m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT03121
|
[1] | |||
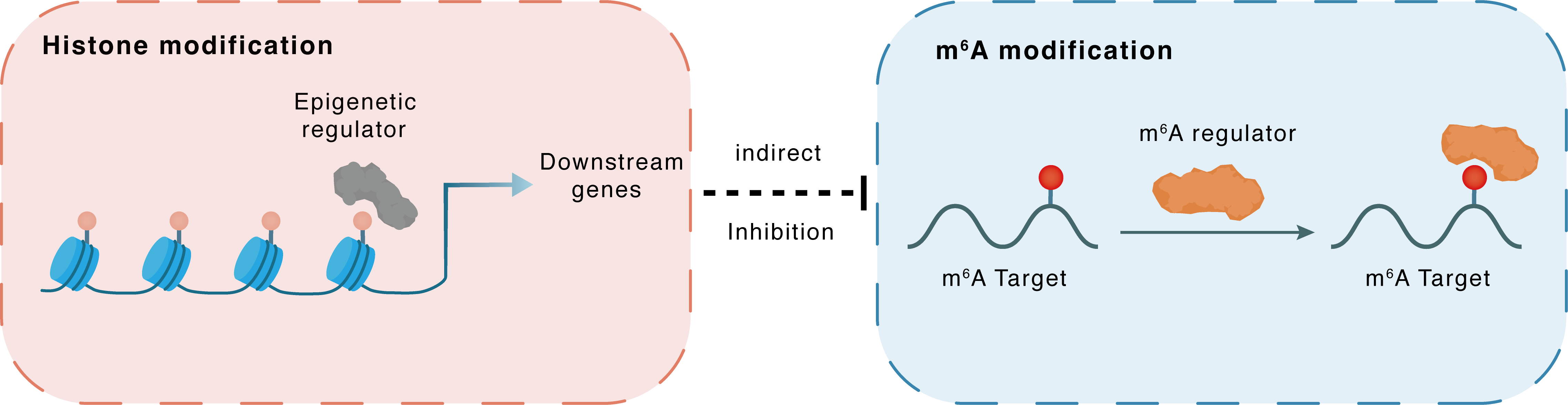 Histone modification
Regulated Target
HDAC2
Downstream Gene
Indirect
Inhibition
m6A modification
ANXA1
ANXA1
YTHDC1
Histone modification
Regulated Target
HDAC2
Downstream Gene
Indirect
Inhibition
m6A modification
ANXA1
ANXA1
YTHDC1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing protein 1 (YTHDC1) | READER | |||
| m6A Target | Annexin A1 (ANXA1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Epigenetic Regulator | Histone deacetylase 2 (HDAC2) | ERASER | View Details | ||
| Crosstalk Relationship | Histone modification → m6A | Inhibition | |||
| Crosstalk Mechanism | Histone modification indirectly regulates m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | We identified a novel YY1/HDAC2/YTHDC1/Annexin A1 (ANXA1) axis modulating the progression and chemosensitivity of ccRCC. | ||||
| Responsed Disease | Clear cell renal cell carcinoma | ICD-11: XH46F1 | |||
| Responsed Drug | Sunitinib | ||||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| Cell Process | Cell proliferation | ||||
In-vitro Model |
786-O | Renal cell carcinoma | Homo sapiens | CVCL_1051 | |
| A-498 | Renal cell carcinoma | Homo sapiens | CVCL_1056 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Annexin A1 (ANXA1) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Difluprednate | Approved | [2] | ||
| Synonyms |
Durezol (TN)
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| Histone deacetylase 2 (HDAC2) | 113 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| CHR-3996 | Phase 1/2 | [3] | ||
| Synonyms |
CCT-075453; CHR-2504; HDAC inhibitors, Chroma Therapeutics; Histone deacetylase inhibitors, Chroma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| PMID29671355-Compound-74 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 119 nM | |||
| External Link | ||||
| PMID29671355-Compound-59 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 nM | |||
| External Link | ||||
| PMID29671355-Compound-55 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 1000 nM | |||
| External Link | ||||
| PMID29671355-Compound-11 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 24 nM | |||
| External Link | ||||
| PMID29671355-Compound-9 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 128 nM | |||
| External Link | ||||
| PMID29671355-Compound-8 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 123000 nM | |||
| External Link | ||||
| PMID29671355-Compound-61 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 45.9 nM | |||
| External Link | ||||
| PMID29671355-Compound-23 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 7450 nM | |||
| External Link | ||||
| PMID29671355-Compound-44 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 150 nM | |||
| External Link | ||||
| PMID29671355-Compound-56 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 54.4 nM | |||
| External Link | ||||
| PMID29671355-Compound-67 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 50000 nM | |||
| External Link | ||||
| PMID29671355-Compound-31 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2 nM | |||
| External Link | ||||
| PMID29671355-Compound-21 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4880 nM | |||
| External Link | ||||
| PMID29671355-Compound-62 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 500 nM | |||
| External Link | ||||
