m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03176
|
[1] | |||
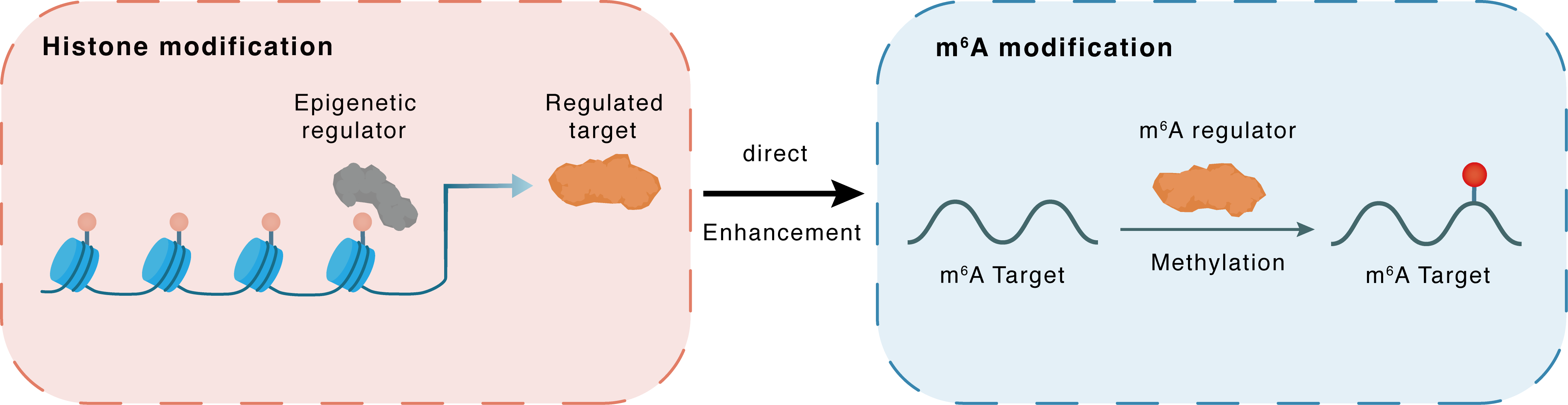 Histone modification
H3K9Ac
CBP
RBM15
Direct
Enhancement
m6A modification
CXCL11
CXCL11
RBM15
Methylation
Histone modification
H3K9Ac
CBP
RBM15
Direct
Enhancement
m6A modification
CXCL11
CXCL11
RBM15
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | RNA-binding motif protein 15 (RBM15) | WRITER | |||
| m6A Target | C-X-C motif chemokine 11 (CXCL11) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | CREB-binding protein (CREBBP) | WRITER | View Details | ||
| Regulated Target | Histone H3 lysine 9 acetylation (H3K9Ac) | View Details | |||
| Downstream Gene | RBM15 | View Details | |||
| Crosstalk Relationship | Histone modification → m6A | Enhancement | |||
| Crosstalk Mechanism | histone modification directly impacts m6A modification through modulating the level of m6A regulator | ||||
| Crosstalk Summary | Epigenetic activation of RBM15 promotes clear cell renal cell carcinoma growth, metastasis and macrophage infiltration by regulating the m6A modification of C-X-C motif chemokine 11 (CXCL11). Enhanced RBM15 was caused by the abundant H3K27ac/Histone H3 lysine 9 acetylation (H3K9ac) of the RBM15 promoter induced by EP300/CREBBP. | ||||
| Responsed Disease | Renal cell carcinoma of kidney | ICD-11: 2C90.0 | |||
| Responsed Drug | SETDB1-TTD-IN-1 | ||||
| Pathway Response | mRNA surveillance pathway | hsa03015 | |||
| RNA degradation | hsa03018 | ||||
| Cell Process | RNA stability | ||||
In-vitro Model |
THP-1 | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0006 | |
| HK-2 [Human kidney] | Normal | Homo sapiens | CVCL_0302 | ||
| 786-O | Renal cell carcinoma | Homo sapiens | CVCL_1051 | ||
| Caki-1 | Clear cell renal cell carcinoma | Homo sapiens | CVCL_0234 | ||
| Caki-2 | Papillary renal cell carcinoma | Homo sapiens | CVCL_0235 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| CREB-binding protein (CREBBP) | 8 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PRI-724 | Phase 1/2 | [2] | ||
| MOA | Modulator | |||
| External Link | ||||
| C 82 | Phase 1/2 | [3] | ||
| Synonyms |
N-(4-Chlorophenyl)-2H-triazol-4-amine; SCHEMBL15831502; C82
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CCS1477 | Phase 1/2 | [4] | ||
| Synonyms |
CCS-1477; CBP-IN-1; 2222941-37-7; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-((1r,4S)-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one; SCHEMBL20094038; SCHEMBL21515367; SCHEMBL22134021; EX-A3687; NSC818619; NSC-818619; HY-111784; CS-0091862; (S)-1-(3,4-Difluorophenyl)-6-(5-(3,5-dimethylisoxazol-4-yl)-1-(trans-4-methoxycyclohexyl)-1H-benzo[d]imidazol-2-yl)piperidin-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FT-7051 | Phase 1 | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pyrrolo-pyrrolone derivative 6 | Patented | [6] | ||
