m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03342
|
[1], [2] | |||
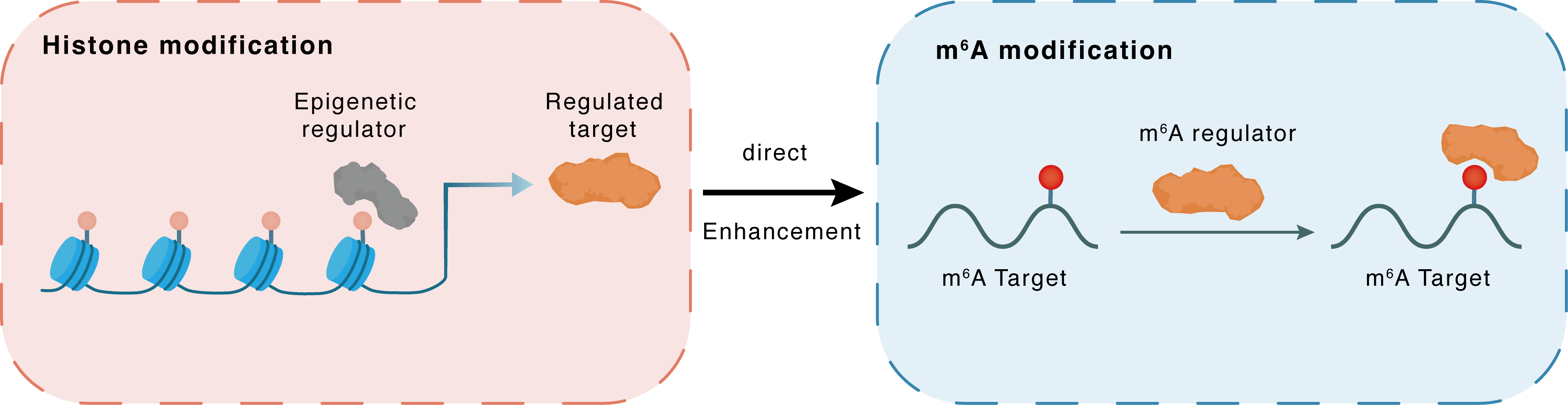 Histone modification
H3K18la
EP300
YTHDF2
Direct
Enhancement
m6A modification
MTOR
MTOR
YTHDF2
Histone modification
H3K18la
EP300
YTHDF2
Direct
Enhancement
m6A modification
MTOR
MTOR
YTHDF2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 2 (YTHDF2) | READER | |||
| m6A Target | Serine/threonine-protein kinase mTOR (MTOR) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone acetyltransferase p300 (P300) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 18 lactylation (H3K18la) | View Details | |||
| Downstream Gene | YTHDF2 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | Histone lactylation activates the transcription of YTHDF2 through p300-mediated Histone H3 lysine 18 lactylation (H3K18la) modification. In colorectal cancer, Glutaminolysis inhibition upregulated ATF4 expression in an m6A-dependent manner to activate pro-survival autophagy through transcriptional activation of the mTOR inhibitor DDIT4. Determined the relationship between FTO alpha-ketoglutarate dependent dioxygenase (FTO), YTH N6-methyladenosine RNA binding protein 2 (YTHDF2), and ATF4. ATF4 transcriptionally upregulated DDIT4 to suppress Serine/threonine-protein kinase mTOR (MTOR), which induced pro-survival autophagy during glutaminolysis inhibition. | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
| Responsed Drug | CB-839 | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Cell Process | RNA decay | ||||
| Cell growth and death | |||||
| Cell autophagy | |||||
In-vitro Model |
SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | |
| HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Histone acetyltransferase p300 (P300) | 2 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| CCS1477 | Phase 1/2 | [3] | ||
| Synonyms |
CCS-1477; CBP-IN-1; 2222941-37-7; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-((1r,4S)-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one; SCHEMBL20094038; SCHEMBL21515367; SCHEMBL22134021; EX-A3687; NSC818619; NSC-818619; HY-111784; CS-0091862; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-(trans-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FT-7051 | Phase 1 | [4] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Serine/threonine-protein kinase mTOR (MTOR) | 72 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Novolimus | Approved | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Temsirolimus | Approved | [6] | ||
| Synonyms |
Torisel
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1760 nM | |||
| External Link | ||||
| Everolimus | Approved | [7] | ||
| Synonyms |
Afinitor; Afinitor (TN); CERTICAN(R); Certican; Certican (TN); Everolimus (JAN/USAN/INN); Everolimus [USAN]; MTOR kinase inhibitors; NVP-RAD-001; RAD 001; RAD-001; RAD-001C; RAD001; RAD001, SDZ-RAD, Certican, Zortress, Afinitor, Everolimus; SDZ-RAD; Zortress
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Zotarolimus | Approved | [8] | ||
| Synonyms |
Abt-578; Zotarolimus (TN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 3.3 nM | |||
| External Link | ||||
| Sirolimus | Approved | [9] | ||
| Synonyms |
