m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02163
|
[1], [2] | |||
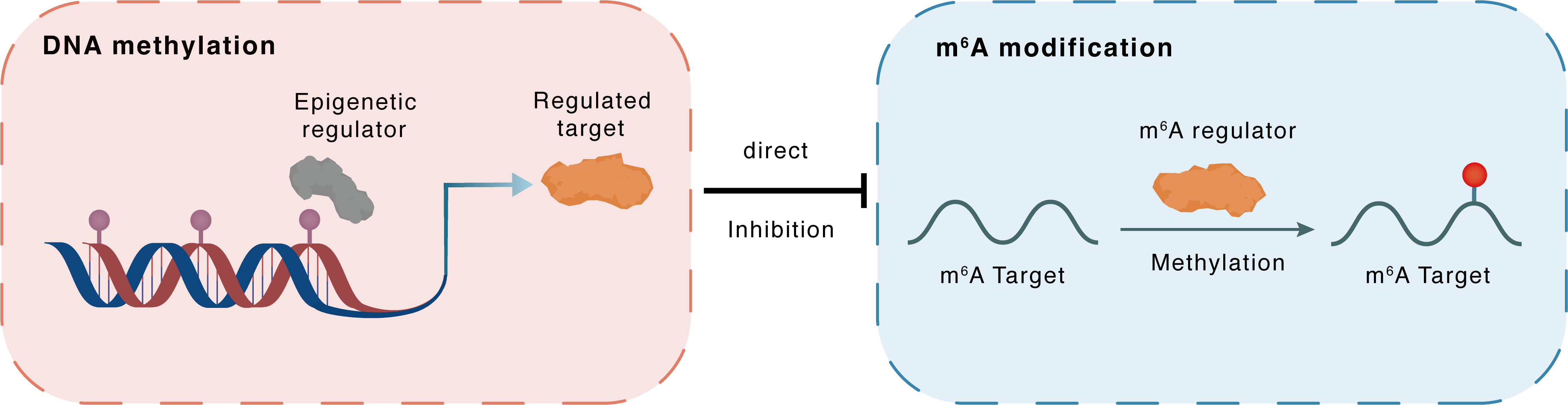 DNA methylation
DNMT3B
METTL3
Direct
Inhibition
m6A modification
AKT1
AKT1
METTL3
Methylation
DNA methylation
DNMT3B
METTL3
Direct
Inhibition
m6A modification
AKT1
AKT1
METTL3
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | RAC-alpha serine/threonine-protein kinase (AKT1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | WRITER | View Details | ||
| Regulated Target | Methyltransferase-like protein 3 (METTL3) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | Methyltransferase-like 3 (METTL3) increased the pri-Let-7b, decreased both the pre-Let-7b and mature Let-7b, attenuating the Let-7b controlling of stem cell renewal. The addition of Metformin increased the bindings of DNA methyltransferase-3a/b (DNMT3A/DNMT3B) to the METTL3 promoter. With the help of the readers of NKAP and HNRNPA2B1, the cluster mediated m6A formation on pri-Let-7b processing increased the mature Let-7b, the key player in suppressing Notch signaling and re-captivating Osimertinib treatment. METTL3-mediated m6A methylation promotes lung cancer progression via activating PI3K/RAC-alpha serine/threonine-protein kinase (AKT1)/mTOR pathway. | ||||
| Responsed Disease | Lung cancer | ICD-11: 2C25 | |||
| Responsed Drug | Metformin | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| PI3K-Akt signaling pathway | hsa04151 | ||||
In-vitro Model |
NCI-H1975 | Lung adenocarcinoma | Homo sapiens | CVCL_1511 | |
| HCC827 | Lung adenocarcinoma | Homo sapiens | CVCL_2063 | ||
| In-vivo Model | 5 × 106 A549 cells overexpressing METTL3 (Lv-METTL3) or control (Lv-Ctrl) were suspended in 100 uL phosphate-buffered saline (PBS), and were subcutaneously injected into mouse lower right flank. Drug treatment started in the Lv-METTL3 group when the tumour volume reached around 100 mm3. Mice were randomly divided into three groups to receive vehicle, GSK2536771 (30 mg/kg) or rapamycin (1 mg/kg). Drugs were administrated daily through intraperitoneal injection for 18 days. Treatment conditions were chosen as previously reported. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DNA (cytosine-5)-methyltransferase 3B (DNMT3B) | 22 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Curcumin | Phase 3 | [3] | ||
| Synonyms |
458-37-7; Diferuloylmethane; Natural yellow 3; Turmeric yellow; Turmeric; Curcuma; Kacha haldi; Gelbwurz; Indian saffron; Curcumin I; Souchet; Halud; Halad; Haidr; Haldar; Merita earth; Yellow Ginger; Terra Merita; Yellow Root; Safran d'Inde; Yo-Kin; Golden seal; Curcuma oil; Orange Root; Oils, curcuma; CI Natural Yellow 3; Curcumine; Hydrastis; Indian turmeric; Yellow puccoon; Turmeric extract; Diferaloylmethane; Kurkumin [Czech]; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione; Tumeric yellow; Turmeric oil
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [4] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| NSC-622444 | Investigative | [3] | ||
| Synonyms |
NSC622444; CHEMBL116347; AC1Q3LXD; AC1L7GK5; SCHEMBL9755151; dichlorinated diacylmethane fragment; ZINC1616868; BDBM50048522; 5,3'-dicarboxy-4,4'-dihydrodiphenylmethane; 5,5''-methylenebis(3-chloro-2-hydroxybenzoic acid); 5,5'-Methylenebis(3-chloro-2-hydroxybenzoic acid); 3,3'-methanediylbis(5-chloro-6-hydroxybenzoic acid); 5-(3-carboxy-5-chloro-4-hydroxybenzyl)-3-chloro-2-hydroxybenzoic acid; 3',3-Dichloro-4',4-dimethoxy-5',5-bis(methoxycarbonyl)-1,1-diphenylmethane
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-138419 | Investigative | [3] | ||
| Synonyms |