| PMID29671355-Compound-43 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 124 nM | |||
| External Link | ||||
| PMID29671355-Compound-25 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 5400 nM | |||
| External Link | ||||
| N8-hydroxy-2-methoxy-N1-phenyloctanediamide | Investigative | [5] | ||
| Synonyms |
CHEMBL251010; SCHEMBL8158442
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N-(4'-acetyl-4-aminobiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1099078; BDBM50317997
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3800 nM | |||
| External Link | ||||
| N-Hydroxy-E-3-(4'-chlorobiphenyl-4-yl)-acrylamide | Investigative | [7] | ||
| Synonyms |
CHEMBL557066; SCHEMBL3292989; SCHEMBL3292983; BDBM50293365
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1860 nM | |||
| External Link | ||||
| N7-hydroxy-2-methoxy-N1-phenylheptanediamide | Investigative | [5] | ||
| Synonyms |
CHEMBL251206; SCHEMBL8143763
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N7-hydroxy-N1-phenyl-2-propoxyheptanediamide | Investigative | [5] | ||
| Synonyms |
CHEMBL251406; SCHEMBL8144856
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N-(4-amino-4'-vinylbiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1096397; BDBM50318002
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 800 nM | |||
| External Link | ||||
| N-(4-amino-3'-methoxybiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1097747; BDBM50317991
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 nM | |||
| External Link | ||||
| KAR-1880 | Investigative | [8] | ||
| Synonyms |
Anti-inflammatory OS-HDI; OS-HDI-2; HDAC 2 inhibitors (topical, dermatitis/psoriasis), Karus
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-amino-5-(pyridin-4-yl)phenyl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1095096
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3900 nM | |||
| External Link | ||||
| N-(4-amino-3'-methylbiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1094108; BDBM50317995
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3100 nM | |||
| External Link | ||||
| N-(3'-acetyl-4-aminobiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1097746; BDBM50317992
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 13000 nM | |||
| External Link | ||||
| N-(4-amino-4'-fluorobiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1098337; SCHEMBL15398027; BDBM50317988
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1400 nM | |||
| External Link | ||||
| N8,2-dihydroxy-N1-phenyloctanediamide | Investigative | [5] | ||
| Synonyms |
CHEMBL251009; SCHEMBL8144564
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N-(2-amino-5-(furan-3-yl)phenyl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1095095; Benzamide, N-[2-amino-5-(3-furanyl)phenyl]-; BDBM50318000
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 39 nM | |||
| External Link | ||||
| N-Hydroxy-E-3-(biphenyl-4-yl)-acrylamide | Investigative | [7] | ||
| Synonyms |
CHEMBL556532; SCHEMBL3292226; SCHEMBL3290139
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 820 nM | |||
| External Link | ||||
| 2-(allyloxy)-N8-hydroxy-N1-phenyloctanediamide | Investigative | [5] | ||
| Synonyms |
CHEMBL402719; SCHEMBL8150833
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| 2-(benzyloxy)-N7-hydroxy-N1-phenylheptanediamide | Investigative | [5] | ||
| Synonyms |
CHEMBL402718; SCHEMBL8152458
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 500 nM | |||