53123-88-9; Rapamune; Rapamycin (Sirolimus); AY-22989; Rapammune; sirolimusum; WY-090217; RAPA; Antibiotic AY 22989; AY 22989; UNII-W36ZG6FT64; CCRIS 9024; CHEBI:9168; SILA 9268A; W36ZG6FT64; HSDB 7284; C51H79NO13; NSC 226080; DE-109; NCGC00021305-05; DSSTox_CID_3582; DSSTox_RID_77091; DSSTox_GSID_23582; Cypher; Supralimus; Wy 090217; Perceiva; RAP; RPM; Rapamycin from Streptomyces hygroscopicus; SIIA 9268A; LCP-Siro; MS-R001; Rapamune (TN); Rapamycin (TN); Sirolimus (RAPAMUNE); Rapamycin C-7, analog 4; Sirolimus (USAN/INN); Sirolimus [USAN:BAN:INN]; Sirolimus, Rapamune,Rapamycin; Heptadecahydro-9,27-dihydroxy-3-[(1R)-2-[(1S,3R,4R)-4-hydroxy; 23,27-Epoxy-3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine; 23,27-Epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine; 23,27-epoxy-3H-pyrido[2,1-c][1,4]oxaazacyclohentriacontine-1,5,11,28,29; 3H-pyrido(2,1-c)(1,4)oxaazacyclohentriacontine-1,5,11,28,29(4H,6H,31H)-pentone; Sirolimus (MTOR inhibitor)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-04449913 | Approved | [10] | ||
| Synonyms |
Glasdegib; 1095173-27-5; PF 04449913; UNII-K673DMO5H9; K673DMO5H9; CHEMBL2043437; Glasdegib (PF-04449913); Glasdegib [USAN:INN]; Glasdegib (USAN/INN); PF-04449913;Glasdegib; GTPL8201; Glasdegib(PF-04449913); EX-A858; MolPort-035-789-706; SFNSLLSYNZWZQG-VQIMIIECSA-N; ZINC68251434; PF-913; BDBM50385635; 2640AH; AKOS027324121; CS-2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ridaforolimus | Phase 3 | [11] | ||
| Synonyms |
Deforolimus; AP 23573; MK 8669; AP-23573; MK-8669; AP23573, MK-8669, Ridaforolimus, Deforolimus
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| INK128 | Phase 2 | [12] | ||
| Synonyms |
1224844-38-5; Sapanisertib; INK-128; INK 128; INK 128 (MLN0128); TAK-228; UNII-JGH0DF1U03; JGH0DF1U03; 5-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)benzo[d]oxazol-2-amine; INK-0128; 3-(2-Amino-5-benzoxazolyl)-1-(1-methylethyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine; C15H15N7O; 5-(4-amino-1-propan-2-ylpyrazolo[3,4-d]pyrimidin-3-yl)-1,3-benzoxazol-2-amine; 5-(4-Amino-1-isopropyl-1H-pyrazolo[3,4-d]-pyrimidin-3-yl)benzo[d]oxazol-2-amine; Sapanisertib (USAN/INN)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1 nM | |||
| External Link | ||||
| OSI-027 | Phase 2 | [13] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| ABI-009 | Phase 2 | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Salirasib | Discontinued in Phase 1/2 | [5] | ||
| Synonyms |
162520-00-5; Farnesylthiosalicylic acid; S-Farnesylthiosalicylic acid; UNII-MZH0OM550M; MZH0OM550M; CHEMBL23293; AK186909; Farnesyl Thiosalicylic Acid; 2-[[(2E,6E)-3,7,11-Trimethyl-2,6,10-dodecatrien-1-yl]thio]benzoic Acid; 2-[(2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienyl]sulfanylbenzoic acid; 2-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trienylthio)benzoic acid; 2-(((2E,6E)-3,7,11-Trimethyl-2,6,10-dodecatrienyl)sulfanyl)benzoic acid; Benzoic acid, 2-(((2E,6E)-3,7,11-trimethyl-2,6,10-dodecatrienyl)thio)-; FTS; Farnesylthiosalicyclic acid; FTS, Thyreos; Ras antagonists, Thyreos; S-trans; Th-101; Trans-farnesylthiosalicylicacid; FTS (oral, cancer), Concordia; Farnesylthiosalicyclic acid (oral, cancer), Concordia; Ras-inhibitors (cancer), Concordia; FTS (oral, cancer), Concordia/Ono; KD032
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| SAR245409 | Phase 2 | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 157 nM | |||
| External Link | ||||
