m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03479
|
[1], [2] | |||
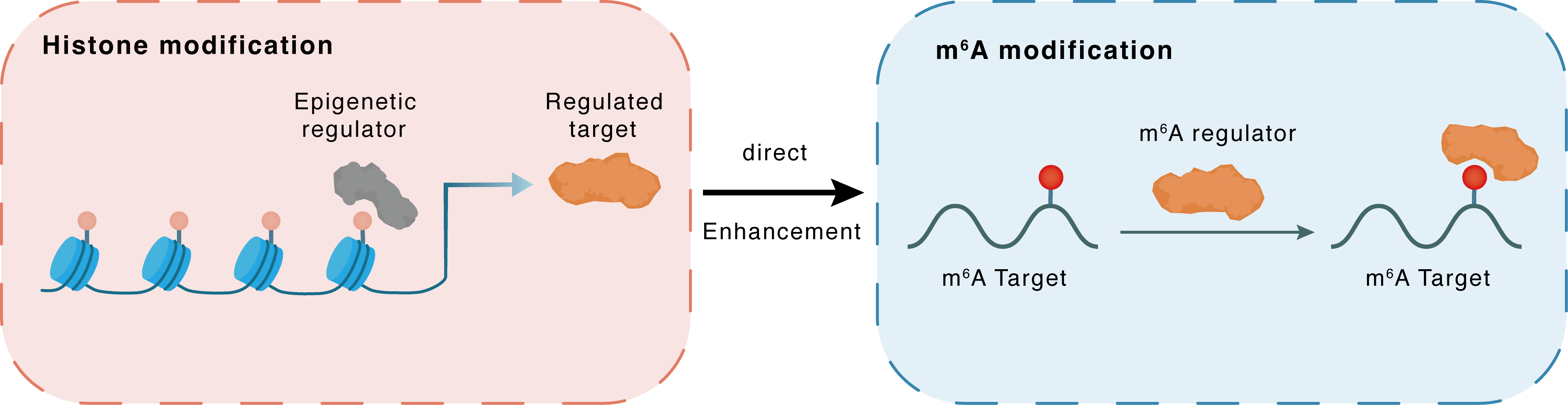 Histone modification
H3K18la
EP300
IGF2BP2
Direct
Enhancement
m6A modification
CD274
CD274
IGF2BP2
Histone modification
H3K18la
EP300
IGF2BP2
Direct
Enhancement
m6A modification
CD274
CD274
IGF2BP2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) | READER | |||
| m6A Target | Programmed cell death 1 ligand 1 (CD274/PD-L1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone acetyltransferase p300 (P300) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 18 lactylation (H3K18la) | View Details | |||
| Downstream Gene | IGF2BP2 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | CTCF interacts with HNRNPU through a FLG-AS1-dependent mechanism, facilitating the recruitment of p300 and activation of the m6A reader IGF2BP2 by Histone H3 lysine 18 lactylation (H3K18la). This activation promotes histone lactylation at the promoter region of IGF2BP2 stimulating the proliferation of PDAC cells. IGF2BP2 enhanced the mRNA stability of CSF1 and MYC. Mechanistically, circMYO1C cyclization was mediated by m6A methyltransferase METTL3. Moreover, methylated RNA immunoprecipitation sequencing (MeRIP-seq) unveiled the remarkable m6A modification on Programmed cell death 1 ligand 1 (CD274/PD-L1) mRNA. Moreover, circMYO1C targeted the m6A site of PD-L1 mRNA to enhance its stability by cooperating with IGF2BP2, thereby accelerating PDAC immune escape. | ||||
| Responsed Disease | Pancreatic cancer | ICD-11: 2C10 | |||
In-vitro Model |
PANC-1 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0480 | |
| Capan-2 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0026 | ||
| BxPC-3 | Pancreatic ductal adenocarcinoma | Homo sapiens | CVCL_0186 | ||
| CFPAC-1 | Cystic fibrosis | Homo sapiens | CVCL_1119 | ||
| In-vivo Model | Male BALB/c nude mice (5-6 weeks) were obtained from Slac Laboratory Animal Center (Shanghai, China) and maintained under pathogen-free conditions. PANC-1 cells (2 × 106 cells suspended in 100 μ l PBS) transfected with circMYO1C knockdown (sh-circMYO1C) or controls (sh-NC) were subcutaneously injected into the flank of nude mice. One week later, the tumor size was measured every three days. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| CD274 molecule (CD274) | 55 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Avelumab | Approved | [3] | ||
| External Link | ||||
| Durvalumab | Approved | [4] | ||
| MOA | Modulator | |||
| External Link | ||||
| RG-7446 | Approved | [5] | ||
| External Link | ||||
| Bavencio | Approved | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Atezolizumab | Approved | [7] | ||
| External Link | ||||
| Sugemalimab | Approved in China | [8] | ||
| External Link | ||||
| MEDI4736 | Phase 3 | [9] | ||
| MOA | Modulator | |||
| External Link | ||||
| MPDL-3280A | Phase 3 | [10] | ||
| MOA | Modulator | |||
| External Link | ||||
| CS1001 | Phase 3 | [11] | ||
| External Link | ||||
| A167 | Phase 3 | [12] | ||
| Synonyms |
KL-A167
Click to Show/Hide
|
|||
| External Link | ||||
| KN046 | Phase 3 | [13] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pidilizumab | Phase 2 | [14] | ||
| Synonyms |
CT-011
Click to Show/Hide
|
|||
| External Link | ||||
| KN035 | Phase 2 | [15] | ||
| Synonyms |
Envafolimab
Click to Show/Hide
|
|||
| External Link | ||||
| CX-072 | Phase 2 | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| INCB86550 | Phase 2 | [17] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [18] | ||
| External Link | ||||
| M7824 | Phase 2 | [19] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| BGB-A333 | Phase 1/2 | [20] | ||
| External Link | ||||
| GS-4224 | Phase 1/2 | [21] | ||
| Synonyms |
Methyl Pyridazine-4-carboxylate; 34231-77-1; PYRIDAZINE-4-CARBOXYLIC ACID METHYL ESTER; 4-PYRIDAZINECARBOXYLIC ACID, METHYL ESTER; MFCD09953488; ACMC-1AJNN; methyl 4-pyridazinecarboxylate; methylpyridazine-4-carboxylate; SCHEMBL1421640; DTXSID30498310; AMY24958; BCP22435; ANW-50355; ZINC12359421; AKOS015854403; Methyl pyridazine-4-carboxylate, 97%; AC-4414; CS-W003697; PB31452; 4-Pyridazinecarboxylic acid methyl ester; AK-48857; SY004472; AB0024323; DB-030309; FT-0717698; W5569; S-2990; J-522632
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [22] | ||
| MOA | Modulator | |||
| External Link | ||||
| LY3300054 | Phase 1 | [19] | ||
| External Link | ||||
| MSB2311 | Phase 1 | [15] | ||
| External Link | ||||
| Anti-PD-L1 | Phase 1 | [23] | ||
| Synonyms |
BMS-936559
Click to Show/Hide
|
|||
| External Link | ||||
| FAZ053 | Phase 1 | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Anti-PD-L1 CSR T cells | Phase 1 | [24] | ||
| MOA | CAR-T-Cell-Therapy | |||
| External Link | ||||
| BMS-986189 | Phase 1 | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cosibelimab | Phase 1 | [25] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| CA-170 | Phase 1 | [26] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PD-L1 t-haNK | Phase 1 | [27] | ||
| External Link | ||||