| External Link | ||||
| N-(4-amino-4'-bromobiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1097748; BDBM50317990
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 600 nM | |||
| External Link | ||||
| N-(2-amino-5-(furan-2-yl)phenyl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1097651
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 43 nM | |||
| External Link | ||||
| 5-(Biphenyl-4-yl)-pentanoic acid N-hydroxyamide | Investigative | [7] | ||
| Synonyms |
CHEMBL541239; SCHEMBL7045815
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 720 nM | |||
| External Link | ||||
| N-(4-aminobiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL271741; 3max; SCHEMBL16380794; BDBM50232053; N-(4-amino-biphenyl-3-yl)-benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 27 nM | |||
| External Link | ||||
| N-(2-amino-5-(benzofuran-2-yl)phenyl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1097063
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10000 nM | |||
| External Link | ||||
| N-(2-aminophenyl)benzamide | Investigative | [6] | ||
| Synonyms |
2'-AMINOBENZANILIDE; 721-47-1; CHEMBL405072; AC1LFSYX; AC1Q5NOK; ACMC-1AE0C; Oprea1_478192; MLS000084661; SCHEMBL407834; N-(2-amino-phenyl)-benzamide; AC1Q514U; Benzamide, N-(2-aminophenyl)-; CTK2H2825; DTXSID60353948; RFDVMOUXHKTCDO-UHFFFAOYSA-N; MolPort-001-783-352; ZINC225957; HMS2355D18; STL497474; BDBM50232046; AKOS000133162; MCULE-5545982713; NE17312; SMR000019009; TC-170926; KB-298435; ST51030142; EN300-31745; SR-01000389415; AE-641/00785046
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3100 nM | |||
| External Link | ||||
| N-(4-amino-4'-methoxybiphenyl-3-yl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1095412; BDBM50317998
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| N-Hydroxy-E-3-(4'-cyanobiphenyl-4-yl)-acrylamide | Investigative | [7] | ||
| Synonyms |
CHEMBL538710; SCHEMBL3292721; SCHEMBL3292715; BDBM50293355
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 330 nM | |||
| External Link | ||||
| N-(2-amino-5-(thiazol-2-yl)phenyl)benzamide | Investigative | [6] | ||
| Synonyms |
CHEMBL1097278
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 390 nM | |||
| External Link | ||||
| N-(2-aminophenyl)quinoxaline-6-carboxamide | Investigative | [9] | ||
| Synonyms |
benzamide-type inhibitor, 20; CHEMBL236060; BDBM19424
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| 7-Biphenyl-4-yl-heptanoic acid hydroxyamide | Investigative | [10] | ||
| Synonyms |
CHEMBL125098; BDBM50222335; 7-(4-Biphenylyl)heptanehydroximic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-4-yloxy)-2-oxo-octanoic acid | Investigative | [11] | ||
| Synonyms |
CHEMBL115049; 436150-72-0; SCHEMBL7368556; CTK1D2674; DTXSID40658342; BDBM50221807; 8-[([1,1'-Biphenyl]-4-yl)oxy]-2-oxooctanoic acid; Octanoic acid, 8-([1,1'-biphenyl]-4-yloxy)-2-oxo-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1,1,1-Trifluoro-8-phenoxy-octan-2-one | Investigative | [12] | ||
| Synonyms |
CHEMBL114796; BDBM50217940
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-heptanoic acid hydroxyamide | Investigative | [11] | ||
| Synonyms |
CHEMBL114184; SCHEMBL3383144
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Phenoxy-heptanoic acid hydroxyamide | Investigative | [10] | ||
| Synonyms |
CHEMBL124322; N-hydroxy-7-phenoxyheptanamide; 7-Phenoxyheptanehydroximic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1,1,1-Trifluoro-8-(4-phenoxy-phenoxy)-octan-2-one | Investigative | [12] | ||