| SF1126 | Phase 2 | [14] | ||
| Synonyms |
CC-1126; SF-1126; L-Serine, N2-(1,4-dioxo-4-((4-(4-oxo-8-phenyl-4H-1-benzopyran-2-yl)morpholinium-4-yl)methoxy)butyl)-L-arginylglycyl-L-alpha-aspartyl-, inner salt
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-05212384 | Phase 2 | [15] | ||
| Synonyms |
PKI-587; 1197160-78-3; Gedatolisib; PKI587; PKI 587; 1-(4-(4-(Dimethylamino)piperidine-1-carbonyl)phenyl)-3-(4-(4,6-dimorpholino-1,3,5-triazin-2-yl)phenyl)urea; PF 05212384; UNII-96265TNH2R; PF-05212384 (PKI-587); CHEMBL592445; 96265TNH2R; N-[4-[[4-(Dimethylamino)-1-piperidinyl]carbonyl]phenyl]-N'-[4-[4,6-di(4-morpholinyl)-1,3,5-triazin-2-yl]phenyl]urea; Gedatolisib (PF-05212384, PKI-587); Urea, N-(4-((4-(dimethylamino)-1-piperidinyl)carbonyl)phenyl)-N'-(4-(4,6-di-4-morpholinyl-1,3,5-triazin-2-yl)phenyl)-
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 0.4 nM | |||
| External Link | ||||
| LY3023414 | Phase 2 | [16] | ||
| MOA | Modulator | |||
| External Link | ||||
| PF-04691502 | Phase 2 | [17] | ||
| Synonyms |
1013101-36-4; PF 04691502; UNII-4W39NS61KI; 4W39NS61KI; 2-amino-8-((1r,4r)-4-(2-hydroxyethoxy)cyclohexyl)-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7(8H)-one; CHEMBL1234354; PF04691502; 2-Amino-8-[trans-4-(2-Hydroxyethoxy)cyclohexyl]-6-(6-Methoxypyridin-3-Yl)-4-Methylpyrido[2,3-D]pyrimidin-7(8h)-One; 2-Amino-8-[4-(2-hydroxyethoxy)cyclohexyl]-6-(6-methoxypyridin-3-yl)-4-methylpyrido[2,3-d]pyrimidin-7-one
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 7.9 nM | |||
| External Link | ||||
| GDC-0980/RG7422 | Phase 2 | [5] | ||
| Synonyms |
Apitolisib; 1032754-93-0; GDC-0980; GDC0980; RG7422; (S)-1-(4-((2-(2-aminopyrimidin-5-yl)-7-methyl-4-morpholinothieno[3,2-d]pyrimidin-6-yl)methyl)piperazin-1-yl)-2-hydroxypropan-1-one; UNII-1C854K1MIJ; GDC-0980 (RG7422); Apitolisib (GDC-0980, RG7422); 1C854K1MIJ; CHEMBL1922094; RG-7422; (2s)-1-(4-{[2-(2-Aminopyrimidin-5-Yl)-7-Methyl-4-(Morpholin-4-Yl)thieno[3,2-D]pyrimidin-6-Yl]methyl}piperazin-1-Yl)-2-Hydroxypropan-1-One; J-502360; C23H30N8O3S
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | Ki = 17 nM | |||
| External Link | ||||
| BEZ235 | Phase 2 | [18] | ||
| Synonyms |
BEZ-235; S14-0511; NVP-BEZ-235; NVP-BEZ235, BEZ235; 2-(4-(2,3-dihydro-3-methyl-2-oxo-8-(quinolin-3-yl)imidazo[4,5-c]quinolin-1-yl)phenyl)-2-methylpropanenitrile
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| MM-141 | Phase 2 | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PQR309 | Phase 2 | [5] | ||
| Synonyms |
Bimiralisib; 1225037-39-7; PI3K-IN-2; PQR-309; UNII-6Z3QHB00LB; 6Z3QHB00LB; 5-(4,6-dimorpholino-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine; 5-[bis(morpholin-4-yl)-1,3,5-triazin-2-yl]-4-(trifluoromethyl)pyridin-2-amine; 5-(4,6-dimorpholin-4-yl-1,3,5-triazin-2-yl)-4-(trifluoromethyl)pyridin-2-amine; Bimiralisib [INN]; Bimiralisib [USAN]; Bimiralisib [WHO-DD]; NCB5; SCHEMBL1309049; GTPL8383; Bimiralisib free base; ADGGYDAFIHSYFI-UHFFFAOYSA-N; EX-A2018; BCP15887; PQR-309(PI3K-IN-2)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 62 nM | |||
| External Link | ||||
| AZD2014 | Phase 2 | [19] | ||
| Synonyms |
