C component IJDQETGUEUJVTB-HNNXBMFYSA-N; 5-amino-1-(4-fluorophenyl)-4-[3-{2(S),3-dihydroxypropoxy}benzoyl]pyrazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 250 nM | |||
| External Link | ||||
| 4-(2-Ethyl-4-m-tolyl-thiazol-5-yl)-pyridine | Investigative | [24] | ||
| Synonyms |
CHEMBL198334; BDBM50173628
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15 nM | |||
| External Link | ||||
| Triazolopyridine | Investigative | [11] | ||
| Synonyms |
CP-808844; 6-[4-(4-FLUOROPHENYL)-1,3-OXAZOL-5-YL]-3-ISOPROPYL[1,2,4]TRIAZOLO[4,3-A]PYRIDINE; 1zzl; AC1NRDFA; Triazolopyridine deriv. 25; SCHEMBL40860; CHEMBL194322; BDBM15414; DB04797; 6-[4-(4-fluorophenyl)-1,3-oxazol-5-yl]-3-(1-methylethyl)[1,2,4]triazolo[4,3-a]pyridine; 6-[4-(4-Fluoro-phenyl)-oxazol-5-yl]-3-isopropyl-[1,2,4]-triazolo[4,3-a]pyridine; 4-(4-fluorophenyl)-5-[3-(propan-2-yl)-[1,2,4]triazolo[3,4-a]pyridin-6-yl]-1,3-oxazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| 3-(Benzyloxy)Pyridin-2-Amine | Investigative | [12] | ||
| Synonyms |
2-Amino-3-benzyloxypyridine; 24016-03-3; 3-Benzyloxy-2-pyridylamine; 2-Pyridinamine, 3-(phenylmethoxy)-; 3-benzyloxypyridin-2-amine; 3-Benzyloxy-2-aminopyridine; 2-amino-3-benzyloxy pyridine; 3-phenylmethoxypyridin-2-amine; 2-Amino-3-(benzyloxy)pyridine; 3-(phenylmethoxy)-2-pyridinamine; MFCD00006316; 3IP; 3-(phenylmethoxy)-2-pyridylamine; 2-Amino-3-benzyloxypyridine, 98.5%; 3-(benzyloxy)pyridin-2-amin; 3fty; EINECS 245-983-9; PubChem2575; 1w7h; ACMC-1COKO; AC1L3JRI; Maybridge1_001801; AC1Q52WQ
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100000 nM | |||
| External Link | ||||
| 6-Benzylsulfanyl-9H-purine | Investigative | [14] | ||
| Synonyms |
Purine, 6-(benzylthio)-; 6-Benzothiopurine; 6-Benzyl MP; 724-34-5; 6-(Benzylthio)purine; 6-Benzylmercaptopurine; 6-Benzyl-MP; SRI 673; NSC 29421; 6-(benzylsulfanyl)-9H-purine; Purine, 6-benzylthio-; 6-((Phenylmethyl)thio)-1H-purine; EINECS 211-965-4; 6-(benzylthio)-9H-purine; AI3-50277; 6-[BENZYLTHIO]-1H-PURINE; 1H-Purine, 6-[(phenylmethyl)thio]-; 1H-Purine, 6-((phenylmethyl)thio)-; 6-[(PHENYLMETHYL)THIO]-1H-PURINE; 6-benzylthiopurine; AC1Q4XUO; CBMicro_000185; 6-(phenylmethylthio)purine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Phenyl-(3-phenyl-1H-indazol-6-yl)-amine | Investigative | [25] | ||
| Synonyms |
CHEMBL383177; N,3-diphenyl-1H-indazol-6-amine; SCHEMBL6582340
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 30 nM | |||
| External Link | ||||
| IN-1130 | Investigative | [26] | ||
| Synonyms |
UNII-KW4O83PQ97; 868612-83-3; CHEMBL492634; KW4O83PQ97; IN 1130; SCHEMBL139815; MolPort-042-665-839; ZINC13985930; BDBM50252542; AKOS032962854; Benzamide, 3-((5-(6-methyl-2-pyridinyl)-4-(6-quinoxalinyl)-1H-imidazol-2-yl)methyl)-; Benzamide, 3-((4-(6-methyl-2-pyridinyl)-5-(6-quinoxalinyl)-1H-imidazol-2-yl)methyl)-; NCGC00386726-01; KB-274188; 3-[[5-(6-Methyl-2-pyridinyl)-4-(6-quinoxalinyl)-1H-imidazol-2-yl]methyl]benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 480 nM | |||
| External Link | ||||
| 4-Phenylsulfanyl-7H-pyrrolo[2,3-d]pyrimidine | Investigative | [14] | ||
| Synonyms |
4-(Phenylsulfanyl)-7H-pyrrolo[2,3-d]pyrimidine; 736142-84-0; 7h-pyrrolo[2,3-d]pyrimidine,4-(phenylthio)-; SCHEMBL191691; CHEMBL360302; TUBUPSQEQADRHY-UHFFFAOYSA-N; ZINC13608572; KB-270401; 7H-Pyrrolo[2,3-d]pyrimidine, 4-(phenylthio)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Tyrphostin ag-1478 | Investigative | [27] | ||