NSC138419; n-[4-(methylamino)benzoyl]glutamic acid; AC1Q5SG8; AC1L5YW4; SCHEMBL5925511; CHEMBL591443; CTK1H0013; 2-[(4-methylaminobenzoyl)amino]pentanedioic acid; A816490; 2-[[4-(methylamino)benzoyl]amino]pentanedioic acid; 2-[[4-(methylamino)phenyl]carbonylamino]pentanedioic acid; 2-[[[4-(methylamino)phenyl]-oxomethyl]amino]pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-319745 | Investigative | [3] | ||
| Synonyms |
61629-60-5; HB 093; BRN 2168571; 4-(2-((5-Chloro-2-methoxybenzoyl)amino)ethyl)hydrocinnamic acid; 3-[4-[2-[(5-CHLORO-2-METHOXY-BENZOYL)AMINO]ETHYL]PHENYL]PROPANOIC ACID; 3-(4-(2-(5-Chlor-2-methoxy-benzamido)-aethyl)phenyl)-propionsaeure [German]; 3-[4-[2-[(5-chloro-2-methoxybenzoyl)amino]ethyl]phenyl]propanoic acid; HYDROCINNAMIC ACID, 4-(2-((5-CHLORO-2-METHOXYBENZOYL)AMINO)ETHYL)-; AC1L2AFL; CHEMBL597112; SCHEMBL11481071; CTK5B3505; DTXSID00210642; AIEFQKOARQRACO-UHFFFAOYSA-N; ZINC1572211; HB-093; NSC319745
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-348926 | Investigative | [3] | ||
| Synonyms |
NSC348926; 2-phthalimidoadipic acid; AC1L7IP1; SCHEMBL9741723; CHEMBL599367; 2-(1,3-dioxoisoindol-2-yl)hexanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-401077 | Investigative | [3] | ||
| Synonyms |
NSC401077; MLS000757170; DNA Methyltransferase Inhibitor; CHEMBL383475; 32675-71-1; 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-3-(1H-indol-3-yl)-propionic acid; 2-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)-3-(1H-indol-3-yl)propanoic acid; 2-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-(1H-indol-3-yl)propanoic acid; 2-(1,3-Dioxo-1,3-dihydro-2H-isoindol-2-yl)-3-(1H-indol-3-yl)propionic acid; SMR000413613; AC1Q71QA; Oprea1_475901; Oprea1_410805; MLS000777218; MLS006011919; SCHEMBL562060
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-tubercidinylhomocysteine | Investigative | [5] | ||
| Synonyms |
CHEMBL552309; 57344-98-6; AC1L3YAS; AC1Q5QMO; (S)-7-(5-S-(3-amino-3-carboxypropyl)-5-thio-beta-D-ribofuranosyl)-7H-pyrrolo(2,3-d)pyrimidin-4-amine; (2s)-2-amino-4-({[(2s,3s,4r,5r)-5-(4-amino-7h-pyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxytetrahydrofuran-2-yl]methyl}sulfanyl)butanoic acid(non-preferred name); BDBM50294482; (2S)-2-amino-4-[[(2S,3S,4R,5R)-5-(4-aminopyrrolo[2,3-d]pyrimidin-7-yl)-3,4-dihydroxyoxolan-2-yl]methylsulfanyl]butanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| NSC-106084 | Investigative | [3] | ||
| Synonyms |
CHEMBL597113; NSC106084; AC1L6H8Q; CTK7J5419; ZINC1868549; BDBM50308983; {4-[5-bromo-2-(carboxymethoxy)benzoyl]phenoxy}acetic acid; 2-(4-bromo-2-(4-(carboxymethoxy)benzoyl)phenoxy)acetic acid; 2-[4-[5-bromo-2-(carboxymethyloxy)benzoyl]phenoxy]acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-154957 | Investigative | [3] | ||
| Synonyms |
NSC154957; AC1L6EF2; CHEMBL586418; 3-benzhydrylsulfanyl-2-formamidopropanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-54162 | Investigative | [3] | ||
| Synonyms |
NSC54162; AC1Q5WTA; AC1L6CM2; CHEMBL611994; 2,2'-{[(2-hydroxyphenyl)methanediyl]disulfanediyl}diacetic acid; ZINC1685025; Acetic acid, (salicylidenedithio)di-; 4265-51-4; Acetic acid, [(o-hydroxybenzylidene)dithio]di-; Acetic acid,2'-[[(2-hydroxyphenyl)methylene]bis(thio)]bis-; 2-[carboxymethylsulfanyl-(2-hydroxyphenyl)methyl]sulfanylacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-57893 | Investigative | [3] | ||
| Synonyms |
MLS002667915; 7399-94-2; 4-[(1h-benzimidazol-2-ylmethyl)(formyl)amino]benzoic acid; NSC57893; AC1L6GFK; AC1Q5TWY; NCIOpen2_002368; CHEMBL599366; 4-[1H-benzimidazol-2-ylmethyl(formyl)amino]benzoic acid; CTK5D9099; DTXSID30288854; HMS3089M13; ZINC1688755; AKOS030547711