| Synonyms |
CHEMBL117916; SCHEMBL7366611; BDBM50217945
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(4-hydroxybiphenyl-3-yl)benzamide | Investigative | [13] | ||
| Synonyms |
CHEMBL269935; SCHEMBL5724398; BDBM50232005
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| 8-Phenyl-octanoic acid hydroxyamide | Investigative | [10] | ||
| Synonyms |
CHEMBL123624; N-Hydroxy-8-phenyloctanamide; SCHEMBL5807174
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-3-yloxy)-1,1,1-trifluoro-octan-2-one | Investigative | [12] | ||
| Synonyms |
CHEMBL116023; SCHEMBL7368359; BDBM50218558
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-aminophenyl)nicotinamide | Investigative | [13] | ||
| Synonyms |
N-(2-Amino-phenyl)-nicotinamide; 436089-31-5; N-(2-aminophenyl)pyridine-3-carboxamide; CHEMBL236678; AC1LMN6K; SCHEMBL18086514; CTK4I7538; DTXSID50360661; CHEBI:125506; ZINC873967; BDBM50220259; 3463AE; AKOS000129725; RTR-042156; MCULE-7933541910; N-(2-aminophenyl)-3-pyridylcarboxamide; ZB014940; ACM436089315; ST086607; ASN 01337807; KB-298440; TR-042156; BC4148434; SR-01000329900; SR-01000329900-1; BRD-K20880473-001-04-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6980 nM | |||
| External Link | ||||
| santacruzamate A | Investigative | [14] | ||
| Synonyms |
CAY10683
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.112 nM | |||
| External Link | ||||
| N-(4-aminobiphenyl-3-yl)nicotinamide | Investigative | [13] | ||
| Synonyms |
CHEMBL255805; BDBM50232035
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 900 nM | |||
| External Link | ||||
| N-(2-amino-5-(thiophen-2-yl)phenyl)nicotinamide | Investigative | [13] | ||
| Synonyms |
CHEMBL256440; SCHEMBL1066609
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 390 nM | |||
| External Link | ||||
| N-(2-aminophenyl)-4-methoxybenzamide | Investigative | [9] | ||
| Synonyms |
AC1LFX2W; Cambridge id 5129152; Oprea1_722128; benzamide-type inhibitor, 22; CHEMBL236061; SCHEMBL5226034; BDBM19426; CTK7A1998; MolPort-001-019-504; BDYVCYUXCNZYRW-UHFFFAOYSA-N; ZINC281656; STK156256; AKOS000130378; MCULE-9183453747; N-(2-Amino-phenyl)4-methoxy-benzamide; N-(2-amino-phenyl)-4-methoxy-benzamide; NCGC00240897-01; N1-(4-methoxybenzoyl)-1,2-benzenediamine; N1-(4-methoxy-benzoyl)-1,2-benzenediamine; ST50908739; N-(2-aminophenyl)(4-methoxyphenyl)carboxamide; SR-01000196394
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 900 nM | |||
| External Link | ||||
| 4-Phenylbutyrohydroxamic acid | Investigative | [15] | ||
| Synonyms |
N-Hydroxy-4-phenylbutanamide; 32153-46-1; NSC131300; UNII-QX182FOM5S; QX182FOM5S; 4-phenylbutanehydroxamic acid; CHEMBL55895; Benzenebutanamide, N-hydroxy-; NSC 131300; AC1Q7DIW; AC1L5RDX; Phenylbutyrylhydroxamic Acid; AC1Q5QD1; N-Hydroxy-4-phenyl-butyramide; 4-Phenylbutyryl hydroxamic acid; SCHEMBL1350853; CTK4G8310; DTXSID60185943; MolPort-011-492-164; UPHXPXYRKPCXHK-UHFFFAOYSA-N; ZINC4962622; STL301752; BDBM50015142; AKOS009266186; MCULE-9765156954; NSC-131300; NE28489; BCB03_000829; EN300-68596
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 430 nM | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid hydroxyamide | Investigative | [16] | ||
| Synonyms |
CHEMBL95959; SCHEMBL3383197; N-hydroxy-8-oxo-8-phenyloctanamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid bis-hydroxyamide | Investigative | [17] | ||