| Synonyms |
AG-1478; 153436-53-4; Tyrphostin AG 1478; N-(3-chlorophenyl)-6,7-dimethoxyquinazolin-4-amine; 175178-82-2; 4-(3-Chloroanilino)-6,7-dimethoxyquinazoline; AG 1478; AG1478; TYRPHOSTIN; 4-Quinazolinamine, N-(3-chlorophenyl)-6,7-dimethoxy-; UNII-SUH0SEZ9HY; SUH0SEZ9HY; AG-1478 hydrochloride; AG-1478 (Tyrphostin AG-1478); CHEMBL7917; CHEBI:75404; N-(3-Chlorophenyl)-6,7-dimethoxy-4-quinazolinamine; NSC-693255; AK-63142; N-(3-chlorophenyl)-6,7-dimethoxy-quinazolin-4-amine; BRD6408; BRD-6408; SR-01000076156; NSC693255
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 560 nM | |||
| External Link | ||||
| RWJ-68354 | Investigative | [28] | ||
| Synonyms |
CHEMBL115769; 2-(4-FLUOROPHENYL)-4-METHOXY-3-(PYRIDIN-4-YL)-1H-PYRROLO[2,3-B]PYRIDIN-6-AMINE; rwj68354; SCHEMBL149547; BDBM15457; BDBM50215267; AKOS000280165
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 52 nM | |||
| External Link | ||||
| GW-788388 | Investigative | [29] | ||
| Synonyms |
452342-67-5; GW788388; 4-(4-(3-(pyridin-2-yl)-1H-pyrazol-4-yl)pyridin-2-yl)-N-(tetrahydro-2H-pyran-4-yl)benzamide; GW 788388; UNII-N14114957J; CHEMBL202887; N14114957J; 4-[4-[3-(Pyridin-2-yl)-1H-pyrazol-4-yl]pyridin-2-yl]-N-(tetrahydropyran-4-yl)benzamide; N-(oxan-4-yl)-4-{4-[3-(pyridin-2-yl)-1H-pyrazol-4-yl]pyridin-2-yl}benzamide; 4-(4-(3-(Pyridin-2-yl)-1H-pyrazol-4-yl)pyridin-2-yl)-N-(tetrahydropyran-4-yl)benzamide; cc-69; SCHEMBL373524; SCHEMBL17926774; CTK8C0589; DTXSID70196444; EX-A122; AOB2606
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7280 nM | |||
| External Link | ||||
| IN-1166 | Investigative | [26] | ||
| Synonyms |
UNII-86NAB50A9A; CHEMBL387748; 86NAB50A9A; SCHEMBL373674; BDBM50214857; Benzonitrile, 3-(((5-(6-methyl-2-pyridinyl)-4-(6-quinoxalinyl)-1H-imidazol-2-yl)methyl)amino)-; 945244-71-3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1030 nM | |||
| External Link | ||||
| Oxindole 94 | Investigative | [30] | ||
| Synonyms |
SB 202190; 152121-30-7; SB202190; SB-202190; FHPI; SB202190 (FHPI); UNII-PVX798P8GI; 4-(4-Fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)-1H-imidazole; CHEBI:79090; 4-(4-fluorophenyl)-2-(4-hydroxyphenyl)-5-(4-pyridyl)imidazole; PVX798P8GI; SB 202190, Immobilized; C20H14FN3O; Phenol, 4-[4-(4-fluorophenyl)-5-(4-pyridinyl)-1H-imidazol-2-yl]-; 4-[4-(4-fluorophenyl)-5-(pyridin-4-yl)-1H-imidazol-2-yl]phenol; 4-[5-(4-fluorophenyl)-4-(pyridin-4-yl)-1H-imidazol-2-yl]phenol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 14.3 nM | |||
| External Link | ||||
| ZM-336372 | Investigative | [31] | ||
| Synonyms |
ZM 336372; 208260-29-1; ZM336372; 3-(dimethylamino)-N-(3-(4-hydroxybenzamido)-4-methylphenyl)benzamide; N-[5-(3-Dimethylaminobenzamido)-2-methylphenyl]-4-hydroxybenzamide; CHEMBL186526; n-(5-(3-dimethylaminobenzamido)-2-methylphenyl)-4-hydroxybenzamide; 3-(dimethylamino)-N-{3-[(4-hydroxybenzoyl)amino]-4-methylphenyl}benzamide; 3-(dimethylamino)-N-[3-[(4-hydroxybenzoyl)amino]-4-methylphenyl]benzamide; 3-(dimethylamino)-N-[3-(4-hydroxybenzamido)-4-methylphenyl]benzamide; Bio1_001346; compound 1 [PMID: 15454231
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 180 nM | |||
| External Link | ||||
| ML-3375 | Investigative | [32] | ||
| Synonyms |
CHEMBL113419; SCHEMBL4460547; SCHEMBL13712659; ZINC13491685; BDBM50119524; 4-(4-(4-fluorophenyl)-2-(methylthio)-1H-imidazol-5-yl)pyridine; 2-(Methylthio)-4-(4-fluorophenyl)-5-(4-pyridinyl)-1H-imidazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 630 nM | |||