| KD033 | Phase 1 | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C-Met/PD-L1 CAR-T Cell | Phase 1 | [29] | ||
| MOA | CAR-T-Cell-Therapy(Dual specific) | |||
| External Link | ||||
| CA-170 | Phase 1 | [19] | ||
| Synonyms |
3-Aminopyrrolidine dihydrochloride; 103831-11-4; pyrrolidin-3-amine dihydrochloride; 3-Pyrrolidinamine, dihydrochloride; 3-Aminopyrrolidine 2HCl; 3-Aminopyrrolidine diHCl; SCHEMBL555933; ACMC-2099s1; ACMC-2099s3; ACMC-20989g; 3-pyrrolidinamine dihydrochloride; CTK0H7226; DTXSID00583661; MolPort-002-343-989; NJPNCMOUEXEGBL-UHFFFAOYSA-N; 3-Amino-pyrrolidine dihydrochloride; KS-00000JI6; ACT01710; ANW-14978; SBB003982; ( -3-Aminopyrrolidine dihydrochloride; AKOS015844825; VP60158; AM85320; VP60228; TRA0055207; TRA0000843; TRA0097
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FS118 | Phase 1 | [15] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [30] | ||
| External Link | ||||
| MCLA-145 | Phase 1 | [31] | ||
| MOA | Agonist | |||
| External Link | ||||
| GEN1046 | Phase 1 | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| ALPN-202 | Phase 1 | [33] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| TAK-252 | Phase 1 | [34] | ||
| Synonyms |
SL-279252
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| INBRX-105 | Phase 1 | [35] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LY3415244 | Phase 1 | [36] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| A337 | Phase 1 | [12] | ||
| External Link | ||||
| IBI318 | Phase 1 | [37] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30107136-Compound-Example2 | Patented | [38] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 150 nM | |||
| External Link | ||||
| PMID30107136-Compound-Example1 | Patented | [38] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| PMID30247903-Compound-General structure7 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CA-327 | Investigative | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure8 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure5 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound5
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure12 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound12
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure9 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure6 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure10 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| YH011 | Investigative | [39] | ||
| External Link | ||||
| YH010 | Investigative | [40] | ||
| External Link | ||||
| RG6084 | Phase 1 | [41] | ||
| External Link | ||||
| Histone acetyltransferase p300 (P300) | 2 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| CCS1477 | Phase 1/2 | [42] | ||
| Synonyms |
CCS-1477; CBP-IN-1; 2222941-37-7; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-((1r,4S)-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one; SCHEMBL20094038; SCHEMBL21515367; SCHEMBL22134021; EX-A3687; NSC818619; NSC-818619; HY-111784; CS-0091862; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-(trans-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FT-7051 | Phase 1 | [43] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C10: Pancreatic cancer | 182 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Atezolizumab | Approved | [19] | ||
| External Link | ||||
| Trimethadione | Approved | [44] | ||
| Synonyms |
Absentol; Absetil; Convenixa; Convexina; Edion; Epidione; Epidone; Epixal; Etydion; Minoaleuiatin; Minoaleviatin; Petidion; Petidon; Petilep; Petimalin; Pitmal; Ptimal; Tioxanona; Tredione; Tricione; Tridilona; Tridion; Tridione; Tridone; Trilidona; Trimedal; Trimedone; Trimetadiona; Trimetadione; Trimethadion; Trimethadionum; Trimethdione; Trimethin; Trimethinum; Trimetin; Trioksal; Trioxanona; Triozanona; Tromedone; Troxidone; Abbott Brand of Trimethadione; Trimetadione [DCIT]; A 2297; Mino-Aleviatin; Neo-Absentol; Tridione (TN); Trimetadiona [INN-Spanish]; Trimethadione [INN:JAN]; Trimethadionum [INN-Latin]; Trimethadione (JP15/INN); 3,3,5-Trimethyl-2,4-diketooxazolidine; 3,5,5,-Trimethyloxazolidine-2,4-dione; 3,5,5-TRIMETHYL-OXAZOLIDINE-2,4-DIONE; 3,5,5-Trimethyl-1,3-oxazolidine-2,4-dione; 3,5,5-Trimethyl-2,4-oxazolidinedione; 3,5,5-Trojmetylooksazolidyno-2,4-dion; 3,5,5-Trojmetylooksazolidyno-2,4-dion [Polish]
Click to Show/Hide
|
|||
| External Link | ||||
| Motixafortide | Approved | [19] | ||
| External Link | ||||
| Uridine triacetate | Approved | [45] | ||
| Synonyms |
PN401
Click to Show/Hide
|
|||
| External Link | ||||
| Bentiromide | Approved | [46] | ||
| Synonyms |
Bentiromide sodium; 41748-47-4; N-Benzoyl-L-tyrosyl-4-aminobenzoic acid sodium salt; NCGC00164607-01; EINECS 255-530-7; DSSTox_CID_26476; DSSTox_RID_81647; DSSTox_GSID_46476; DTXSID6046476; CHEMBL3188891; Tox21_112229; AKOS024373587; ACM41748474; Sodium (S)-4-((2-(benzoylamino)-3-(4-hydroxyphenyl)-1-oxopropyl)amino)benzoate; CAS-41748-47-4; FT-0771579; ST51012404; N-Benzoyl-L-tyrosine p-amidobenzoic acid sodium salt; sodium (S)-4-(2-benzamido-3-(4-hydroxyphenyl)propanamido)benzoate; N-Benzoyl-L-tyrosine p-amidobenzoic acid so
Click to Show/Hide
|
|||