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-56071 | Investigative | [3] | ||
| Synonyms |
32230-52-7; NSC56071; AC1L6EJV; AC1Q7ES0; NCIOpen2_007380; CHEMBL596910; CTK4G8394; DTXSID80288485; ZINC1686711; 2,2'-[piperazine-1,4-diylbis(carbonothioylsulfanediyl)]diacetic acid; AKOS030574801; Acetic acid,2,2'-[1,4-piperazinediylbis(carbonothioylthio)]bis- (9CI); 2-[4-(carboxymethylsulfanylcarbothioyl)piperazine-1-carbothioyl]sulfanylacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-137546 | Investigative | [3] | ||
| Synonyms |
NSC137546; CHEMBL591202; AC1L5Y49; AKOS008984447; 2-[(2,6-dichlorobenzoyl)amino]pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-623548 | Investigative | [3] | ||
| Synonyms |
2581-36-4; NSC 408488; o-Cresotic acid, 5,5'-methylenedi-; 2,3-CRESOTIC ACID, 5,5'-METHYLENEDI-; UNII-S3D8KC88KC; 5,5'-Methylenedi-2,3-cresotic acid; NSC 623548; BRN 3433298; S3D8KC88KC; CHEMBL113835; 5,5'-Methylenedi-o-cresotic acid; NSC623548; NSC408488; 5,5'-Methylenebis(2-hydroxy-3-methylbenzoic acid); 2, 5,5'-methylenedi-; AC1L29YK; Oprea1_231968; 2-10-00-00398 (Beilstein Handbook Reference); SCHEMBL9755153; CTK4F6504; DTXSID90180466; o-Cresotic acid,5'-methylenedi-; MolPort-000-698-522; ZINC4028795; STL511095
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-345763 | Investigative | [3] | ||
| Synonyms |
7-(8-hydroxyquinolin-5-yl)-4,7-dioxoheptanoic acid; NSC345763; AC1L7HSU; CHEMBL597114
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NSC-158324 | Investigative | [3] | ||
| Synonyms |
Acediasulfone; UNII-30YP2YHH8W; 30YP2YHH8W; CHEMBL48396; N-[4-[(4-AMINOPHENYL)SULPHONYL]PHENYL]GLYCINE; 2-[4-(4-aminophenyl)sulfonylanilino]acetic acid; Acediasulfonum; N-(4-((4-Aminophenyl)sulphonyl)phenyl)glycine; EINECS 201-243-7; AC1L25EF; ZINC862; SCHEMBL143660; CTK5E7379; DTXSID00229991; CHEBI:135300; BDBM50099670; AKOS027327086; DB08926; Glycine,N-[4-[(4-aminophenyl)sulfonyl]phenyl]-; {4-[(4-aminophenyl)sulfonyl]anilino}acetic acid; 2-(4-(4-aminophenylsulfonyl)phenylamino)acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (L-)-S-adenosyl-L-homocysteine | Investigative | [6] | ||
| Synonyms |
S-Adenosyl-L-homocysteine; S-adenosylhomocysteine; 979-92-0; AdoHcy; S-(5'-adenosyl)-L-homocysteine; adenosylhomocysteine; Formycinylhomocysteine; Adenosyl-L-homocysteine; S-(5'-deoxyadenosin-5'-yl)-L-homocysteine; 2-S-adenosyl-L-homocysteine; 5'-Deoxy-S-adenosyl-L-homocysteine; S-adenosyl-homocysteine; S-Adenosyl Homocysteine; L-S-Adenosylhomocysteine; L-Homocysteine, S-(5'-deoxyadenosin-5'-yl)-; adenosylhomo-cys; adenosyl-homo-cys; UNII-8K31Q2S66S; (S)-5'-(S)-(3-Amino-3-carboxypropyl)-5'-thioadenosine; BRN 5166233; SAH
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| NSC-622445 | Investigative | [3] | ||
| Synonyms |
5,5'-Methylenedisalicylic acid; 122-25-8; 5,5'-Methylenebis(2-hydroxybenzoic acid); Methylenebis(salicylic acid); 5,5-Methylenebis(salicylic acid); UNII-2KF4FVV76N; 5,5-Methylenedisalicylic acid; 5-(3-Carboxy-4-hydroxybenzyl)salicylic acid; 4,4'-Dihydroxy-3,3'-dicarboxydiphenylmethane; 3,3'-Dicarboxy-4,4'-dihydroxydiphenylmethane; NSC 14778; 2KF4FVV76N; 4,4'-Dihydroxydiphenylmethane-3,3'-dicarboxylic acid; 3,3'-Methylenebis(6-hydroxybenzoic acid); CHEMBL115145; Benzoic acid, 3,3'-methylenebis[6-hydroxy-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 17000 nM | |||
| External Link | ||||
| RAC-alpha serine/threonine-protein kinase (AKT1) | 40 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Capivasertib | Approved | [7] | ||
| Synonyms |