| External Link | ||||
| 6-o-tolylquinazolin-2-amine | Investigative | [33] | ||
| Synonyms |
CHEMBL215952; SCHEMBL5494575; RAWAQXBVMDKSIO-UHFFFAOYSA-N; ZINC35049815
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3903 nM | |||
| External Link | ||||
| ML-3403 | Investigative | [32] | ||
| Synonyms |
p38 MAP Kinase Inhibitor III; ML3403; 549505-65-9; ML 3403; 4-(4-(4-fluorophenyl)-2-(methylthio)-1H-imidazol-5-yl)-N-(1-phenylethyl)pyridin-2-amine; CHEMBL111364; (RS)-{4-[5-(4-Fluorophenyl)-2-methylsulfanyl-3H-imidazol-4-yl]pyridin-2-yl}-(1-phenylethyl)amine]; K00568a; AC1O4WCF; SCHEMBL2896072; GTPL6014; ml-3043; SCHEMBL15013352; MolPort-023-278-904; HMS3229M04; {4-[5-(4-Fluoro-phenyl)-2-methylsulfanyl-3H-imidazol-4-yl]-pyridin-2-yl}-(1-phenyl-ethyl)-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 380 nM | |||
| External Link | ||||
| L-779450 | Investigative | [34] | ||
| Synonyms |
303727-31-3; L-779,450; 2-chloro-5-(2-phenyl-5-(pyridin-4-yl)-1H-imidazol-4-yl)phenol; L779450; CHEMBL373011; 2-chloro-5-(2-phenyl-5-pyridin-4-yl-1H-imidazol-4-yl)phenol; 2-(Phenyl)-4-(3-hydroxy-4-chlorophenyl)-5-(4-pyridyl)-1H-imidazole; 2-Chloro-5-[2-phenyl-5-(4-pyridinyl)-1H-imidazol-4-yl]phenol; C20H14ClN3O; L 779450; 2-chloro-5-[2-phenyl-5-(pyridin-4-yl)-1H-imidazol-4-yl]phenol; 2-chloro-5-[2-phenyl-4-(pyridin-4-yl)-1H-imidazol-5-yl]phenol; 2-chloro-5-(2-phenyl-4-(pyridin-4-yl)-1H-imidazol-5-yl)phenol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ML-3163 | Investigative | [35] | ||
| Synonyms |
ML 3163; CHEMBL111456; 4-[5-(4-Fluorophenyl)-2-(4-methanesulfinyl-benzylsulfanyl)-3H-imidazol-4-yl]pyridine; SCHEMBL4106398; BDBM50114690; IN1229; 4-[5-(4-Fluoro-phenyl)-2-(4-methanesulfinyl-benzylsulfanyl)-1H-imidazol-4-yl]-pyridine; 4-(2-(4-(methylsulfinyl)benzylthio)-4-(4-fluorophenyl)-1H-imidazol-5-yl)pyridine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4000 nM | |||
| External Link | ||||
| KT-5720 | Investigative | [36] | ||
| Synonyms |
KT 5720; KT5720; 108068-98-0; GTPL337; ZINC3873013; KT 5720, > hexyl (15R,16R,18S)-16-hydroxy-15-methyl-3-oxo-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8,10,12,20,22,24-nonaene-16-carboxylate; (9S,10S,12R)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3 inverted exclamation marka,2 inverted exclamation marka,1 inverted exclamation marka-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PD-0173956 | Investigative | [37] | ||
| Synonyms |
UNII-YR2DP5GJ1Y; YR2DP5GJ1Y; PD173956; PD-173956; AC1NS9BQ; 305820-76-2; SCHEMBL1334768; BDBM6569; CHEMBL574059; Pyrido(2,3-d)pyrimidin-7(8H)-one, 6-(2,6-dichlorophenyl)-2-((4-fluorophenyl)amino)-8-methyl-; 6-(2,6-dichlorophenyl)-2-(4-fluoroanilino)-8-methylpyrido[2,3-d]pyrimidin-7-one; 6-(2,6-dichlorophenyl)-2-[(4-fluorophenyl)amino]-8-methyl-7H,8H-pyrido[2,3-d]pyrimidin-7-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 50 nM | |||
| External Link | ||||
| PD-0166326 | Investigative | [37] | ||
| Synonyms |
PD-166326
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| STAUROSPORINONE | Investigative | [36] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro31-8220 | Investigative | [36] | ||
| Synonyms |