| External Link | ||||
| Olaparib | Approved | [19] | ||
| Synonyms |
AZD 2281; AZD2281; AZD-2281; Acylpiperazine analogue, 47; KU-0059436; KU-59436; Olaparib, KU-0059436, AZD2281,KU0059436, AZD2281; 4-[(3-{[4-Cyclopropylcarbonyl)piperazin-4-yl]carbonyl}-4-fluorophenyl)methyl]phtalazin-1(2H)-one; 4-[3-(4-Cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one
Click to Show/Hide
|
|||
| External Link | ||||
| Streptozocin | Approved | [47] | ||
| Synonyms |
Estreptozocina; STREPTOZOTOCIN; STRZ; Streptozocine; Streptozocinium; Streptozocinum; Streptozosin; Zanosar; Alkylating agent; Binds to DNA; Streptozocinium [Latin]; Streptozocine [INN-French]; Streptozocinum [INN-Latin]; Zanosar (TN); Streptozocin (USAN/INN); Streptozocin, Zanosar, STZ,Streptozotocin;N-(Methylnitrosocarbamoyl)-alpha-D-glucosamine; N-D-Glucosyl-(2)-N'-nitrosomethylharnstoff; N-D-Glucosyl-(2)-N'-nitrosomethylurea; D-Glucose, 2-deoxy-2-(((methylnitrosoamino)carbonyl)amino)-(9CI); 1-methyl-1-nitroso-3-[(2S,3R,4R,5S,6R)-2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl]urea; 2-Deoxy-2-(((methylnitrosoamino)carbonyl)amino)-D-glucopyranose; 2-Deoxy-2-(3-methyl-3-nitrosoureido)-D-glucopyranose; 2-Deoxy-2[[(methylnitrosoamino)-carbonyl]amino]-D-glucopyranose; 2-deoxy-2-{[methyl(nitroso)carbamoyl]amino}-alpha-D-glucopyranose
Click to Show/Hide
|
|||
| External Link | ||||
| Plazomicin | Phase 3 | [48] | ||
| Synonyms |
ACHN-490; UNII-LYO9XZ250J; 1154757-24-0; LYO9XZ250J; Plazomicin [USAN:INN]; Plazomicin (USAN); ZINC68150640; DB12615; D10151; D-Streptamine,
Click to Show/Hide
|
|||
| External Link | ||||
| Taxol | Approved | [19] | ||
| Synonyms |
C47H51NO14; weekly paclitaxel; Micellar Paclitaxel; Paclitaxel [USAN:INN:BAN]; SCHEMBL15000506; Benzenepropanoic acid, beta-(benzoylamino)-alpha-hydroxy-, 6,12b-bis(acetyloxy)-12-(benzoyloxy)-2a,3,4,4a,5,6,9,10,11,12,12a,12b-dodecahydro-4,11-dihydroxy-4a,8,13,13-tetramethyl-5-oxo-7,11-methano-1H-cyclodeca(3,4)benz(1,2-b)oxet-9-yl ester, (2aR-(2aalpha,4beta,4abeta,6beta,9alpha(alphaR*,betaS*),11alpha,12alpha,12aalpha,12balpha))-
Click to Show/Hide
|
|||
| External Link | ||||
| Ibrutinib | Phase 3 | [19] | ||
| Synonyms |
PCI-32765; Ibrutinib (BTK inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Erlotinib | Approved | [49] | ||
| Synonyms |
Erlotinin; Tarceva; Erlotinib Base; OSI 744; R 1415; CP 358,774; CP-358774; Erlotinib(Tarceva); Tarceva (TN); CP-358,774; Erlotinib, OS-774; N-(3-ethynylphenyl)[6,7-bis(2-methoxyethoxy)quinazolin-4-yl]amine; N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)-4-quinazolinamine; N-(3-Ethynylphenyl)-6,7-bis(2-methoxyethoxy)quinazolin-4-amine; [6,7-BIS(2-METHOXY-ETHOXY)QUINAZOLINE-4-YL]-(3-ETHYNYLPHENYL)AMINE; [6,7-Bis-(2-methoxy-ethoxy)-quinazolin-4-yl]-(3-ethynyl-phenyl)-amine; 4-[(3-Ethynylphenyl)amino]-6,7-bis(2-methoxyethoxy)quinazoline
Click to Show/Hide
|
|||
| External Link | ||||
| Ruxolitinib | Approved | [50] | ||
| Synonyms |
Ruxolitinib (JAK inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Nivolumab | Approved | [19] | ||
| External Link | ||||
| Coenzyme Q10 | Phase 2 | [19] | ||
| Synonyms |
CoQ10; Coenzyme Q10 (oral formulation); CoQ10 platform technology, Ryan (Receptagen); Coenzyme Q10 (oral formulation), Receptagen
Click to Show/Hide
|
|||
| External Link | ||||
| Aglatimagene besadenovec | Phase 1/2 | [19] | ||
| External Link | ||||
| Zolbetuximab | Phase 3 | [51] | ||
| Synonyms |
IMAB362
Click to Show/Hide
|
|||
| External Link | ||||
| AC-1204 | Phase 3 | [52] | ||
| Synonyms |
isoindoline hydrochloride; 32372-82-0; 2,3-Dihydroisoindole hydrochloride; 2,3-dihydro-1H-isoindole hydrochloride; 2,3-Dihydro-1H-isoindole HCl; Isoindoline HCl salt; 1H-Isoindole, 2,3-dihydro-, hydrochloride; Isoindoline hydrochloride, 97%; Isoindolinehydrochloride; Isoindoline, HCl; ISOINDOLINE HCL; AC1Q38WR; dihydroisoindole hydrochloride; KSC491I3F; AMBZ0192; SCHEMBL4702076; CTK3J1432; DTXSID50487241; MolPort-003-986-749; NOVIRODZMIZUPA-UHFFFAOYSA-N; BH168; CS-D1516; ACT08858; ACN-S003258; KS-000001RA
Click to Show/Hide
|
|||
| External Link | ||||
| Radiosensitizer gene therapy | Phase 3 | [53] | ||
| Synonyms |
Radiosensitizer gene therapy (prostate cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| Glufosfomide | Phase 3 | [54] | ||
| External Link | ||||
| Yttrium (90Y) clivatuzumab tetraxetan | Phase 3 | [55] | ||
| Synonyms |
PAM4 mAb; Yttrium Y 90 clivatuzumab tetraxetan; Anti-MUC1 PAM4 monoclonal antibody; Clivatuzumab tetraxetan-[90Y]; HPAM4-Cide; IMMU-107; PAM-4; PAM4-Y-90; Yttrium-90-hPAM4; 90Y-clivatuzumab tetraxetan; 90Y-hPAM4
Click to Show/Hide
|
|||
| External Link | ||||
| Y-90 Clivatuzumab | Phase 3 | [56] | ||
| External Link | ||||
| Civacir | Phase 3 | [57] | ||
| External Link | ||||
| GV1001 | Phase 3 | [58] | ||
| External Link | ||||
| Masitinib | Phase 3 | [19] | ||
| Synonyms |