1009298-59-2; Vistusertib; AZD-2014; AZD 2014; UNII-0BSC3P4H5X; 0BSC3P4H5X; cc-551; 3-[2,4-Bis((3S)-3-methylmorpholin-4-yl)pyrido[5,6-e]pyrimidin-7-yl]-N-methylbenzamide; CHEMBL2336325; 3-[2,4-Bis((3S)-3-methyLmorpholin-4-yl)pyrido-[5,6-e]pyrimidin-7-yl]-N-methylbenzamide; C25H30N6O3; 3-(2,4-bis((S)-3-methylmorpholino)pyrido[2,3-d]pyrimidin-7-yl)-N-methylbenzamide; 3-[2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-N-methylbenzamide; Vistusertib [INN]; Vistusertib [USAN]; Vistusertib (JAN/INN)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2.8 nM | |||
| External Link | ||||
| CC-223 | Phase 1/2 | [20] | ||
| Synonyms |
GW 791343 HYDROCHLORIDE; GW791343 trihydrochloride; 309712-55-8; 1019779-04-4; GW791343 HCl; GW791343 (trihydrochloride); GW791343; GW-791343; 2-[(3,4-Difluorophenyl)amino]-N-[2-methyl-5-(1-piperazinylmethyl)phenyl]-acetamide trihydrochloride; GW791343 (HCL); GW-791343 hydrochloride; C20H27Cl3F2N4O; GW 791343 Trihydrochloride; C20H24F2N4O.3ClH; CTK8F0044; EX-A438; GW 791343 HCl; WSBRAHWNJBXXJM-UHFFFAOYSA-N; MolPort-023-219-209; BCP23425; AKOS024457596; CS-1030; BCP9000749; API0008007; HY-15470; BCP0726000290; RT-017402; KB-272661
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| BGT226 | Phase 1/2 | [21] | ||
| Synonyms |
BGT-226 free base; 915020-55-2; UNII-ZXE7F2GMJJ; BGT226 free base; ZXE7F2GMJJ; BGT-226; 8-(6-Methoxypyridin-3-yl)-3-methyl-1-[4-(piperazin-1-yl)-3-trifluoromethylphenyl]-1,3-dihydroimidazo[4,5-c]quinolin-2-one; CHEBI:71967; BGT 226; NVPBGT226; 8-(6-methoxypyridin-3-yl)-3-methyl-1-[4-(piperazin-1-yl)-3-(trifluoromethyl)phenyl]-1,3-dihydro-2H-imidazo[4,5-c]quinolin-2-one; 8-(6-Methoxy-pyridin-3-yl)-3-methyl-1-(4-piperazin-1-yl-3-trifluoromethyl-phenyl)-1,3-dihydro-imidazo[4,5-c]quinolin-2-one; NPV-BGT226; SCHEMBL146939
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| ME-344 | Phase 1/2 | [22] | ||
| Synonyms |
NV-128; NV-344; MTOR inhibitor (cancer), Novogen; MTOR inhibitors (cancer), Marshall Edwards
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BI 860585 | Phase 1 | [23] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LAM-001 | Phase 1 | [24] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-3078 | Phase 1 | [25] | ||
| MOA | Modulator | |||
| External Link | ||||
| GDC-0349 | Phase 1 | [26] | ||
| Synonyms |
MTORC1/2 inhibitors
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3.8 nM | |||
| External Link | ||||
| CERC 006 | Phase 1 | [27] | ||
| Synonyms |
(+/-)-Cyclohexanecarboxylic acid, 4-(4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo(5,1-f)(1,2,4)triazin-7-yl)-, trans-; (1r,4r)-4-(4-Amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl)cyclohexane-1-carboxylic acid; (1r,4r)-4-(4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl)cyclohexanecarboxylic acid; (1r,4r)-4-(4-Amino-5-(7-methoxy-1H-indol-2-yl)imidazo-[5,1-f][1,2,4]triazin-7-yl)cyclohexanecarboxylic acid; 1187559-66-5; 25MKH1SZ0M; 4-(4-Amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f] [1,2,4]Triazin-7-yl)cyclohexanecarboxylic Acid; 4-[(5Z)-4-amino-5-(7-methoxyindol-2-ylidene)-1H-imidazo[5,1-f][1,2,4]triazin-7-yl]cyclohexane-1-carboxylic acid; 4-[4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl]cyclohexane-1-carboxylic acid; 4-[4-amino-5-(7-methoxy-2-indolylidene)-1H-imidazo[5,1-f][1,2,4]triazin-7-yl]-1-cyclohexanecarboxylic acid; 936890-98-1; 936890-98-1 (free acid); A-1065; AC-31517; AEVI-006; AKOS030238938; AKOS037643584; AM81260; AS-17003; ASP 