Bisindolylmaleimide IX; ro 31-8220; 125314-64-9; Ro 31 8220; Ro 318220; UNII-W9A0B5E78O; Ro-318220; Ro-31-8220; CHEMBL6291; W9A0B5E78O; CHEBI:38912; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl carbamimidothioate; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl imidothiocarbamate; CHEMBL1591531; Carbamimidothioic acid, 3-(3-(2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl)-1H-indol-1-yl)propyl; bisindolymaleimide IX
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| KN-62 | Investigative | [36] | ||
| Synonyms |
KN-62 (non-isomeric); GTPL6001; HMS3229A04; CCG-206863
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CI-1040 | Investigative | [36] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4,5,6,7-tetrabromo-1H-benzo[d][1,2,3]triazole | Investigative | [38] | ||
| Synonyms |
4,5,6,7-tetrabromobenzotriazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| B-Octylglucoside | Investigative | [12] | ||
| Synonyms |
29836-26-8; Octyl-beta-D-glucopyranoside; OCTYL BETA-D-GLUCOPYRANOSIDE; Octyl beta-D-glucoside; Octyl glucoside; Octyl b-D-Glucopyranoside; n-Octyl-beta-D-Glucoside; 1-O-n-Octyl-beta-D-glucopyranoside; Octyl-beta-D-glucoside; beta-D-Octyl glucoside; 1-O-Octyl-beta-D-glucopyranoside; (2R,3S,4S,5R,6R)-2-(hydroxymethyl)-6-(octyloxy)tetrahydro-2H-pyran-3,4,5-triol; UNII-V109WUT6RL; n-Octyl glucoside; n-Octyl-beta-D-glucopyranoside; Octyl-beta-D-Glucopyranose; Octyl; V109WUT6RL
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [39] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Bisindolylmaleimide-I | Investigative | [36] | ||
| Synonyms |
Bisindolylmaleimide i; 133052-90-1; GF 109203X; GF109203X; Go 6850; GF-109203X; RBT205 INHIBITOR; Go-6850; UNII-L79H6N0V6C; Bisindolylmaleimide I (GF 109203X); CHEMBL7463; 3-{1-[3-(DIMETHYLAMINO)PROPYL]-1H-INDOL-3-YL}-4-(1H-INDOL-3-YL)-1H-PYRROLE-2,5-DIONE; 3-(1-(3-(Dimethylamino)propyl)-1H-indol-3-yl)-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione; L79H6N0V6C; QMGUOJYZJKLOLH-UHFFFAOYSA-N; 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl)maleimide; GF-109203; Go6850
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RO-316233 | Investigative | [36] | ||
| Synonyms |
119139-23-0; bisindolylmaleimide iv; 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-dione; Arcyriarubin A; 3,4-Bis(3-indolyl)maleimide; 3,4-Di-1H-indol-3-yl-1H-pyrrole-2,5-dione; UNII-MBK3OO5K8T; BIM IV; 3,4-bis(1H-indol-3-yl)pyrrole-2,5-dione; MBK3OO5K8T; CHEMBL266487; 3,4-bis(1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione; DQYBRTASHMYDJG-UHFFFAOYSA-N; 2,3-bis(1H-Indol-3-yl)maleimide; 1H-Pyrrole-2,5-dione, 3,4-di-1H-indol-3-yl-; Ro-31-6233; AK-15401; 3,4-bis(3-indolyl)-1H-pyrrole-2,5-dione; Bisindoylmaleimide; Bisindolyl deriv. 3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-lysine methyltransferase SMYD2 (SMYD2) | 6 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| AZ505 | Preclinical | [40] | ||
| Synonyms |
AZ 505; AZ-505
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 120 nM | |||
| External Link | ||||
| LLY-507 | Preclinical | [41] | ||
| Synonyms |
1793053-37-8; LLY507; CHEMBL3414623; 5-Cyano-2'-{4-[2-(3-Methyl-1h-Indol-1-Yl)ethyl]piperazin-1-Yl}-N-[3-(Pyrrolidin-1-Yl)propyl]biphenyl-3-Carboxamide; 3-cyano-5-(2-{4-[2-(3-methyl-1H-indol-1-yl)ethyl]piperazin-1-yl}phenyl)-N-[3-(pyrrolidin-1-yl)propyl]benzamide; 3-Cyano-5-[2-[4-[2-(3-methyl-1H-indol-1-yl)ethyl]piperazin-1-yl]phenyl]-N-[3-(pyrrolidin-1-yl)propyl]benzamide; GTPL8239; SCHEMBL19760400; EX-A899; LLY 507; MolPort-042-624-530; BCP17114; s7575; BDBM50075102; ZINC231558920; AKOS027470175; CS-5126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 15 nM | |||