1143532-39-1; AZD-5363; capivasertib; AZD 5363; UNII-WFR23M21IE; WFR23M21IE; cc-638; 4-Amino-N-[(1s)-1-(4-Chlorophenyl)-3-Hydroxypropyl]-1-(7h-Pyrrolo[2,3-D]pyrimidin-4-Yl)piperidine-4-Carboxamide; C21H25ClN6O2; (S)-4-AMINO-N-(1-(4-CHLOROPHENYL)-3-HYDROXYPROPYL)-1-(7H-PYRROLO[2,3-D]PYRIMIDIN-4-YL)PIPERIDINE-4-CARBOXAMIDE; 4-Amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-4-piperidinecarboxamide; 4-Piperidinecarboxamide, 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| GDC-0068 | Phase 3 | [8] | ||
| Synonyms |
RG7440
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 157 nM | |||
| External Link | ||||
| Enzastaurin | Phase 3 | [8] | ||
| Synonyms |
LY317615; LE-0014; LY317615, Enzastaurin; 3-(1-methyl-1H-indol-3-yl)-4-{1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]-1H-indol-3-yl}-1H-pyrrole-2,5-dione; 3-(1-methylindol-3-yl)-4-[1-[1-(pyridin-2-ylmethyl)piperidin-4-yl]indol-3-yl]pyrrole-2,5-dione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2110183 | Phase 2 | [9] | ||
| MOA | Modulator | |||
| External Link | ||||
| RX-0201 | Phase 2 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Trametinib + 2141795 | Phase 2 | [9] | ||
| MOA | Modulator | |||
| External Link | ||||
| PTX-200 | Phase 2 | [8] | ||
| Synonyms |
Plant-derived antiparkinsonian, Phytrix
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CMX-2043 | Phase 2 | [11] | ||
| MOA | Modulator | |||
| External Link | ||||
| ARQ 092 | Phase 2 | [12] | ||
| Synonyms |
Miransertib
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CI-1033 | Phase 2 | [13] | ||
| Synonyms |
Canertinib; Canertinib HCl; Canertinib dihydrochloride; Canertinib dihydrochloride [USAN]; CI1033; PD 183805; Canertinib dihydrochloride (USAN); PD-0183805; PD-183805; Canertinib, PD-183805, CI1033, PD183805; N-[4-(3-Chloro-4-fluorophenylamino)-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]acrylamide dihydrochloride; N-[4-[(3-Chloro-4-fluorophenyl)amino]-7-[3-(4-morpholinyl)propoxy]-6-quinazolinyl]-2-propenamide dihydrochloride; N-[4-(3-chloro-4-fluoroanilino)-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]prop-2-enamide; N-[4-(3-chloro-4-fluoroanilino)-7-(3-morpholin-4-ylpropoxy)quinazolin-6-yl]prop-2-enamide dihydrochloride; N-(4-(3-chloro-4-fluorophenyl)amino)-7-(3-morpholin-4-yl)propoxy)quinazolin-6-yl)prop-2-enamide dihydrochloride; N-{4-[(3-chloro-4-fluorophenyl)amino]-7-[3-(morpholin-4-yl)propoxy]quinazolin-6-yl}prop-2-enamide; N-(4-((3-Chloro-4-fluorophenyl)amino)-7-(3-(morpholin-4-yl)propoxy)quinazolin-6-yl)prop-2-enamide; 2-Propenamide, N-(4-((3-chloro-4-fluorophenyl) amino)-7-(3-(4-morpholinyl) propoxy)-6-quinazolinyl)-, dihydrochloride; 2-Propenamide, N-(4-((3-chloro-4-fluorophenyl)amino)-7-(3-(4-morpholinyl)propoxy)-6-quinazolinyl)-, dihydrochloride
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Triciribine prodrug | Phase 1/2 | [9] | ||
| Synonyms |
TSR-826; Triciribine prodrug (oral, cancer); Triciribine prodrug (oral, cancer), TSRL
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-754807 | Phase 1 | [14] | ||
| Synonyms |
1001350-96-4; BMS 754807; BMS754807; UNII-W9E3353E8J; CHEMBL575448; CHEBI:88339; W9E3353E8J; 1-{4-[(3-cyclopropyl-1H-pyrazol-5-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl}-N-(6-fluoropyridin-3-yl)-2-methyl-L-prolinamide; W-204348; J-501009; 2-Pyrrolidinecarboxamide, 1-[4-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl]-N-(6-fluoro-3-pyridinyl)-2-methyl-, (2S)-;2-Pyrrolidinecarboxamide, 1-[4-[(5-cyclopropyl-1H-pyrazol-3-yl)amino]pyrrolo[2,1-f][1,2,4]triazin-2-yl]-N-(6-fluoro-3-pyridin