790299-79-5; AB1010; Masatinib; Masitinib (AB1010); Masivet; AB-1010; AB 1010; UNII-M59NC4E26P; Masitinib [INN]; M59NC4E26P; 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-thiazolyl]amino]phenyl]benzamide; CHEMBL1908391; CHEBI:63450; Masitinib (INN); N-(4-Methyl-3-((4-(pyridin-3-yl)thiazol-2-yl)amino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide; Q-201339; C28H30N6OS; N-(4-methyl-3-(4-(pyridin-3-yl)thiazol-2-ylamino)phenyl)-4-((4-methylpiperazin-1-yl)methyl)benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| Glufosfamide | Phase 3 | [19] | ||
| Synonyms |
Glucosylifostamide mustard; D 19575; D-19575; Glc-IPM; Glucosyl-ifosfamide mustard; Beta-D-Glucopyranose 1-(N,N'-bis(2-chloroethyl)phosphorodiamidate; Beta-D-Glucopyranose, 1-(N,N'-bis(2-chloroethyl)phosphorodiamidate); (2S,3R,4S,5S,6R)-2-bis(2-chloroethylamino)phosphoryloxy-6-(hydroxymethyl)oxane-3,4,5-triol
Click to Show/Hide
|
|||
| External Link | ||||
| Sapacitabine | Phase 3 | [19] | ||
| Synonyms |
CYC682
Click to Show/Hide
|
|||
| External Link | ||||
| Pelareorep | Phase 2 | [16] | ||
| External Link | ||||
| Pamrevlumab | Phase 3 | [59] | ||
| External Link | ||||
| GRASPA | Phase 1 | [60] | ||
| Synonyms |
L-asparaginase (erythrocyte-encapsulated, acute lymphoblastic leukemia/solid tumor), ERYtech
Click to Show/Hide
|
|||
| External Link | ||||
| Pancreas algenpantucel-L | Phase 3 | [61] | ||
| Synonyms |
HyperAcute (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| CPI-613 | Phase 3 | [62] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Zarnestra | Phase 3 | [63] | ||
| Synonyms |
JAN; Tipifarnib; Tipifarnib [USAN]; R 115777; R115777; R-11577; R-115777; Tipifarnib (USAN/INN); Zarnestra, IND 58359, R115777, Tipifarnib; (R)-6-(Amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl)-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone; (R)-R115777; 2 (1H))-Quinolinone,6-(amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl)-4-(3-chlorophenyl)-1-methyl-, 2(1H)-quinolinone; 6-[(R)-amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2(1H)-one; 6-[(R)-amino-(4-chlorophenyl)-(3-methylimidazol-4-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2-one; 6-[(S)-AMINO(4-CHLOROPHENYL)(1-METHYL-1H-IMIDAZOL-5-YL)METHYL]-4-(3-CHLOROPHENYL)-1-METHYLQUINOLIN-2(1H)-ONE
Click to Show/Hide
|
|||
| External Link | ||||
| MM-398 | Phase 3 | [64] | ||
| External Link | ||||
| Marimastat | Phase 3 | [65] | ||
| Synonyms |
Marimastat [USAN]; BB 2516; BB-2516; Marimastat (USAN/INN); (2R,3S)-N-[(2S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl]-N',3-dihydroxy-2-(2-methylpropyl)butanediamide; (2S,3R)-3-(((1S)-2,2-Dimethyl-1-(methylcarbamoxy)propyl)carboyl)-2-hydroxy-5-methylhexanohydroxamic acid; (2S,3R)-3-(((1S)-2,2-Dimethyl-1-(methylcarbamoyl)propyl)carbamoyl)-2-hydroxy-5-methylhexanohydroxamic acid; (2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl]-N(1),2-dihydroxy-3-(2-methylpropyl)butanediamide
Click to Show/Hide
|
|||
| External Link | ||||
| AM0010 | Phase 3 | [66] | ||
| External Link | ||||
| ANX-510 | Phase 3 | [67] | ||
| External Link | ||||
| Napabucasin | Phase 3 | [19] | ||
| Synonyms |
83280-65-3; UNII-Z1HHM49K7O; 2-acetylnaphtho[2,3-b]furan-4,9-dione; Z1HHM49K7O; 2-Acetylnaphtho(2,3-b)furan-4,9-dione; 2-Acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione; Napabucasin [USAN:INN]; Napabucasin (BBI608); 2-Acetylfuranonaphthoquinone; CHEMBL64130; Napabucasin (JAN/USAN/INN); SCHEMBL1883845; Napabucasin - BBI 608/ FNQ; 2-Acetylfuro-1,4-naphthoquinone; DPHUWDIXHNQOSY-UHFFFAOYSA-N; MolPort-039-101-321; EX-A1314; ZINC13306865; s7977; AKOS027470201; DB12155; CS-1747; ACN-053294; HY-13919
Click to Show/Hide
|
|||
| External Link | ||||
| Algenpantucel-L | Phase 3 | [68] | ||
| Synonyms |
HyperAcute pancreas (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| OT-101 | Phase 2/3 | [19] | ||
| External Link | ||||
| RP101 | Phase 2/3 | [69] | ||
| Synonyms |
SCHEMBL15589316; CHEMBL3703295; BDBM149820; US8975415,
Click to Show/Hide
|
|||
| External Link | ||||
| NLG8189 | Phase 2/3 | [19] | ||
| Synonyms |
1-Methyl-D-tryptophan; Indoximod; 110117-83-4; D-Tryptophan, 1-methyl-; D-1MT; Indoximod (NLG-8189); D-1-methyltryptophan; UNII-TX5CYN1KMZ; D-(+)-1-Methyltryptophan; TX5CYN1KMZ; (R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methylindol-3-yl)propanoic acid; NSC-721782; (2R)-2-amino-3-(1-methyl-3-indolyl)propanoic acid; 1-MT; (2R)-2-azanyl-3-(1-methylindol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; D-l-Methyltryptophan; Indoximod [USAN:INN]; NLG-8189; NLG 8189
Click to Show/Hide
|
|||
| External Link | ||||
| LY2157299 | Phase 2/3 | [19] | ||
| Synonyms |
Galunisertib; 700874-72-2; LY 2157299; LY-2157299; UNII-3OKH1W5LZE; 4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide; 3OKH1W5LZE; Galunisertib (LY2157299); AK-79916; 4-[5,6-Dihydro-2-(6-methyl-2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-6-quinolinecarboxamide; 4-(2-(6-Methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo-[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide; 4-[2-(6-methylpyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline-6-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| SM-88 | Phase 2/3 | [70] | ||
| External Link | ||||
| SiG12D-LODER | Phase 2 | [19] | ||
| External Link | ||||
| BNT141 | Phase 2 | [71] | ||