| External Link | ||||
| EPZ032597 | Preclinical | [42] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| EPZ033294 | Preclinical | [42] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| A-893 | Preclinical | [43] | ||
| Synonyms |
1868232-32-9; (R)-N-cyclohexyl-3-((3,4-dichlorophenethyl)amino)-N-(2-((2-hydroxy-2-(3-oxo-3,4-dihydro-2H-benzo[b][1,4]oxazin-8-yl)ethyl)amino)ethyl)propanamide; CHEMBL3590526; N-Cyclohexyl-N~3~-[2-(3,4-Dichlorophenyl)ethyl]-N-(2-{[(2r)-2-Hydroxy-2-(3-Oxo-3,4-Dihydro-2h-1,4-Benzoxazin-8-Yl)ethyl]amino}ethyl)-Beta-Alaninamide; SCHEMBL17476248; EX-A2769; BDBM50095537; AKOS030235552; ZINC230499113; ACN-037539; AC-29886; HY-19563; CS-0015655; Q27454706; 4GQ; N-Cyclohexyl-3-[2-(3,4-dichlorophenyl)ethylamino]-N-[2-[[(2R)-2-hydroxy-2-(3-oxo-4H-1,4-benzoxazin-8-yl)ethyl]amino]ethyl]propanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BAY 598 | Preclinical | [44] | ||
| Synonyms |
BAY-598; 1906919-67-2; CHEMBL3818617; 1906919-67-2 (S-isomer); (S,E)-N-(1-(N'-cyano-N-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; BAY 598 - Bio-X; BAY598; GTPL8953; EX-A1835; BDBM50180955; ZINC504786915; AC-31567; BS-16389; HY-19546; CS-0015642; J3.601.000B; Q27074893; (S)-N-(1-(N'-Cyano-N-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; (S,E)-N-(1-(N-cyano-N'-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; (S,Z)-N-(1-(N-cyano-N'-(3-(difluoromethoxy)phenyl)carbamimidoyl)-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl)-N-ethyl-2-hydroxyacetamide; N-[(4S)-1-[(Cyanoamino)[[3-(difluoromethoxy)phenyl]imino]methyl]-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl]-N-ethyl-2-hydroxyacetamide; N-[(4S)-1-[(Z)-N'-cyano-N-[3-(difluoromethoxy)phenyl]carbamimidoyl]-3-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-4-yl]-N-ethyl-2-hydroxyacetamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [45] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [46] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [47] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [48] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [48] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [49] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [48] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [46] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [50] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [51] | ||
| External Link | ||||
| CV301 | Phase 2 | [52] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [53] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [54] | ||
| External Link | ||||
| RG7221 | Phase 2 | [55] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [56] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [57] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [58] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [59] | ||
| External Link | ||||
| MGD007 | Phase 1 | [55] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [60] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [48] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [61] | ||
| External Link | ||||
| Nimesulide | Terminated | [62] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [63] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [64] | ||
| External Link | ||||
References