7486; ASP4786; ASP7486; ASP-7486; BCP02613; BCP9001034; BDBM185151; BRD-K94294671-003-01-3; CCG-268721; CERC 006; CERC006; CERC-006; CHEBI:91363; CHEMBL2132692; CHEMBL3120215; CS-0257; Cyclohexanecarboxylic acid, 4-(4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo(5,1-f)(1,2,4)triazin-7-yl)-, trans-; Cyclohexanecarboxylic acid, 4-[4-amino-5-(7-methoxy-1h-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl]-, trans-; DB12387; DTXSID901025951; EX-A143; F17371; HMS3656H05; HMS3748I11; HY-10423; J-523839; JROFGZPOBKIAEW-HAQNSBGRSA-N; LS-14875; MLS006011006; NCGC00250395-01; NCGC00386179-01; NCGC00386179-04; NCGC00387858-03; NSC800810; NSC-800810; OSI 027; OSI027; OSI-027; Q27163231; Q27253978; s2624; SB19259; SCHEMBL20482333; SCHEMBL22594988; SCHEMBL22787096; SCHEMBL976795; SCHEMBL976796; SMR004702804; SW220246-1; trans-4-(4-Amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl)cyclohexanecarboxylic acid; TRANS-4-[4-AMINO-5-(7-METHOXY-1H-INDOL-2-YL)IMIDAZO[5,1-F][1,2,4]TRIAZIN-7-YL]CYCLOHEXANECARBOXYLIC ACID; trans-4-[4-amino-5-(7-methoxy-1H-indol-2-yl)imidazo[5,1-f][1,2,4]triazin-7-yl]-cyclohexanecarboxylic acid; trans-4-[4-amino-5-(7-methoxy-1H-indol-2-yl)-imidazo[5,1-f][1,2,4]triazin-7-yl]-cyclohexanecarboxylic acid; UNII-25MKH1SZ0M
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DS-7423 | Phase 1 | [28] | ||
| MOA | Modulator | |||
| External Link | ||||
| PWT-33597 | Phase 1 | [29] | ||
| Synonyms |
PI3 kinase alpha/mTOR dual inhibitor (cancer), Pathway Therapeutics
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| VS-5584 | Phase 1 | [5] | ||
| Synonyms |
5-(9-Isopropyl-8-methyl-2-morpholino-9H-purin-6-yl)pyrimidin-2-amine; 1246560-33-7; VS-5584 (SB2343); UNII-W71J4X250V; SB-2343; SB2343; CHEMBL3393066; W71J4X250V; 5-(8-methyl-2-morpholin-4-yl-9-propan-2-ylpurin-6-yl)pyrimidin-2-amine; C17H22N8O; QYBGBLQCOOISAR-UHFFFAOYSA-N; SCHEMBL539098; GTPL8382; EX-A288; DTXSID10677328; MolPort-035-757-944; HMS3652B16; BCP08247; 2797AH; ZINC95644685; s7016; VS5584; BDBM50059635; AKOS024465057; 5-(9-isopropyl-8-methyl-2-morpholin-4-yl-9H-purin-6-yl)-pyrimidin-2-ylamine; SB16877
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| PMID25726713-Compound-49 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-51 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-48 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-47 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-50 | Patented | [30] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1 nM | |||
| External Link | ||||
| AZD8055 | Discontinued in Phase 1/2 | [31] | ||
| Synonyms |
1009298-09-2; AZD-8055; AZD 8055; [5-[2,4-Bis((3S)-3-methylmorpholin-4-yl)pyrido[2,3-d]pyrimidin-7-yl]-2-methoxyphenyl]methanol; (5-(2,4-bis((S)-3-methylmorpholino)pyrido[2,3-d]pyrimidin-7-yl)-2-methoxyphenyl)methanol; UNII-970JJ37FPW; 970JJ37FPW; CHEMBL1801204; AK109550; (5-(2,4-Bis((S)-3-methylmorpholino)pyrido-[2,3-d]pyrimidin-7-yl)-2-methoxyphenyl)methanol; (5-(2,4-bis((3S)-3-methylmorpholin-4-yl)pyrido(2,3-d)pyrimidin-7-yl)-2-methoxyphenyl)methanol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| TAFA-93 | Discontinued in Phase 1 | [32] | ||
| Synonyms |
MTOR inhibitor, Isotechnika; Rapamycin prodrug, Isotechnika; Transplant rejection therapy, Isotechnika
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SCR-44001 | Terminated | [33] | ||
| Synonyms |