| Synonyms |
Suberohydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 29 nM | |||
| External Link | ||||
| ST-2986 | Investigative | [18] | ||
| Synonyms |
CHEMBL471041; SCHEMBL3444455; BDBM50278219
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11000 nM | |||
| External Link | ||||
| 9,9,9-Trifluoro-8-oxo-nonanoic acid phenylamide | Investigative | [12] | ||
| Synonyms |
9,9,9-Trifluoro-8-Oxo-N-Phenylnonanamide; CHEMBL113537; 2gh6; SCHEMBL2702892; KRCXZGYVOZSCSF-UHFFFAOYSA-N; BDBM50121062; DB07553
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1400 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid phenylamide | Investigative | [19] | ||
| Synonyms |
Thiol-SAHA (t-SAHA); CHEMBL325676; SCHEMBL14821761; BDBM152692
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1300 nM | |||
| External Link | ||||
| 6-benzenesulfinylhexanoic acid hydroxamide | Investigative | [20] | ||
| Synonyms |
6-(benzenesulfinyl)hexanoic acid hydroxyamide; 875737-03-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-succinamide | Investigative | [21] | ||
| Synonyms |
CHEMBL193959
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-4-ylamide | Investigative | [19] | ||
| Synonyms |
CHEMBL112311
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-phenylacetylamino-benzamide | Investigative | [22] | ||
| Synonyms |
CHEMBL356824; 656261-23-3; SCHEMBL675578; CTK1J6158; DTXSID40458440; ZINC13533297; AKOS030583151; Benzeneacetamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(2-Bromo-acetylamino)-hexanoic acid phenylamide | Investigative | [19] | ||
| Synonyms |
CHEMBL344920; 651767-99-6; SCHEMBL3736839; CTK1J8444; DTXSID50432973; HWYLREOMBVUGJQ-UHFFFAOYSA-N; BDBM50222416; ZINC13587789; AKOS030603042; N-Phenyl-6-(bromoacetylamino)hexanamide; Hexanamide, 6-[(bromoacetyl)amino]-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(phenylacetylamino-methyl)-benzamide | Investigative | [23] | ||
| Synonyms |
CHEMBL143674; SCHEMBL673760
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [24] | ||
| Synonyms |
CHEMBL126355; BDBM50222394
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-4-ylamide | Investigative | [25] | ||
| Synonyms |
SCHEMBL8082656; CHEMBL165162; ZINC13472304
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(6-Mercapto-hexyl)-benzamide | Investigative | [19] | ||
| Synonyms |
CHEMBL112364; BDBM50223650
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Hydroxy-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [25] | ||
| Synonyms |
CHEMBL167455
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((R)-2-phenyl-butyrylamino)-benzamide | Investigative | [22] | ||
| Synonyms |
SCHEMBL675474
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-benzenesulfonylhexanoic acid hydroxamide | Investigative | [20] | ||
| Synonyms |
CHEMBL203207
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 9-(Biphenyl-4-yloxy)-1,1,1-trifluoro-nonan-2-one | Investigative | [12] | ||
| Synonyms |
SCHEMBL7373122; CHEMBL116578
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Thioacetic acid S-(6-phenylcarbamoyl-hexyl) ester | Investigative | [19] | ||
| Synonyms |
CHEMBL111806; SCHEMBL14812153
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Butyrylamino-N-hydroxy-benzamide | Investigative | [23] | ||
| Synonyms |
CHEMBL142254; 656261-22-2; Benzamide, N-hydroxy-4-[(1-oxobutyl)amino]-; SCHEMBL675234; CTK1J6159; DTXSID90461262