| Synonyms |
PMID26924192-Compound-39
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 91 nM | |||
| External Link | ||||
| ischemin | Investigative | [7] | ||
| Synonyms |
MS120
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5000 nM | |||
| External Link | ||||
| SGC-CBP30 | Investigative | [8] | ||
| Synonyms |
1613695-14-9; (s)-4-(1-(2-(3-chloro-4-methoxyphenethyl)-5-(3,5-dimethylisoxazol-4-yl)-1h-benzo[d]imidazol-1-yl)propan-2-yl)morpholine; 2-[2-(3-chloro-4-methoxyphenyl)ethyl]-5-(dimethyl-1,2-oxazol-4-yl)-1-[(2S)-2-(morpholin-4-yl)propyl]-1H-1,3-benzodiazole; 2-[2-(3-Chloro-4-Methoxyphenyl)ethyl]-5-(3,5-Dimethyl-1,2-Oxazol-4-Yl)-1-[(2s)-2-(Morpholin-4-Yl)propyl]-1h-Benzimidazole; 2LO; C28H33ClN4O3; GTPL7529; SCHEMBL17512896; CHEMBL3622373; AOB4800; MolPort-035-395-859; BDBM188519; EX-A2159; ZINC96170456; s7256
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 30 nM | |||
| External Link | ||||
| I-CBP112 | Investigative | [9] | ||
| Synonyms |
1640282-31-0; I-CBP 112; CHEMBL3774655; 1-[7-(3,4-Dimethoxyphenyl)-9-[[(3S)-1-methylpiperidin-3-yl]methoxy]-2,3,4,5-tetrahydro-1,4-benzoxazepin-4-yl]propan-1-one; 1-[7-(3,4-dimethoxyphenyl)-9-{[(3S)-1-methylpiperidin-3-yl]methoxy}-2,3,4,5-tetrahydro-1,4-benzoxazepin-4-yl]propan-1-one; GTPL8236; SCHEMBL17620385; MolPort-035-765-871; EX-A2474; ZINC96024493; BDBM50151663; AKOS024458402; CS-6146; NCGC00350526-04; HY-19541; I-CBP112, >
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 440 nM | |||
| External Link | ||||
| 2C90: Renal cell carcinoma | 76 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Atezolizumab | Approved | [10] | ||
| External Link | ||||
| Tivozanib | Approved | [11] | ||
| Synonyms |
ASP4130/AV-951
Click to Show/Hide
|
|||
| External Link | ||||
| Sorafenib | Approved | [12] | ||
| Synonyms |
Nexavar; Sorafenibum; Sorafenib [INN]; Nexavar (TN); Sorafenib (INN); N-[4-Chloro-3-(trifluoromethyl)phenyl]-N'-[4-[2-(N-methylcarbamoyl)-4-pyridyloxy]phenyl]urea; N-(4-Chloro-3-(trifluoromethyl)phenyl)-N'-(4-(2-(N-methylcarbamoyl)-4-pyridyloxy)phenyl)urea; N-(4-chloro-3-(trifluoromethyl)phenyl)-N'-(4-(2-(N-methylcar bamoyl)-4-pyridyloxy)phenyl)urea; 4(4-{3-[4-Chloro-3-(trifluoromethyl)phenyl]ureido}phenoxy)-N(sup 2)-methylpyridine-2-carboxamide; 4-(4-((((4-Chloro-3-(trifluoromethyl)phenyl)amino)carbonyl)amino)phenoxy)-N-methyl-2-pyridinecarboxamide; 4-(4-(3-(4-chloro-3-trifluoromethylphenyl)ureido)phenoxy)pyridine-2-carboxyllic acid methyamide-4-methylbenzenesulfonate; 4-(4-{3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido}phenoxy)-N(sup 2)-methylpyridine-2-carboxamide; 4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)phenoxy]-N-methylpyridine-2-carboxamide; 