| External Link | ||||
| BNT321 | Phase 2 | [72] | ||
| Synonyms |
MVT-5873
Click to Show/Hide
|
|||
| External Link | ||||
| BPM 31510 | Phase 2 | [73] | ||
| External Link | ||||
| GC4711 | Phase 2 | [74] | ||
| Synonyms |
UNII-FW5T90VM32; FW5T90VM32; GC-4419 dipropionate; Bis-propionato(gc4419); Avasopasem manganese dipropionate; GC-4711; 2248030-85-3; Manganese(II), bis-propionato((4aS,13aS,17aS,21aS)-1,2,3,4,4a,5,6,12,13,13a,14,15,16,17,17a,18,19,20,21,21a-eicosahydro-11,7-nitrilo-7H-dibenzo(b,H)(1,4,7,10)tetraaza-cycloheptadecine-kn5,kn13,kn18,kn21,kn22)-,
Click to Show/Hide
|
|||
| External Link | ||||
| CYTO-401 | Phase 2 | [75] | ||
| External Link | ||||
| Zenocutuzumab | Phase 2 | [76] | ||
| External Link | ||||
| Cabiralizumab | Phase 2 | [19] | ||
| External Link | ||||
| VS-6063 | Phase 2 | [19] | ||
| Synonyms |
Defactinib hydrochloride; 1073160-26-5; Defactinib (hydrochloride); UNII-L2S469LM49; Defactinib hydrochloride [USAN]; L2S469LM49; Defactinib hydrochloride (USAN); Benzamide, N-methyl-4-[[4-[[[3-[methyl(methylsulfonyl)amino]-2-pyrazinyl]methyl]amino]-5-(trifluoromethyl)-2-pyrimidinyl]amino]-, hydrochloride; Defactinib HCl; Benzamide, N-methyl-4-((4-(((3-(methyl(methylsulfonyl)amino)-2-pyrazinyl)methyl)amino)-5-(trifluoromethyl)-2-pyrimidinyl)amino)-, hydrochloride (1:1); Benzamide, N-methyl-4-[[4-[[[3-[methyl(methylsu
Click to Show/Hide
|
|||
| External Link | ||||
| MENK | Phase 2 | [54] | ||
| External Link | ||||
| CO-101 | Phase 2 | [77] | ||
| Synonyms |
methyl 2-(dimethylcarbamoyl)benzoate; 26593-43-1; Phthalamic acid, N,N-dimethyl-, methyl ester; CO 101; BRN 2504723; N,N-Dimethylphthalamic acid methyl ester; N,N-Dimethylphthalamic acid, methyl ester; AC1Q5ZAU; 2-09-00-00601 (Beilstein Handbook Reference); AC1L4V19; CTK4F8203; DTXSID50181156; 2-methoxycarbonyl-N,N-dimethylbenzamide; LS-109082; Benzoic acid,2-[(dimethylamino)carbonyl]-, methyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| MENK | Phase 2 | [19] | ||
| Synonyms |
IRT-101
Click to Show/Hide
|
|||
| External Link | ||||
| TL-118 | Phase 2 | [78] | ||
| Synonyms |
Hamsa 1; TL-111; TL-112; Combination anti-angiogenic therapy (oral suspension, solid tumors), Tiltan Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| Antroquinonol | Phase 2 | [19] | ||
| Synonyms |
Hocena; Fungal extract (cancer), Golden Biotechnology
Click to Show/Hide
|
|||
| External Link | ||||
| NPC-1C | Phase 2 | [19] | ||
| Synonyms |
Ensituximab
Click to Show/Hide
|
|||
| External Link | ||||
| Necuparanib | Phase 2 | [79] | ||
| External Link | ||||
| CCX872 | Phase 2 | [19] | ||
| External Link | ||||
| GC4419 | Phase 1/2 | [19] | ||
| Synonyms |
Avasopasem manganese; UNII-EY1WA413UL; EY1WA413UL; Avasopasem manganese [USAN]; SC-72325A; M-40419; 435327-40-5; Manganese, dichloro((4aS,13aS,17aS,21aS)-1,2,3,4,4a,5,6,12,13,13a,14,15,16,17,17a,18,19,20,21,21a-eicosahydro-7,11-nitrilo-7H-dibenzo(b,H)-5,13,18,21-tetraazacycloheptadecine-kappaN5,kappaN13,kappaN18,kappaN21,kappaN22)-, (pb-7-11-2344'3')-
Click to Show/Hide
|
|||
| External Link | ||||
| Istiratumab | Phase 2 | [16] | ||
| External Link | ||||
| GI-4000 | Phase 2 | [80] | ||
| External Link | ||||
| OCV-101 | Phase 2 | [81] | ||
| Synonyms |
OTS-11101
Click to Show/Hide
|
|||
| External Link | ||||
| RX-3117 | Phase 2 | [19] | ||
| Synonyms |
Antimetabolite (cancer), Rexahn; Antimetabolite (cancer), Rexahn/ Teva
Click to Show/Hide
|
|||
| External Link | ||||
| Ensitiximab | Phase 2 | [54] | ||
| External Link | ||||
| GI-4000 + gemcitabine | Phase 2 | [82] | ||
| External Link | ||||
| BC-819 | Phase 2 | [83] | ||
| External Link | ||||
| IRT-102 | Phase 2 | [84] | ||
| External Link | ||||
| LE-DT | Phase 2 | [85] | ||
| Synonyms |
Liposomal docetaxel
Click to Show/Hide
|
|||
| External Link | ||||
| TH-302 | Phase 2 | [19] | ||
| Synonyms |
evofosfamide; 918633-87-1; TH 302; TH302; UNII-8A9RZ3HN8W; Evofosfamide(TH 302); n,n'-bis(2-bromoethyl)phosphorodiamidic acid (1-methyl-2-nitro-1h-imidazol-5-yl)methyl ester; 8A9RZ3HN8W; compound 3b; Evofosfamide;HAP-302; Phosphorodiamidic acid, N,N'-bis(2-bromoethyl)-, (1-methyl-2-nitro-1H-imidazol-5-yl)methyl ester; 2-bromo-N-[(2-bromoethylamino)-[(3-methyl-2-nitroimidazol-4-yl)methoxy]phosphoryl]ethanamine; Evofosfamide [USAN:INN]; Evofosfamide(TH-302); C9H16Br2N5O4P; CHEMBL260046; SCHEMBL2357174
Click to Show/Hide
|
|||
| External Link | ||||
| Demcizumab | Phase 2 | [54] | ||
| External Link | ||||
| Anti-PSCA mab | Phase 2 | [86] | ||
| External Link | ||||
| ALT-803 | Phase 2 | [16] | ||
| Synonyms |
IL-15 agonist/ IL-15R alpha-Fc fusion complex (cancer), Altor BioScience
Click to Show/Hide
|
|||
| External Link | ||||
| ARQ 761 | Phase 2 | [19] | ||
| External Link | ||||
| Reolysinpelareorep | Phase 2 | [19] | ||
| External Link | ||||
| PBI-05204 | Phase 2 | [19] | ||
| External Link | ||||
| PCI-27483 | Phase 2 | [87] | ||
| External Link | ||||
| RX-0201 | Phase 2 | [54] | ||
| External Link | ||||
| CP-613 | Phase 2 | [88] | ||
| External Link | ||||
| CART 19 | Preclinical | [89] | ||
| External Link | ||||