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Naphthalen-2-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [24] | ||
| Synonyms |
CHEMBL127328
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid biphenyl-3-ylamide | Investigative | [19] | ||
| Synonyms |
CHEMBL320323
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-hydroxyphenyl)-3H-1,2-dithiole-3-thione | Investigative | [26] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(2-phenyl-butyrylamino)-benzamide | Investigative | [22] | ||
| Synonyms |
SCHEMBL676079
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Dimethylamino-N-(6-mercapto-hexyl)-benzamide | Investigative | [19] | ||
| Synonyms |
CHEMBL324126
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid pyridin-3-ylamide | Investigative | [19] | ||
| Synonyms |
CHEMBL332246; Heptanamide, 7-mercapto-N-3-pyridinyl-; BDBM50223653
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Phenoxy-hexane-1-thiol | Investigative | [19] | ||
| Synonyms |
CHEMBL109796; 6-phenoxyhexane-1-thiol; 1-Hexanethiol, 6-phenoxy-; SCHEMBL5679745; MolPort-020-180-823; BDBM50223652; AKOS018584222; MCULE-9521857089
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Benzoylamino-N-hydroxy-benzamide | Investigative | [22] | ||
| Synonyms |
SCHEMBL673678; CHEMBL191227
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Chloro-N-(5-hydroxycarbamoyl-pentyl)-benzamide | Investigative | [16] | ||
| Synonyms |
CHEMBL143734; NSC718168; AC1L8L82; SCHEMBL13039735; ZINC5579677; BDBM50082664; NSC-718168; NCI60_040737; 6-(4-Chlorobenzoylamino)hexanehydroxamic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-(Biphenyl-4-yloxy)-1,1,1-trifluoro-octan-2-one | Investigative | [12] | ||
| Synonyms |
CHEMBL112148; SCHEMBL7364383; BDBM50218532
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-3-yloxy)-1-oxazol-2-yl-heptan-1-one | Investigative | [24] | ||
| Synonyms |
CHEMBL127351; SCHEMBL7365180; HWZHDGRMABBYOV-UHFFFAOYSA-N; BDBM50222367; 7-((1,1'-biphenyl)-3-yloxy)-1-(1 ,3-oxazol-2-yl)-1-heptanone
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-Mercapto-hexanoic acid phenylamide | Investigative | [19] | ||
| Synonyms |
CHEMBL109654; Hexanamide, 6-mercapto-N-phenyl-; SCHEMBL14254925; BDBM50027600
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2220 nM | |||
| External Link | ||||
| Cyclostellettamine derivative | Investigative | [27] | ||
| Synonyms |
CHEMBL88332
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(5-Hydroxycarbamoyl-pentyl)-4-nitro-benzamide | Investigative | [16] | ||
| Synonyms |
CHEMBL139999; SCHEMBL1232700; BDBM50082661; ZINC13472309
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-Mercapto-pentanoic acid phenylamide | Investigative | [19] | ||
| Synonyms |
N-Phenyl-5-mercaptovaleramide; CHEMBL114344; Pentanamide, 5-mercapto-N-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Octanedioic acid hydroxyamide pyridin-2-ylamide | Investigative | [25] | ||
| Synonyms |
SCHEMBL8090513; CHEMBL164872; ZINC13472303
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-(2-Mercapto-ethyl)-N'-phenyl-oxalamide | Investigative | [21] | ||
| Synonyms |
CHEMBL193979
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(methylsulfonylthio)ethyl 2-propylpentanoate | Investigative | [26] | ||
| Synonyms |
CHEMBL271677; SCHEMBL4156413
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (E)-8-Biphenyl-4-yl-1-oxazol-2-yl-oct-7-en-1-one | Investigative | [24] | ||
| Synonyms |