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methyl-pyridine-2-carboxamide; 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]phenoxy]-N-methylpyridine-2-carboxamide; 4-[4-[[[[4-chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]phenoxy]-N-methyl-2-pyridinecarboxamide; 4-{4-[({[4-CHLORO-3-(TRIFLUOROMETHYL)PHENYL]AMINO}CARBONYL)AMINO]PHENOXY}-N-METHYLPYRIDINE-2-CARBOXAMIDE; Sorafenib (Pan-TK inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Lenvatinib | Approved | [10] | ||
| Synonyms |
E 7080; E-7080, E7080; 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Belzutifan | Approved | [13] | ||
| Synonyms |
MK-6482
Click to Show/Hide
|
|||
| External Link | ||||
| Reniale | Approved | [14] | ||
| Synonyms |
Autologous renal tumor cell vaccine (renal cell carcinoma), LipoNova
Click to Show/Hide
|
|||
| External Link | ||||
| Romidepsin | Approved | [15] | ||
| Synonyms |
Chromadax; Istodax; Antibiotic FR 901228; FK 228; FK228; FR 901228; FR901228; HDInhib_000006; Chromadax (TN); FK-228; FK-901228; FR-901228; Istodax (TN); Romidepsin (USAN); Cyclo((2Z)-2-amino-2-butenoyl-L-valyl-(3S,4E)-3-hydroxy-7-mercapto-4-heptenoyl-D-valyl-D-cysteinyl), cyclic (35)-disulfide; L-Valine, N-((3S,4E)-3-hydroxy-7-mercapto-1-oxo-4-heptenyl)-D-valyl-D-cysteinyl-(2Z)-2-amino-2-butenoxyl-, (4-1)-lactone, cyclic (1-2)-disulfide; (1S,4S,7Z,10S,16E,21R)-7-Ethylidene-4,21-bis(1-methylethyl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo(8.7.6)tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetraazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone; (1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-di(propan-2-yl)-2-oxa-12,13-dithia-5,8,20,23-tetrazabicyclo[8.7.6]tricos-16-ene-3,6,9,19,22-pentone
Click to Show/Hide
|
|||
| External Link | ||||
| Avelumab | Approved | [10] | ||
| External Link | ||||
| Everolimus | Approved | [16] | ||
| Synonyms |
Afinitor; Afinitor (TN); CERTICAN(R); Certican; Certican (TN); Everolimus (JAN/USAN/INN); Everolimus [USAN]; MTOR kinase inhibitors; NVP-RAD-001; RAD 001; RAD-001; RAD-001C; RAD001; RAD001, SDZ-RAD, Certican, Zortress, Afinitor, Everolimus; SDZ-RAD; Zortress
Click to Show/Hide
|
|||
| External Link | ||||
| Pazopanib HCl | Approved | [17] | ||
| Synonyms |
Votrient (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Temsirolimus | Approved | [18] | ||
| Synonyms |
Torisel
Click to Show/Hide
|
|||
| External Link | ||||
| Pembrolizumab | Approved | [10] | ||
| External Link | ||||
| CreaVax-RCC | Approved | [19] | ||
| Synonyms |
Autologous DC vaccines (renal cell carcinoma), CreaGene; Autologous dendritic cell vaccines (renal cell carcinoma), CreaGene
Click to Show/Hide
|
|||
| External Link | ||||
| Aldesleukin | Approved | [20] | ||
| Synonyms |