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ARQ 751 | Phase 1 | [8] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| M2698 | Phase 1 | [8] | ||
| Synonyms |
HXAUJHZZPCBFPN-QGZVFWFLSA-N; 1379545-95-5; SCHEMBL15262358; EX-A1187; AKOS030627134; M2698(MSC-2363318A)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID28460551-Compound-6 | Patented | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Squalestatin 1 | Terminated | [16] | ||
| Synonyms |
Zaragozic acid A; Squalestatin; 142561-96-4; ZARAGOZIC ACIDS A; UNII-1117HVX02L; CHEMBL280978; CHEBI:75170; 1117HVX02L; 1S-((4S-acetoxy-5R-methyl-3-methylene-6-phenylhexyl)-6-(E)-4S,6S-dimethyloct-2-enoyloxy)-4,7S-dihydroxy-2,8-dioxabicyclo[321]octane-3S,4S,5R-tricarboxylic acid; L-erythro-L-glycero-D-altro-7-Trideculo-7,4-furanosonic acid, 2,7-anhydro-3,4-di-C-carboxy-8,9,10,12,13-pentadeoxy-10-methylene-12-(phenylmethyl)-, 11-acetate 5-(4,6-dimethyl-2-octenoate), (5(2E,4S,6S),7S)-; Squalestatin 1, Glaxo; Zaragozic acid A, Glaxo
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MYRIOCIN | Investigative | [16] | ||
| Synonyms |
thermozymocidin; 35891-70-4; ISP-I; ISP-1; UNII-YRM4E8R9ST; (2S,3R,4R,6E)-2-Amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxo-6-eicosenoic acid; YRM4E8R9ST; Myriocin, Mycelia sterilia; Myriocin from Mycelia sterilia; CHEBI:582124; NCGC00163597-02; NCGC00163597-03; DSSTox_CID_26360; DSSTox_RID_81561; (2S,3R,4R,6E)-2-amino-3,4-dihydroxy-2-(hydroxymethyl)-14-oxoicos-6-enoic acid; DSSTox_GSID_46360; [2S,3R,4R]-(E)-2-Amino-3,4-dihydroxy-2-[hydroxymethyl]-14-oxo-6-eicosenoic Acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LD-101 | Investigative | [9] | ||
| Synonyms |
AKT-SI-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| A-674563 | Investigative | [17] | ||
| Synonyms |
A 674563; A674563
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 11 nM | |||
| External Link | ||||
| Lactoquinomycin | Investigative | [18] | ||
| Synonyms |
SCHEMBL12324296
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 149 nM | |||
| External Link | ||||
| VLI-27 | Investigative | [9] | ||
| Synonyms |
AKT inhibitor (pancreatic cancer), NovaLead Pharma
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| A-443654 | Investigative | [19] | ||
| Synonyms |
A-4436554
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.16 nM | |||
| External Link | ||||
| NU-1001-41 | Investigative | [9] | ||
| Synonyms |
Anti-phospho-AKT monoclonal antibodies (cancer), Nuclea Biotechnologies
Click to Show/Hide
|
|||
| External Link | ||||
| 4,5,6-trihydroxy-3-methylphthalide | Investigative | [20] | ||
| Synonyms |
CHEMBL486813; AGUVVAYMPQDJDX-UHFFFAOYSA-; BDBM50242174; 3-methyl-4,5,6-trihydroxy-phthalide; 4,5,6-Trihydroxy-3-methylisobenzofuran-1(3H)-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19700 nM | |||
| External Link | ||||
| ALM-301 | Investigative | [9] | ||
| Synonyms |
Akt inhibitors (cancer), Almac
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BX-517 | Investigative | [21] | ||
| Synonyms |
BX517; 850717-64-5; UNII-SYV8VN8W5K; SYV8VN8W5K; pdk-1 inhibitors; BX517(PDK1 inhibitor2); Indolinone based inhibitor, 4i; SCHEMBL5567818; CHEMBL228654; 5-Ureido-3-(1-(pyrrol-2-yl)ethylidene)indolin-2-one; BDBM17004; MolPort-046-033-615; BCP16225; EX-A2243; ZINC14962724; AKOS032945106; CS-6066; Urea, N-(2,3-dihydro-2-oxo-3-((3Z)-1-(1H-pyrrol-2-yl)ethylidene)-1H-indol-5-yl)-; Urea, N-(2,3-dihydro-2-oxo-3-(1-(1H-pyrrol-2-yl)ethylidene)-1H-indol-5-yl)-; Urea, (2,3-dihydro-2-oxo-3-(1-(1H-pyrrol-2-yl)ethylidene)-1H-indol-5-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Inositol 1,3,4,5-Tetrakisphosphate | Investigative | [22] | ||