| VT-122 | Phase 1 | [54] | ||
| External Link | ||||
| PEGPH20 | Phase 2 | [90] | ||
| External Link | ||||
| Ficlatuzumab | Phase 2 | [19] | ||
| Synonyms |
AV-299
Click to Show/Hide
|
|||
| External Link | ||||
| Tigatuzumab | Phase 2 | [91] | ||
| External Link | ||||
| CRS-207 | Phase 2 | [92] | ||
| External Link | ||||
| CAP1-6D | Phase 2 | [93] | ||
| Synonyms |
Modified CEA peptide (pancreatic cancer), University of Chicago
Click to Show/Hide
|
|||
| External Link | ||||
| SGT-53 | Phase 2 | [19] | ||
| Synonyms |
P53 gene stimulator (solid tumor), Synergene Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Tarextumab | Phase 2 | [94] | ||
| External Link | ||||
| BVD-523 | Phase 2 | [19] | ||
| External Link | ||||
| Ocaperidone | Phase 2 | [16] | ||
| Synonyms |
Ocaperidona; 129029-23-8; UNII-26HUS7139V; 3-(2-(4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino)ethyl)-2,9-dimethyl-4H-pyrido(1,2-a)pyrimidin-4-one; 26HUS7139V; Ocaperidonum; Ocaperidonum [INN-Latin]; Ocaperidona [INN-Spanish]; 4H-Pyrido[1,2-a]pyrimidin-4-one,3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-2,9-dimethyl-; Ocaperidone (USAN); Ocaperidone [USAN:INN:BAN]; 3-[2-[4-(6-Fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl]ethyl]-2,9-dimethyl-4H-pyrido[1,2-a]pyrimidin-4-one; 3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2,9-dimethylpyrido[1,2-a]pyrimidin-4-one; 8-[2-[4-(6-fluorobenzo[d]isoxazol-3-yl)-1-piperidyl]ethyl]-2,9-dimethyl-6,10-diazabicyclo[440]deca-2,4,8,10-tetraen-7-one; FG-3019
Click to Show/Hide
|
|||
| External Link | ||||
| Encapsulated live cells converting ifosfamide | Phase 2 | [19] | ||
| External Link | ||||
| LY2603618 | Phase 2 | [95] | ||
| Synonyms |
Rabusertib; 911222-45-2; LY 2603618; LY-2603618; UNII-3S9L1NU6U7; 3S9L1NU6U7; IC-83; ly2603618 IC-83; (S)-1-(5-bromo-4-methyl-2-(morpholin-2-ylmethoxy)phenyl)-3-(5-methylpyrazin-2-yl)urea; n-(5-bromo-4-methyl-2-((2s)-2-morpholinylmethoxy)phenyl)-n'-(5-methyl-2-pyrazinyl)urea; LY2603618 (IC-83); Rabusertib [USAN:INN]; 3-(5-Bromo-4-methyl-2-[(2s)-morpholin-2-ylmethoxy]phenyl)-1-(5-methylpyrazin-2-yl)urea; N-[5-Bromo-4-methyl-2-[(2S)-2-morpholinylmethoxy)phenyl]-N'-(5-methyl-2-pyrazinyl)urea
Click to Show/Hide
|
|||
| External Link | ||||
| Salirasib | Discontinued in Phase 1/2 | [96] | ||
| Synonyms |
162520-00-5; Farnesylthiosalicylic acid; S-Farnesylthiosalicylic acid; UNII-MZH0OM550M; MZH0OM550M; CHEMBL23293; AK186909; Farnesyl Thiosalicylic Acid; 2-[[(2E,6E)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-yl]thio]benzoic Acid; 2-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]sulfanylbenzoic acid; 2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienylthio)benzoic acid; 2-(((2E,6E)-3,7,11-Trimethyl-2,6,10-dodecatrienyl)sulfanyl)benzoic acid; Benzoic acid, 2-(((2E,6E)-3,7,11-trimethyl-2,6,10-dodecatrienyl)thio)-; FTS; Farnesylthiosalicyclic acid; FTS, Thyreos; Ras antagonists, Thyreos; S-trans; Th-101; Trans-farnesylthiosalicylicacid; FTS (oral, cancer), Concordia; Farnesylthiosalicyclic acid (oral, cancer), Concordia; Ras-inhibitors (cancer), Concordia; FTS (oral, cancer), Concordia/Ono; KD032
Click to Show/Hide
|
|||
| External Link | ||||
| MM-141 | Phase 2 | [19] | ||
| External Link | ||||
| GB1275 | Phase 1/2 | [97] | ||
| External Link | ||||
| ABTL0812 | Phase 1/2 | [98] | ||
| Synonyms |
(9Z,12Z)-2-Hydroxy-9,12-octadecadienoic acid; (9Z,12Z)-2-hydroxyoctadeca-9,12-dienoic acid; (9Z,12Z)-2-hydroxyoctadecadienoic acid; (alpha)-Hydroxylinoleic acid; .ALPHA.-HYDROXYLINOLEIC ACID; 0DE74TJ7EZ; 2-hydroxy-9Z,12Z-Octadecadienoic acid; 2-hydroxylinoleic acid; 57818-44-7; 9,12-Octadecadienoic acid, 2-hydroxy-, (9Z,12Z)-; 9,12-Octadecadienoic acid, 2-hydroxy-, (Z,Z)-; ABTL0812; ABTL-0812; a-Hydroxylinoleic acid; AKOS040740632; alpha-Hydroxylinoleic acid; CHEBI:136927; CS-7178; DTXSID301258077; hydroxylinoleic acid; HY-U00141; LMFA02000290; MS-24253; s9611; SCHEMBL320069; UNII-0DE74TJ7EZ
Click to Show/Hide
|
|||
| External Link | ||||
| GP-2250 | Phase 1/2 | [99] | ||
| External Link | ||||
| Delolimogene mupadenorepvec | Phase 1/2 | [100] | ||
| Synonyms |
LOAd703
Click to Show/Hide
|
|||
| External Link | ||||
| GSK3145095 | Phase 1/2 | [101] | ||
| Synonyms |
1622849-43-7; CHEMBL4452233; (S)-5-benzyl-N-(7,9-difluoro-2-oxo-2,3,4,5-tetrahydro-1H-benzo[b]azepin-3-yl)-4H-1,2,4-triazole-3-carboxamide; UNII-B4D3WPS7JY; B4D3WPS7JY; SCHEMBL17312826; BCP31015; EX-A3069; BDBM50502339; s8845; GSK-3145095; HY-111946; CS-0094287; GSK 3145095; FC1=CC2=C(NC(=O)[C@H](CC2)NC(=O)C2=NN=C(CC3=CC=CC=C3)N2)C(F)=C1; (S)-5-Benzyl-N-(7,9-difluoro-2-oxo-2,3,4,5-tetrahydro-1hbenzo(b)azepin-3-yl)-1H-1,2,4-triazole-3-carboxamide (7,7-dimethyl-2- oxobicyclo(2.2.1)heptan-1-yl)
Click to Show/Hide
|
|||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [102] | ||
| External Link | ||||
| ETBX-011 cancer vaccine | Phase 1/2 | [80] | ||
| External Link | ||||
| BPX-601 | Phase 1/2 | [103] | ||
| External Link | ||||
| NANT | Phase 1/2 | [19] | ||
| External Link | ||||
| BrevaRex | Phase 1/2 | [104] | ||
| Synonyms |
BrevaRex MAb; monoclonal antibody