Proleukin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sifalimumab | Approved | [21] | ||
| Synonyms |
Cci 779; CHEBI:79699; Torisel (TN); 42-[3-Hydroxy-2-(hydroxymethyl)-2-methylpropanoate]rapamycin; Temsirolimus (JAN/USAN/INN); 162635-04-3; DB06287; C15182; D06068; J-524319; (1R,2R,4S)-4-[(2R)-2-[(1R,9S,12S,15R,18R,19R,21R,23S,30S,32S,35R)-1,18-dihydroxy-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-2,3,10,14,20-pentaoxo-11,36-dioxa-4-azatricyclo[30.3.1.0; {4,9}]hexatriaconta-16,24,26,28-tetraen-12-yl]propyl]-2-methoxycyclohexyl 3-hydroxy-2-(hydroxymethyl)-2-methylpropanoate
Click to Show/Hide
|
|||
| External Link | ||||
| BOL-303259-X | Phase 3 | [22] | ||
| Synonyms |
Latanoprostene bunod; Latanoprostene bunod); NCX-116; PF-03187207; PF-3187207; BOL-303259-X1; NO donors (glaucoma), NicOx/Pfizer; NO-donating prostaglandin F2-alpha analogs (glaucoma), Pfizer; NO-donating prostaglandin F2-alpha analogs (glaucoma), NicOx/Pfizer; Nitric oxide-donating prostaglandin F2-alpha analogs (eye disease), NicOx/Pfizer; NO-donating prostaglandin F2-alpha analogs (glaucoma), NicOx/ Bausch & Lomb
Click to Show/Hide
|
|||
| External Link | ||||
| Axitinib | Approved | [23] | ||
| Synonyms |
AG 013736; AG013736; AG-013736; AG-13736; AG-013736, Axitinib; N-methyl-2-[[3-[(E)-2-pyridin-2-ylethenyl]-1H-indazol-6-yl]sulfanyl]benzamide; N-methyl-2-((3-((1E)-2-(pyridin-2-yl)ethenyl)-1H-indazol-6-yl)sulfanyl)benzamide; Axitinib (VEGFR inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Nivolumab | Approved | [10] | ||
| External Link | ||||
| HBI-8000 | Phase 1/2 | [10] | ||
| Synonyms |
CS055; SCHEMBL5500152; GTPL8305; AKOS026750315; N-(2-amino-5-fluorophenyl)-4-{[3-(pyridin-3-yl)prop-2-enamido]methyl}benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| Human coagulation factor X | BLA submitted | [24] | ||
| External Link | ||||
| Trebananib | Phase 3 | [25] | ||
| External Link | ||||
| Abexinostat | Phase 3 | [26] | ||
| Synonyms |
PCI-24781; 783355-60-2; PCI 24781; CRA-024781; CRA 024781; UNII-IYO470654U; 3-((dimethylamino)methyl)-N-(2-(4-(hydroxycarbamoyl)phenoxy)ethyl)benzofuran-2-carboxamide; CRA-02478; Abexinostat(PCI-24781); PCI-24781 (Abexinostat); Abexinostat (PCI-24781); IYO470654U; 3-[(Dimethylamino)methyl]-N-[2-[4-[(hydroxyamino)carbonyl]phenoxy]ethyl]-2-benzofurancarboxamide; 3-((Dimethylamino)methyl)-N-(2-(4-(hydroxycarbamoyl)-phenoxy)ethyl)benzofuran-2-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Vitespen | Phase 3 | [27] | ||
| Synonyms |
Oncophage (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| AMD-070 | Phase 3 | [10] | ||
| Synonyms |
AMD 070; AMD070; AMD11070; S14-0353; N-(1H-benzoimidazol-2-ylmethyl)-N-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine; N'-(1H-Benzo[d]imidazol-2-ylmethyl)-N'-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine Trihydrobromide Dihydrate