MTOR pathway inhibitors (cancer); PI3K modulators, BioImage; SCR-0044001; SCR-0334654; SCR-0335319; TOP-216; MTOR pathway inhibitors (cancer), TopoTarget; MTOR pathway inhibitors (cancer), BioImage/TopoTarget
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (4-(6-morpholino-9H-purin-2-yl)phenyl)methanol | Investigative | [34] | ||
| Synonyms |
CHEMBL594669; SCHEMBL4442909
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4500 nM | |||
| External Link | ||||
| Rapamycin complexed with immunophilin FKBP12 | Investigative | [35] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C-16-(S)-3-methylindolerapamycin | Investigative | [36] | ||
| Synonyms |
CHEMBL503885
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| PF-05094037 | Investigative | [5] | ||
| Synonyms |
PF-05171310; PF-05181059; MTOR inhibitors (cancer), Pfizer
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SX-MTR1 | Investigative | [5] | ||
| Synonyms |
MTOR modulators (small peptide mimetics, bladder cancer), Serometrix
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 2-(6-morpholino-9H-purin-2-yl)phenol | Investigative | [34] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2500 nM | |||
| External Link | ||||
| P-2281 | Investigative | [5] | ||
| Synonyms |
MTOR inhibitor (ulcerative colitis), Piramal
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| X-387 | Investigative | [5] | ||
| Synonyms |
MTOR inhibitors (cancer), Shanghai Institute of Materia Medica
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(6-morpholino-9H-purin-2-yl)phenol | Investigative | [34] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 450 nM | |||
| External Link | ||||
| EM-101 | Investigative | [5] | ||
| Synonyms |
EM-100 series; LY-3; LY-303511; MTOR pathway inhibitors (cancer), Emiliem; MTOR pathway inhibitors (cancer), NIH
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| EC-0845 | Investigative | [5] | ||
| Synonyms |
MTOR modulator (inflammatory disease), Endocyte
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| 4-(2-(thiophen-2-yl)-9H-purin-6-yl)morpholine | Investigative | [34] | ||
| Synonyms |
CHEMBL604876; SCHEMBL4439490
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2800 nM | |||
| External Link | ||||
| SB-2280 | Investigative | [5] | ||
| Synonyms |
SB-2602; Selective mTOR inhibitors (cancer); Selective mTOR inhibitors (cancer), S*BIO
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(2-(thiophen-3-yl)-9H-purin-6-yl)morpholine | Investigative | [34] | ||
| Synonyms |
CHEMBL608095; SCHEMBL4438208
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3900 nM | |||
| External Link | ||||
| torin 1 | Investigative | [37] | ||
| Synonyms |
Torin-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.32 nM | |||
| External Link | ||||
| EC-0565 | Investigative | [5] | ||
| Synonyms |
Folate-everolimus conjugate (inflammation), Endocyte
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| OXA-01 | Investigative | [5] | ||
| Synonyms |
MTORC1/mTORC2 inhibitor (cancer) OSI Pharmaceuticals
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| AR-mTOR-26 | Investigative | [5] | ||
| Synonyms |
AR-mTOR-1; MTORC1/2 inhibitors (cancer); MTORC1/2 inhibitors (cancer), Array BioPharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-chloro-N-(6-cyanopyridin-3-yl)propanamide | Investigative | [38] | ||