| Synonyms |
Inositol-1,3,4,5-tetraphosphate; Ins-1,3,4,5-P4; 1D-myo-inositol 1,3,4,5-tetrakisphosphate; Inositol-1,3,4,5-tetrakisphosphate; inositol-(1,3,4,5)-tetrakisphosphate; Inositol 1,3,4,5-tetraphosphate; myo-Inositol-1,3,4,5-tetrakisphosphate; CHEMBL23552; D-myo-inositol 1,3,4,5-tetrakisphosphate; CHEBI:16783; myo-Inositol, 1,3,4,5-tetrakis(dihydrogen phosphate); 1D-myo-inositol 1,3,4,5-tetrakis(dihydrogen phosphate); Ins(1,3,4,5)P4; 1bwn; 4IP; 102850-29-3; myo-Inositol 1,3,4,5-tetraphosphate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (Z)-3-((1H-pyrrol-2-yl)methylene)indolin-2-one | Investigative | [21] | ||
| Synonyms |
oxindole i; CHEMBL86755; 3-(1H-Pyrrol-2-ylmethylene)-1,3-dihydroindol-2-one; oxindole 1; AC1NZGXV; K00027; Indolinone based inhibitor, 1; SCHEMBL1162655; SCHEMBL13819612; BDBM17015; MolPort-023-197-743; SEZFNTZQMWJIAI-FLIBITNWSA-N; ZINC3874586; HSCI1_000049; NCGC00343760-01; BRD-K51816706-001-01-7; (3Z)-3-(1H-pyrrol-2-ylmethylidene)-1H-indol-2-one; 3-[(1H-Pyrrole-2-yl)methylene]-1H-indole-2(3H)-one; Z-(1H-Pyrrol-2-ylmethylene)-1,3-dihydro-indol-2-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2300 nM | |||
| External Link | ||||
| Akt inhibitor VIII | Investigative | [23] | ||
| Synonyms |
isozyme-selective, Akti-1/2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 58 nM | |||
| External Link | ||||
| SB-747651A | Investigative | [24] | ||
| Synonyms |
CHEMBL188434; compound 26; SCHEMBL4719834; GTPL8130; BDBM24996; oxadiazole-containing compound, 9; MBCJUIJWPYUEBX-UHFFFAOYSA-N; ZINC13998530; NCGC00273984-05; NCGC00273984-03; SB-747651; 4-{1-ethyl-7-[(piperidin-4-ylamino)methyl]-1H-imidazo[4,5-c]pyridin-2-yl}-1,2,5-oxadiazol-3-amine; [2-(4-Amino-furazan-3-yl)-1-ethyl-1H-imidazo[4,5-c]pyridin-7-ylmethyl]-piperidin-4-yl-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| PMID20005102C1 | Investigative | [25] | ||
| Synonyms |
GTPL8181; BDBM50305878; B99
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 nM | |||
| External Link | ||||
| STAUROSPORINONE | Investigative | [26] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro31-8220 | Investigative | [26] | ||
| Synonyms |
Bisindolylmaleimide IX; ro 31-8220; 125314-64-9; Ro 31 8220; Ro 318220; UNII-W9A0B5E78O; Ro-318220; Ro-31-8220; CHEMBL6291; W9A0B5E78O; CHEBI:38912; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl carbamimidothioate; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl imidothiocarbamate; CHEMBL1591531; Carbamimidothioic acid, 3-(3-(2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl)-1H-indol-1-yl)propyl; bisindolymaleimide IX
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-536924 | Investigative | [27] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| KN-62 | Investigative | [26] | ||
| Synonyms |
KN-62 (non-isomeric); GTPL6001; HMS3229A04; CCG-206863
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CI-1040 | Investigative | [26] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4,5,6,7-tetrabromo-1H-benzo[d][1,2,3]triazole | Investigative | [28] | ||
| Synonyms |
4,5,6,7-tetrabromobenzotriazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [29] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Bisindolylmaleimide-I | Investigative | [26] | ||
| Synonyms |
Bisindolylmaleimide i; 133052-90-1; GF 109203X; GF109203X; Go 6850; GF-109203X; RBT205 INHIBITOR; Go-6850; UNII-L79H6N0V6C; Bisindolylmaleimide I (GF 109203X); CHEMBL7463; 3-{1-[3-(DIMETHYLAMINO)PROPYL]-1H-INDOL-3-YL}-4-(1H-INDOL-3-YL)-1H-PYRROLE-2,5-DIONE; 3-(1-(3-(Dimethylamino)propyl)-1H-indol-3-yl)-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione; L79H6N0V6C; QMGUOJYZJKLOLH-UHFFFAOYSA-N; 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl)maleimide; GF-109203; Go6850