Click to Show/Hide
|
|||
| External Link | ||||
| Nivolumab + ipilimumab | Phase 3 | [28] | ||
| External Link | ||||
| Savolitinib | Phase 3 | [10] | ||
| Synonyms |
1313725-88-0; AZD-6094; AZD6094; UNII-2A2DA6857R; CHEMBL3334567; 2A2DA6857R; Savolitinib [INN]; Volitinib(Savolitinib); Savolitinib [USAN:INN]; GTPL9918; SCHEMBL12489208; EX-A845; BDBM50023342; ZINC149738712; AKOS030526403; DB12048; compound 28 [PMID: 25148209]; HY-15959; AS-35250; 1H-1,2,3-Triazolo(4,5-b)pyrazine, 1-((1S)-1-imidazo(1,2-a)pyridin-6-ylethyl)-6-(1-methyl-1H-pyrazol-4-yl)-; KB-333895; FT-0700162; J-690125; 4-{1-[(1S)-1-{imidazo[1,2-a]pyri
Click to Show/Hide
|
|||
| External Link | ||||
| Rocapuldencel-T | Phase 3 | [29] | ||
| External Link | ||||
| Cotellic | Phase 3 | [10] | ||
| Synonyms |
Cobimetinib fumarate; Cobimetinib hemifumarate; Xl-518 hemifumarate; GDC-0973 hemifumarate; UNII-6EXI96H8SV; 6EXI96H8SV; Cobimetinib fumarate [USAN]; 1369665-02-0; Cobimetinib fumarate (USAN); Cotellic (TN); CHEMBL2364607; CHEBI:90853; Methanone, (3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)phenyl)(3-hydroxy-3-(2S)-2-piperidinyl-1-azetidinyl)-, (2E)-2-butenedioate (2:1); D10615; bis[(2S)-2-{1-[3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzoyl]-3-hydroxyazetidin-3-yl}piperidin-1-ium] (2E)-but-2-enedioate
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986205 | Phase 3 | [10] | ||
| Synonyms |
KRTIYQIPSAGSBP-KLAILNCOSA-N; 1923833-60-6; BMS986205; UNII-0A7729F42K; 0A7729F42K; GTPL9707; SCHEMBL18826792; SCHEMBL17740982; SCHEMBL19105151; EX-A2606; AKOS032954040; HY-101560; CS-0021719; Q29213697; (R)-N-(4-chlorophenyl)-2-((1s,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide; (2R)-N-(4-chlorophenyl)-2-[4-(6-fluoroquinolin-4-yl)cyclohexyl]propanamide; (2R)-N-(4-Chlorophenyl)-2-(4-(6-fluoro-4-quinolyl)cyclohexyl)propanamide, cis; Cyclohexaneacetamide, N-(4-chlorophenyl)-4-(6-fluoro-4-quinolinyl)-alpha-methyl-, cis-(alphaR)-
Click to Show/Hide
|
|||
| External Link | ||||
| TKI258 | Phase 3 | [30] | ||
| Synonyms |
Dovitinib; 405169-16-6; CHIR-258; TKI-258; Chir 258; 4-Amino-5-fluoro-3-[5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]quinolin-2(1H)-one; CHIR258; Dovitinib (TKI-258, CHIR-258); UNII-I35H55G906; CHEMBL522892; 804551-71-1; I35H55G906; TKI 258; 1027263-12-2; (3Z)-4-Amino-5-fluoro-3-[5-(4-methyl-1-piperazinyl)-1,3-dihydro-2H-benzimidazol-2-ylidene]-2(3H)-quinolinone; C21H21FN6O; 4-Amino-5-fluoro-3-(5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl)quinolin-2(1H)-one
Click to Show/Hide
|
|||
| External Link | ||||
| AM0010 | Phase 3 | [28] | ||
| External Link | ||||
| IMA-901 | Phase 3 | [31] | ||
| Synonyms |