| Synonyms |
1112994-35-0; SCHEMBL1483919; CHEMBL446834; VFOLQYOVUCHHET-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(6-morpholino-9H-purin-2-yl)phenol | Investigative | [34] | ||
| Synonyms |
CHEMBL593515; SCHEMBL4443377; FUDQNOGEMXSUSQ-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1650 nM | |||
| External Link | ||||
| AP-21967 | Investigative | [36] | ||
| Synonyms |
CHEMBL525042; SCHEMBL18176922; C-16-(S)-7-methylindolerapamycin
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| 4-(2-(1H-indol-6-yl)-9H-purin-6-yl)morpholine | Investigative | [34] | ||
| Synonyms |
CHEMBL611630
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 150 nM | |||
| External Link | ||||
| Torin2 | Investigative | [39] | ||
| Synonyms |
Torin 2; 1223001-51-1; Torin-2; 9-(6-aminopyridin-3-yl)-1-[3-(trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2-one; 9-(6-AMINO-3-PYRIDINYL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-BENZO[H]-1,6-NAPHTHYRIDIN-2(1H)-ONE; CHEMBL1765602; C24H15F3N4O; CHEBI:90682; 9-(6-Aminopyridin-3-Yl)-1-[3-(Trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2(1h)-One; 9-(6-AMINOPYRIDIN-3-YL)-1-(3-(TRIFLUOROMETHYL)PHENYL)BENZO[H][1,6]NAPHTHYRIDIN-2(1H)-ONE; 9-(6-Aminopyridin-3-yl)-1-(3-(trifluoromethyl)-phenyl)benzo[h][1,6]naphthyridin-2(1H)-one; BENZO[H]-1,6-NAPHTHYRIDIN-2(1H)-ONE, 9-(6-AMINO-3-PYRIDINYL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-; 9-(6-Amino-3-pyridinyl)-1-[3-(trifluoromethyl)phenyl]benzo[h]-1,6-naphthyridin-2(1H)-one; cc-275; MLS006011167; GTPL8839; SCHEMBL6876328; AOB3537; DTXSID00679917; EX-A431; HMS3265O05; HMS3265O06; HMS3265P05; HMS3265P06; HMS3651N13; BCP02612; ABP000908; BDBM50341209; MFCD18782652; NSC775727; s2817; ZINC71318831; AKOS024458055; CCG-265003; CS-0236; NSC-775727; PB34957; NCGC00263216-01; NCGC00263216-02; NCGC00263216-09; NCGC00263216-13; 9-(6-AMINO-PYRIDIN-3-YL)-1-(3-TRIFLUOROMETHYL-PHENYL)-1H-BENZO[H][1,6]NAPHTHYRIDIN-2-ONE; AC-31520; AK171126; AS-74405; HY-13002; SMR004702936; AB0035864; DB-084736; FT-0700124; SW218309-2; Y0293; Q-4148; J-519481; BRD-K68174511-001-01-7; Q27089008; 9-(6-amino-3-pyridyl)-1-[3-(trifluoromethyl)phenyl]benzo[h][1,6]naphthyridin-2-one; 17G; 9-(6-Amino-3-pyridinyl)-1-[3-(trifl uoromethyl)phenyl]-benzo[h]-1,6-naphthyridin-2(1H) -one; 9-(6-AMINOPYRIDIN-3-YL)-1-[3-(TRIFLUOROMETHYL)PHENYL]-1H,2H-BENZO[H]1,6-NAPHTHYRIDIN-2-ONE
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HM-5016699 | Investigative | [5] | ||
| Synonyms |
Dual PI3K/mTOR inhibitor (cancer); Dual PI3K/mTOR inhibitor (cancer), Hutchison
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PF-03772304 | Investigative | [40] | ||
| Synonyms |
MTOR inhibitors, Biotica; MTOR inhibitors, Wyeth; PF-04979064; PF-05017255; PF-05168899; WYE-125132; WYE-132; WYE-178; WYE-354; WYE-600; WYE-687; Imidazolo and pyrazolopyrimidine derivatives (cancer), Pfizer; Imidazolopyrimidine derivatives (cancer), Pfizer; Imidazolopyrimidine derivatives (cancer), Wyeth; Non-rapamycin mTOR/PI3K inhibitors (cancer); PI3K/mTOR signalling inhibitors (cancer), Wyeth; Non-rapamycin mTOR/PI3K inhibitors (cancer), Pfizer; 5H-pyrrolo[3,2-d]pyridimine analogs (cancer), Wyeth
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PP-242 | Investigative | [41] | ||
| Synonyms |
PP242; TORKinib