Click to Show/Hide
|
|||
| External Link | ||||
| DCVax-Pancreas | Phase 1/2 | [105] | ||
| Synonyms |
Dendritic cell-based immunotherapy (pancreatic cancer), Northwest Biotherapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| PEGylated hyaluronidase (human recombinant) | Phase 1/2 | [106] | ||
| Synonyms |
PEGylated hyaluronidase; PEGylated hyaluronidase (human recombinant) (intravenous, stroke/cancer), Halozyme
Click to Show/Hide
|
|||
| External Link | ||||
| CAR-T cells targeting mesothelin | Phase 1/2 | [107] | ||
| External Link | ||||
| MALP-2S | Phase 1/2 | [108] | ||
| External Link | ||||
| Anti-Mesothelin CAR-T cells | Phase 1/2 | [109] | ||
| External Link | ||||
| Anti-MUC1 CAR T Cells | Phase 1/2 | [110] | ||
| External Link | ||||
| Anti-HER2 CAR-T | Phase 1/2 | [111] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [112] | ||
| External Link | ||||
| Anti-MUC1 AR20.5 | Phase 1/2 | [16] | ||
| External Link | ||||
| AR20.5 | Phase 1/2 | [19] | ||
| External Link | ||||
| G0-203-2c | Phase 1/2 | [113] | ||
| External Link | ||||
| LOAd703 | Phase 1/2 | [19] | ||
| External Link | ||||
| Anti-mesothelin CAR transduced PBL | Phase 1/2 | [114] | ||
| External Link | ||||
| M9241 | Phase 1 | [115] | ||
| Synonyms |
NHS-IL12
Click to Show/Hide
|
|||
| External Link | ||||
| NBF-006 | Phase 1 | [116] | ||
| External Link | ||||
| AB680 | Phase 1 | [117] | ||
| Synonyms |
AB-680; UNII-J6K8WSV73A; J6K8WSV73A; CHEMBL4471306; 2105904-82-1; (((((2R,3S,4R,5R)-5-(6-chloro-4-(((S)-1-(2-fluorophenyl)ethyl)amino)-1H-pyrazolo[3,4-b]pyridin-1-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methoxy)(hydroxy)phosphoryl)methyl)phosphonic acid; [[(2~{R},3~{S},4~{R},5~{R})-5-[6-chloranyl-4-[[(1~{S})-1-(2-fluorophenyl)ethyl]amino]pyrazolo[3,4-b]pyridin-1-yl]-3,4-bis(oxidanyl)oxolan-2-yl]methoxy-oxidanyl-phosphoryl]methylphosphonic acid; SCHEMBL19100484; GTPL10707; BDBM50527134; HY-125286; CS-0090231; [[(2R,3S,4R,5R)-5-[6-chloro-4-[[(1S)-1-(2-fluorophenyl)ethyl]amino]pyrazolo[3,4-b]pyridin-1-yl]-3,4-dihydroxyoxolan-2-yl]methoxy-hydroxyphosphoryl]methylphosphonic acid; QDH
Click to Show/Hide
|
|||
| External Link | ||||
| STAT400 | Phase 1 | [118] | ||
| External Link | ||||
| CEND-1 | Phase 1 | [119] | ||
| Synonyms |
iRGD; UNII-Z8MXU5GH4Q; Z8MXU5GH4Q; iRGD-peptide; 1392278-76-0; Internalized-arginylglycylaspartic acid cyclic peptide; Q48988348; L-Cysteine, L-cysteinyl-L-arginylglycyl-L-alpha-aspartyl-L-lysylglycyl-L-prolyl-L-alpha-aspartyl-, cyclic (1->9)-disulfide
Click to Show/Hide
|
|||
| External Link | ||||
| CAR-T Cells targeting EGFRvIII | Phase 1 | [120] | ||
| External Link | ||||
| HuCART-meso cells | Phase 1 | [121] | ||
| External Link | ||||
| OCV-105 | Phase 1 | [122] | ||
| Synonyms |
Cancer vaccine (pancreas), Otsuka/OncoTherapy
Click to Show/Hide
|
|||
| External Link | ||||
| SBP-101 | Phase 1 | [54] | ||
| Synonyms |
diethyl dihydroxyhomospermine
Click to Show/Hide
|
|||
| External Link | ||||
| RG7882 | Phase 1 | [16] | ||
| External Link | ||||
| CART-meso-19 T cells | Phase 1 | [123] | ||
| External Link | ||||
| MOv19-BBz CAR T cells | Phase 1 | [124] | ||
| External Link | ||||
| Anti-MUC1 AR20.5 mab | Phase 1 | [125] | ||
| External Link | ||||
| Anti-CEA-CAR T | Phase 1 | [126] | ||
| External Link | ||||
| MVT-5873 | Phase 1 | [16] | ||
| External Link | ||||
| CART-meso cells | Phase 1 | [127] | ||
| External Link | ||||
| CAR-20/19-T Cells | Phase 1 | [128] | ||
| External Link | ||||
| CARTmeso/19 | Phase 1 | [129] | ||
| External Link | ||||
| CAR-T Cells targeting Mesothelin | Phase 1 | [120] | ||
| External Link | ||||
| CAR-T Cells targeting CEA | Phase 1 | [120] | ||
| External Link | ||||
| HLA-A*2402-restricted KIF20A and VEGFR-1 epitope peptide vaccine | Phase 1 | [130] | ||
| Synonyms |
HLA-A*2402-restricted KIF20A and VEGFR-1 epitope peptide vaccine (pancreatic cancer, subcutaneous)
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-hCD70 CAR transduced PBL | Phase 1 | [131] | ||
| External Link | ||||
| MORAb-066 | Phase 1 | [132] | ||
| Synonyms |
Anti-tissue factor monoclonal antibody (pancreatic tumor), Morphotek
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-MUC1 mab | Phase 1 | [133] | ||
| External Link | ||||
| Anti-meso-CAR vector transduced T cells | Phase 1 | [134] | ||
| External Link | ||||
| CRS-207 + GVAX | Phase 2 | [54] | ||
| External Link | ||||
| Autologous T cells transfected with chimeric anti-mesothelin immunoreceptor SS1 | Phase 1 | [135] | ||
| External Link | ||||
| Meso-CART | Phase 1 | [136] | ||
| External Link | ||||
| ASG-5ME | Phase 1 | [137] | ||
| External Link | ||||
| Anti-CD133-CAR vector-transduced T cells | Phase 1 | [138] | ||
| External Link | ||||
| IRX4204 | Phase 1 | [19] | ||
| Synonyms |
220619-73-8; CHEMBL75133; UNII-877M97Z38Y; VTP-194204; 877M97Z38Y; KB-145960; SCHEMBL3437269; MolPort-042-665-869; ZINC1550770; IRX-4204; 3-Methyl-5-[2-methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-cyclopropyl]-penta-2,4-dienoic acid; BDBM50101445; DB11806; VTP 194204; (+)-VTP-194204; AGN 4204; (2E,4E)-3-Methyl-5-[(1S,2S)-2-methyl-2-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-cyclopropyl]-penta-2,4-dienoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| AbGn-107 | Phase 1 | [19] | ||