TUMAP vaccine (RCC), immatics; Tumor-associoated peptide vaccine (renal cell carcinoma), immatics
Click to Show/Hide
|
|||
| External Link | ||||
| NKTR 214 | Phase 3 | [28] | ||
| External Link | ||||
| TRC105 | Phase 2 | [28] | ||
| External Link | ||||
| CAP-232 | Phase 2a | [32] | ||
| Synonyms |
TT-232; UNII-49D4Q4254Z; TT 232; 49D4Q4254Z; 147159-51-1; Phe-cys-tyr-trp-lys-cys-thr-NH2 (2-6)-disulfide; TLN 232; CAP 232; Phenylalanyl-cysteinyl-tyrosyl-tryptophyl-lysyl-cysteinyl-threoninamide (2-6)-disulfide; AC1OCF7X; CHEMBL539934; TLN-232; L-Threoninamide, D-phenylalanyl-L-cysteinyl-L-tyrosyl-D-tryptophyl-L-lysyl-L-cysteinyl-, cyclic (2-6)-disulfide; TT2-32; ZINC169289417; AKOS024458270; DB12088; NCGC00249606-01
Click to Show/Hide
|
|||
| External Link | ||||
| CMN-001 | Phase 2 | [33] | ||
| External Link | ||||
| DS-3201b | Phase 2 | [34] | ||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| External Link | ||||
| AGS-16C3F | Phase 2 | [10] | ||
| External Link | ||||
| TV-Kidney-1 | Phase 2 | [29] | ||
| External Link | ||||
| IMO-2055 | Phase 2 | [35] | ||
| External Link | ||||
| Anti-LAG3 | Phase 2 | [28] | ||
| External Link | ||||
| AGS-003 | Phase 2 | [36] | ||
| External Link | ||||
| Ispinesib | Phase 2 | [37] | ||
| Synonyms |
SB-715992; Ispinesib (SB-715992 /CK0238273); SB-715992, CK0238273,Ispinesib; N-(3-aminopropyl)-N-[(1R)-1-(3-benzyl-7-chloro-4-oxoquinazolin-2-yl)-2-methylpropyl]-4-methylbenzamide
Click to Show/Hide
|
|||
| External Link | ||||
| Ilixadencel | Phase 2 | [10] | ||
| External Link | ||||
| CRLX101 | Phase 2 | [10] | ||
| External Link | ||||
| FolateImmune | Phase 2 | [38] | ||
| Synonyms |
Folate-FITC; EC-17; UNII-V7YQ6134AE; V7YQ6134AE; EC17 (Folate-FITC); Folate-fluorescein conjugate; DTXSID40207031; ZMTAPBHUSYTHBY-PMERELPUSA-N; EC 17; DB12559; 910661-23-3; 1159606-35-5
Click to Show/Hide
|
|||
| External Link | ||||
| Varlilumab | Phase 2 | [10] | ||
| External Link | ||||
| TVI-Kidney-1 | Phase 2 | [39] | ||
| External Link | ||||
| OSI-027 | Phase 2 | [40] | ||
| External Link | ||||
| XL880 | Phase 2 | [41] | ||
| Synonyms |
GSK 089; GSK 1363089; GSK1363089; XL 880; GSK1363089, GSK089, foretinib, EXEL-2880, XL880; 88Z; MET inhibitors
Click to Show/Hide
|
|||
| External Link | ||||
| Girentuximab I-124 | Phase 2 | [42] | ||
| Synonyms |
Redectane (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Autologous renal cell carcinoma vaccine | Phase 2 | [43] | ||
| External Link | ||||
| RX-0201 | Phase 2 | [10] | ||
| External Link | ||||
| Human interleukin-2 | Phase 2 | [44] | ||
| Synonyms |
Pulmoleukin; Human interleukin-2 (inhaled, renal cell carcinoma); Human IL-2 (inhaled, renal cell carcinoma),Immunservice; Human interleukin-2 (inhaled, renal cell carcinoma), Immunservice
Click to Show/Hide