CHEMBL126465; SCHEMBL7368197; SCHEMBL7368201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-((S)-2-phenyl-butyrylamino)-benzamide | Investigative | [22] | ||
| Synonyms |
SCHEMBL676080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(3-phenyl-propionylamino)-benzamide | Investigative | [28] | ||
| Synonyms |
N-hydroxy-4-(3-phenylpropanamido)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(5-phenyl-pentanoylamino)-benzamide | Investigative | [22] | ||
| Synonyms |
SCHEMBL7311087
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Oxo-8-phenyl-octanoic acid | Investigative | [25] | ||
| Synonyms |
8-Oxo-8-phenyloctanoic acid; 7-Benzoylheptanoic acid; 24314-23-6; Benzeneoctanoic acid, h-oxo-; 7-BENZOYL HEPTANOIC ACID; AC1L6TSB; SCHEMBL3381106; 8-keto-8-phenyl-caprylic acid; CHEMBL162423; 8-Oxo-8-phenyloctanoic acid #; CTK4F3363; DTXSID40305602; UMCSRRHQLAVYRS-UHFFFAOYSA-N; ZINC2168376; 7009f; NSC171230; AKOS016022495; NSC-171230; MCULE-7202530747; ACM24314236; ST50825837
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(4-phenyl-butyrylamino)-benzamide | Investigative | [22] | ||
| Synonyms |
CHEMBL143336; 656261-24-4; SCHEMBL674421; CTK1J6157; DTXSID30433908; ZINC13533300; AKOS030583673; n-hydroxy-4-(4-phenylbutyryl-amino)benzamide; Benzenebutanamide, N-[4-[(hydroxyamino)carbonyl]phenyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-phenylsulfanylhexanoic acid hydroxamide | Investigative | [20] | ||
| Synonyms |
Hexanamide, N-hydroxy-6-(phenylthio)-; CHEMBL203028; SCHEMBL7317658
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ST-2987 | Investigative | [18] | ||
| Synonyms |
CHEMBL471042; SCHEMBL3444989; SCHEMBL3444984; BDBM50278220
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2670 nM | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid quinolin-3-ylamide | Investigative | [19] | ||
| Synonyms |
CHEMBL112234
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5-(4-Chloro-phenyl)-pentanoic acid hydroxyamide | Investigative | [29] | ||
| Synonyms |
CHEMBL84288
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 8-Mercapto-octanoic acid phenylamide | Investigative | [19] | ||
| Synonyms |
8-mercapto-N-phenyloctanamide; CHEMBL326433; ZINC13609343
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 754 nM | |||
| External Link | ||||
| N-(6-Hydroxycarbamoyl-hexyl)-benzamide | Investigative | [25] | ||
| Synonyms |
CHEMBL57107; 174664-71-2; SCHEMBL573254; CTK0A7470; DTXSID00433435; BDBM50220823; ZINC13490043; 7-(Benzoylamino)heptanehydroxamic acid; AKOS030580013; Benzamide, N-[7-(hydroxyamino)-7-oxoheptyl]-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(Biphenyl-4-yloxy)-1,1,1-trifluoro-heptan-2-one | Investigative | [12] | ||
| Synonyms |
CHEMBL326529; SCHEMBL7365237; BDBM50217957
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-Mercapto-heptanoic acid benzothiazol-2-ylamide | Investigative | [19] | ||
| Synonyms |
CHEMBL178779
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| N-Hydroxy-4-(pentanoylamino-methyl)-benzamide | Investigative | [23] | ||
| Synonyms |
CHEMBL143102
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PSAMMAPLIN A | Investigative | [16] | ||
| Synonyms |
110659-91-1; Bisprasin; NSC614495; AC1O46WI; SCHEMBL364511; ZINC150352860; NSC-614495; B723735K022; J-002461; Benzenepropanamide, N,N'-(dithiodi-2,1-ethanediyl)bis(3-bromo-4-hydroxy-alpha-(hydroxyimino)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
References