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| CU-906 | Investigative | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| P-6915 | Investigative | [5] | ||
| Synonyms |
PI3K/mTOR inhibitors (cancer), Piramal
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-Morpholin-4-yl-pyrimido[2,1-a]isoquinolin-4-one | Investigative | [42] | ||
| Synonyms |
Compound 401; 168425-64-7; 2-morpholino-4H-pyrimido[2,1-a]isoquinolin-4-one; 2-(morpholin-4-yl)pyrimido[2,1-a]isoquinolin-4-one; CHEMBL179242; 2-(4-MORPHOLINYL)-4H-PYRIMIDO[2,1-A]ISOQUINOLIN-4-ONE; Compound401; SCHEMBL10092321; KS-00001DEG; CTK4D2994; DTXSID20434626; MolPort-023-276-726; HMS3229D15; EX-A1016; BCP04303; BDBM50159620; ZINC13608047; AKOS016369524; CS-5624; NCGC00378805-02; HY-19341; KB-224235; M2537; B7337; S-7713; J-010456
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5300 nM | |||
| External Link | ||||
| 2-(2-Methyl-morpholin-4-yl)-benzo[h]chromen-4-one | Investigative | [42] | ||
| Synonyms |
CHEMBL435507; SCHEMBL3545107
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4800 nM | |||
| External Link | ||||
| Ethyl 1-[(1H-benzimidazol-2(3H)one-5-yl)sulfonyl]-1H-pyrrole-2-carboxylate | Investigative | [42] | ||
| Synonyms |
2-(Morpholin-4-yl)-benzo[h]chromen-4-one; 154447-35-5; NU7026; NU 7026; DNA-PK Inhibitor II; NU-7026; 2-morpholino-4H-benzo[h]chromen-4-one; LY293646; LY-293646; 2-(4-Morpholinyl)-4H-naphthol[1,2-b]pyran-4-one; 2-(4-morpholinyl)-4H-naphtho[1,2-b]pyran-4-one; CHEMBL104468; AK186905; DNA-Dependent Protein Kinase Inhibitor II; 2-morpholin-4-ylbenzo[h]chromen-4-one; SCHEMBL610237; ZINC9230; GTPL5959; KS-00000XHI; CTK0E7833; CHEBI:92165; DTXSID10432010; AOB2835; MolPort-009-019-548; HMS3229C11; EX-A1100; BCP04736; IN1364; s2893
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6400 nM | |||
| External Link | ||||
| PP121 | Investigative | [41] | ||
| Synonyms |
PP-121; PP 121
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [43] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [44] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [45] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [14] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [14] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [46] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [14] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [44] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [47] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [48] | ||
| External Link | ||||
| CV301 | Phase 2 | [49] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [50] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [51] | ||
| External Link | ||||
| RG7221 | Phase 2 | [52] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [53] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [54] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [55] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [56] | ||
| External Link | ||||
| MGD007 | Phase 1 | [52] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [57] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [14] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [58] | ||
| External Link | ||||
| Nimesulide | Terminated | [59] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [60] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [61] | ||
| External Link | ||||
References