|
|||
| External Link | ||||
| CB-839 | Phase 2 | [10] | ||
| External Link | ||||
| ATN-161 | Phase 2 | [45] | ||
| Synonyms |
PHSCN; PHSCN, University of Michigan; Angiogenesis inhibitors (cancer), Attenuon; Integrin antagonists (cancer), Attenuon; Ac-PHSCN-NH2, University of Michigan
Click to Show/Hide
|
|||
| External Link | ||||
| TV1-Kidney-1 | Phase 2 | [46] | ||
| External Link | ||||
| CCT301-38 (targeting ROR2) | Phase 1/2 | [47] | ||
| External Link | ||||
| LAG525 | Phase 1/2 | [10] | ||
| External Link | ||||
| CCT301-59 (targeting ROR2) | Phase 1/2 | [47] | ||
| External Link | ||||
| Anti-C-met CAR-T cells | Phase 1/2 | [48] | ||
| External Link | ||||
| CDX-014 | Phase 1/2 | [10] | ||
| External Link | ||||
| IL-2 XL | Phase 1/2 | [49] | ||
| Synonyms |
IL-2 XL (controlled release, Medusa); IL-2 XL (controlled release, Medusa), Flamel; Interleukin-2 (long-acting, Medusa), Flamel
Click to Show/Hide
|
|||
| External Link | ||||
| ARO-HIF2 | Phase 1 | [50] | ||
| External Link | ||||
| AGS-16 mab | Phase 1 | [51] | ||
| Synonyms |
AGS-16M18
Click to Show/Hide
|
|||
| External Link | ||||
| Neovastat | Phase 1 | [52] | ||
| External Link | ||||
| Biomed 101 | Discontinued in Phase 1 | [53] | ||
| Synonyms |
SC-41930; SC 41930; 120072-59-5; CHEMBL14823; CGS 24115; 7-(3-(4-Acetyl-3-methoxy-2-propylphenoxy)propoxy)-3,4-dihydro-8-propyl-2H-1-benzopyran-2-carboxylic acid; 2H-1-Benzopyran-2-carboxylic acid, 7-(3-(4-acetyl-3-methoxy-2-propylphenoxy)propoxy)-3,4-dihydro-8-propyl-; 2H-1-Benzopyran-2-carboxylicacid, 7-[3-(4-acetyl-3-methoxy-2-propylphenoxy)propoxy]-3,4-dihydro-8-propyl-; 7-(3-(4-acetyl-3-methoxy-2-propylphenoxy)propoxy)-8-propylchroman-2-carboxylic acid; SC-41390
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 172 | Phase 1 | [54] | ||
| External Link | ||||
| Anti-hCD70 CAR transduced PBL | Phase 1 | [55] | ||
| External Link | ||||
| Veglin | Phase 1 | [56] | ||
| External Link | ||||
| IL-7/CD80-expressing allogeneic RCC-26 tumor cell vaccine | Phase 1 | [57] | ||
| Synonyms |
RCC-26 tumor cell vaccine; IL-7/CD80-expressing allogeneic RCC-26 tumorcell vaccine (renal cell carcinoma)
Click to Show/Hide
|
|||
| External Link | ||||
| Vorsetuzumab mafodotin | Discontinued in Phase 1 | [58] | ||
| External Link | ||||
| BCL-004 | Investigative | [59] | ||
| External Link | ||||
| GM-CAIX | Investigative | [59] | ||
| Synonyms |
GM-CSF/cancer antigen chimeric protein (renal cancer), Kite
Click to Show/Hide
|
|||
| External Link | ||||
| GS-168 | Investigative | [59] | ||
| Synonyms |
GS-168A
Click to Show/Hide
|
|||
| External Link | ||||
| STF-62247 | Investigative | [59] | ||
| Synonyms |
Renal cell carcinoma therapy, Stanford University
Click to Show/Hide
|
|||
| External Link | ||||
References