| External Link | ||||
| LMB-100 | Phase 1/2 | [19] | ||
| External Link | ||||
| PLX7486 | Phase 1 | [139] | ||
| External Link | ||||
| CAR-T Cells targeting HER2 | Phase 1 | [120] | ||
| External Link | ||||
| CAR-T Cells targeting MUCI | Phase 1 | [120] | ||
| External Link | ||||
| SEL-403 | Phase 1 | [19] | ||
| External Link | ||||
| CAR-T Cells targeting PSCA | Phase 1 | [120] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [140] | ||
| External Link | ||||
| MVT-1075 | Phase 1 | [19] | ||
| External Link | ||||
| CAR-CLD18 T cell | Clinical trial | [141] | ||
| External Link | ||||
| CART-meso cells | Clinical trial | [142] | ||
| External Link | ||||
| PMID28460551-Compound-1 | Patented | [143] | ||
| External Link | ||||
| Tanomastat | Discontinued in Phase 3 | [144] | ||
| Synonyms |
Tanomastat (USAN/INN); (2S)-4-[4-(4-chlorophenyl)phenyl]-4-oxo-2-(phenylsulfanylmethyl)butanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Larotaxel | Discontinued in Phase 3 | [145] | ||
| Synonyms |
Benzenepropanoic acid; PNU 100940; XRP 9881; XRP9881
Click to Show/Hide
|
|||
| External Link | ||||
| Apricoxib | Discontinued in Phase 2 | [146] | ||
| Synonyms |
TG01
Click to Show/Hide
|
|||
| External Link | ||||
| Lintitript | Discontinued in Phase 2 | [147] | ||
| Synonyms |
SR 27897; SR 27897B; SR27897; SR-27897; SR-27897B; 1-((2-(4-(2-Chlorophenyl)thiazol-2-yl)aminocarbonyl)indolyl)acetic acid; 2-((4-(o-Chlorophenyl)-2-thiazolyl)carbamoyl)indole-1-acetic acid; 2-[2-[[4-(2-chlorophenyl)-1,3-thiazol-2-yl]carbamoyl]indol-1-yl]acetic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Merbarone | Discontinued in Phase 2 | [148] | ||
| Synonyms |
NSC-336628
Click to Show/Hide
|
|||
| External Link | ||||
| LY293111 | Discontinued in Phase 2 | [149] | ||
| Synonyms |
Etalocib; 161172-51-6; UNII-THY6RIW44R; LY 293111; THY6RIW44R; CHEMBL329123; LY-193111; 2-[3-[3-[2-ethyl-4-(4-fluorophenyl)-5-hydroxyphenoxy]propoxy]-2-propylphenoxy]benzoic acid; VML295; Etalocib [USAN:INN]; Etalocib (USAN); GTPL2948; SCHEMBL1649516; CTK8E7596; C33H33FO6; VML 295; DTXSID70167073; YFIZRWPXUYFCSN-UHFFFAOYSA-N; MolPort-009-019-411; ZINC3930629; AC1L4328; PDSP2_001221; BDBM50029450; PDSP1_001237; 1758AH; DB12850; RT-013626; D04074; L001468; J-009797; Benzoic acid, 2-(3-(3-((5-ethyl-4'-fluoro-2-hydroxy(1,1'-bipheny
Click to Show/Hide
|
|||
| External Link | ||||
| HMN-214 | Discontinued in Phase 1 | [150] | ||
| Synonyms |
N-(4-methoxyphenyl)sulfonyl-N-[2-[(E)-2-(1-oxidopyridin-4-yl)ethenyl]phenyl]acetamide; (E)-4-(2-(2-(N-Acetyl-N-((p-methoxyphenyl)sulfonyl)amino)phenyl)ethenyl)pyridine 1-oxide
Click to Show/Hide
|
|||
| External Link | ||||
| RG7600 | Discontinued in Phase 1 | [151] | ||
| External Link | ||||
| IC261 | Preclinical | [152] | ||
| Synonyms |
IC-261; IC 261
Click to Show/Hide
|
|||
| External Link | ||||
| ANAVEX 1007 | Preclinical | [153] | ||
| External Link | ||||
| IPH-4201 | Terminated | [154] | ||
| Synonyms |
MAb-16D10; MAb-J28; FAPP-targeting mAb (pancreatic cancer), Innate Pharma; FAPP-targeting mAb (pancreatic cancer), Universite de la Mediterranee/ INSERM; Feto-acinar pancreatic protein-targeting monoclonal antibodies (pancreatic cancer), Innate Pharma; Feto-acinar pancreatic protein-targeting monoclonal antibodies (pancreatic cancer), Universite de la Mediterranee/ INSERM
Click to Show/Hide
|
|||
| External Link | ||||
| MesoTarg | Investigative | [155] | ||
| External Link | ||||
| PAT-PM-1 | Investigative | [155] | ||
| Synonyms |
PM-1; Human monoclonal antibody (pancreatic cancer), Patrys; Human MAb (pancreas cancer), OncoMab/ Acceptys; Human monoclonal antibody (pancreatic cancer), OncoMab/ Acceptys; PM-1 antibody, OncoMab/ Acceptys
Click to Show/Hide
|
|||
| External Link | ||||
| OP-04 | Investigative | [155] | ||
| Synonyms |
OP-04 program (prodrug, pancreatic cancer); OP-04 program (prodrug, pancreatic cancer), Onco-Pharmakon
Click to Show/Hide
|
|||
| External Link | ||||
| VLI-27 | Investigative | [156] | ||
| Synonyms |
AKT inhibitor (pancreatic cancer), NovaLead Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| GS-326 | Investigative | [155] | ||
| Synonyms |
GS-326C
Click to Show/Hide
|
|||
| External Link | ||||
| PX-12 | Phase 2 | [157] | ||
| External Link | ||||
| Prodigiosin | Investigative | [155] | ||
| External Link | ||||
| Pbi-shPDX-1 LP | Investigative | [155] | ||
| External Link | ||||
| Gastrin 17C diphtheria toxoid conjugate | Investigative | [155] | ||
| Synonyms |
Gastrin 17C diphtheria toxoid conjugate (pancreatic cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| HS-P1 | Investigative | [155] | ||
| Synonyms |
HS-310; Endoplasmin modulator (pancreas tumor, HeatShock/fusion protein/antigen), Heat Biologics; Gp-96-Ig + unspecified tumor antigen secreting live cell vaccine (pancreas tumor, HeatShock), Heat Biologics
Click to Show/Hide
|
|||
| External Link | ||||
References