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RO-316233 | Investigative | [26] | ||
| Synonyms |
119139-23-0; bisindolylmaleimide iv; 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-dione; Arcyriarubin A; 3,4-Bis(3-indolyl)maleimide; 3,4-Di-1H-indol-3-yl-1H-pyrrole-2,5-dione; UNII-MBK3OO5K8T; BIM IV; 3,4-bis(1H-indol-3-yl)pyrrole-2,5-dione; MBK3OO5K8T; CHEMBL266487; 3,4-bis(1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione; DQYBRTASHMYDJG-UHFFFAOYSA-N; 2,3-bis(1H-Indol-3-yl)maleimide; 1H-Pyrrole-2,5-dione, 3,4-di-1H-indol-3-yl-; Ro-31-6233; AK-15401; 3,4-bis(3-indolyl)-1H-pyrrole-2,5-dione; Bisindoylmaleimide; Bisindolyl deriv. 3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C25: Lung cancer | 52 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Adagrasib | Approved | [30] | ||
| Synonyms |
2326521-71-3; MRTX-849; UNII-8EOO6HQF8Y; 8EOO6HQF8Y; 2-((S)-4-(7-(8-Chloronaphthalen-1-yl)-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile; CHEMBL4594350; SCHEMBL20974691; GTPL10888; Kras G12C inhibitor MRTX849; BCP31538; EX-A3258; MRTX 849; MFCD32263433; s8884; compound 20 [PMID: 32250617]; BS-16211; HY-130149; CS-0105265; 2-Piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2S)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2S)-
Click to Show/Hide
|
|||
| External Link | ||||
| Amivantamab | Approved | [31] | ||
| External Link | ||||
| Mobocertinib | Approved | [32] | ||
| Synonyms |
1847461-43-1; TAK-788; TAK788; AP32788; UNII-39HBQ4A67L; 39HBQ4A67L; propan-2-yl 2-[4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxy-5-(prop-2-enamido)anilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Mobocertinib [INN]; Mobocertinib [USAN]; AP-32788; Mobocertinib (TAK788); Mobocertinib(TAK-788); SCHEMBL17373133; GTPL10468; BDBM368374; BCP31045; EX-A3392; US10227342, Example 10; MFCD32669806; NSC825519; s6813; TAK-788;AP32788; WHO 11183; NSC-825519; example 94 [WO2015195228A1]; HY-135815; CS-0114256; TAK-788;TAK 788; AP 32788; 5-Pyrimidinecarboxylic acid, 2-((4-((2-(dimethylamino)ethyl)methylamino)-2-methoxy-5-((1-oxo-2-propen-1-yl)amino)phenyl)amino)-4-(1-methyl-1H-indol-3-yl)-, 1-methylethyl ester; C(C=C)(=O)NC=1C(=CC(=C(C=1)NC1=NC=C(C(=N1)C1=CN(C2=CC=CC=C12)C)C(=O)OC(C)C)OC)N(C)CCN(C)C; Isopropyl 2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Isopropyl 2-(5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenylamino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Propan-2-yl 2-(5-(acryloylamino)-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyanilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Sugemalimab | Approved in China | [33] | ||
| External Link | ||||
| Sacituzumab govitecan | Approved | [34] | ||
| Synonyms |
1491917-83-9; 1535963-91-7; 1796566-95-4; CYSTEINYL CL2A-SN-38; DA64T2C2IO; DTXSID401335985; EX-A4354; F82944; GOVITECAN CYSTEINYL CONJUGATE; hRS 7SN38; hRS7-SN38; IMMU 132; IMMU-132; M9BYU8XDQ6; Sacituzumab govitecan; Sacituzumab govitecan [USAN]; sacituzumab-govitecan; Satralizumab linker; SN-38 CYSTEINYL CONJUGATE; UNII-M9BYU8XDQ6
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [35] | ||
| External Link | ||||
| Tepotinib | Approved | [36] | ||
| Synonyms |
1100598-32-0; EMD 1214063; UNII-1IJV77EI07; Tepotinib (EMD 1214063); EMD1214063; 1IJV77EI07; MSC-2156119J; Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]-; C29H28N6O2; Tepotinib [INN]; Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]; Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo
Click to Show/Hide
|
|||
| External Link | ||||
| Sotorasib | Approved | [37] | ||
| Synonyms |
AMG-510; AMG510; AMG-510 racemate; 2252403-56-6; AMG 510; Kras G12C inhibitor 9; 2296729-00-3; UNII-2B2VM6UC8G; 2B2VM6UC8G; CHEMBL4535757; 2296729-00-3 (racemate); 4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one; Sotorasib [INN]; 6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-((2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl)pyrido(2,3-d)pyrimidin-2-one; AMG510 racemate; Sotorasib [USAN]; AMG-510(racemate); Kras mutant-targeting AMG 510; SCHEMBL20560375; GTPL10678; AMG 510 pound>>AMG-510; AMY16918; BCP30452; BCP33368; EX-A3538; BDBM50514402; NSC818433; s8830; WHO 11370; DB15569; NSC-818433; BS-16684; HY-114277; CS-0081316; compound (R)-38 [PMID: 31820981]; (1m)-6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)-4-((2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl)pyrido(2,3-d)pyrimidin-2(1H)-one; (1S)-4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 2296729-66-1; Pyrido(2,3-d)pyrimidin-2(1H)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(1-methylethyl)-3-pyridinyl)-4-((2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Entrectinib | Approved | [38] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Repotrectinib | Approved | [39] | ||
| Synonyms |
FIKPXCOQUIZNHB-RRKGBCIJSA-N; SCHEMBL20438940; TPX 0005; BCP19778
Click to Show/Hide
|
|||
| External Link | ||||
| MYL-1402O | Phase 3 | [40] | ||
| Synonyms |
bevacizumab biosimilar
Click to Show/Hide
|
|||
| External Link | ||||
| AB154 | Phase 3 | [41] | ||
| Synonyms |
Domvanalimab
Click to Show/Hide
|
|||
| External Link | ||||
| Datopotamab deruxtecan | Phase 3 | [42] | ||
| External Link | ||||
| CS1001 | Phase 3 | [43] | ||
| External Link | ||||
| JDQ443 | Phase 3 | [44] | ||
| Synonyms |
(S)-JDQ-443; 1-(6-((4S)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl)-2-azaspiro[3.3]heptan-2-yl)prop-2-en-1-one; 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-inda zol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]- 2-Propen-1-one; 1-[6-[(4R)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-2-propen-1-one; 1-[6-[4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methylindazol-5-yl)pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl]prop-2-en-1-one; 1-{6-[(4M)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5- methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2- azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 1-{6-[(4M)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 2653994-08-0; 2653994-10-4; 2-Propen-1-one, 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-; AKOS040757949; AT36708; BDBM50579985; CHEMBL5077861; compound 5 [PMID: 35404998]; CS-0226220; CS-0311034; EX-A5693; example 1a [WO2021120890A1]; GLXC-25533; GTPL11715; HY-139612A; JDQ 443; JDQ 443 [WHO-DD]; JDQ443; JDQ-443; MS-29737; NSC846146; NSC-846146; NVP-JDQ443; NVP-JDQ-443; Opnurasib; opnurasib [INN]; -PROPEN-1-ONE, 1-(6-((4R)-4-(5-CHLORO-6-METHYL-1H-INDAZOL-4-YL)-5-METHYL-3-(1-METHYL-1H-INDAZOL-5-YL)-1H-PYRAZOL-1-YL)-2-AZASPIRO(3.3)HEPT-2-YL)-; Q3W0H3V1LQ; SCHEMBL23533580
Click to Show/Hide
|
|||
| External Link | ||||
| TRS003 | Phase 3 | [45] | ||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [46] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| RG6058 | Phase 3 | [47] | ||
| Synonyms |
Tiragolumab
Click to Show/Hide
|
|||
| External Link | ||||
| GSK4069889 | Phase 2 | [48] | ||
| Synonyms |
TSR-022
Click to Show/Hide
|
|||
| External Link | ||||
| APL-101 | Phase 2 | [49] | ||
| Synonyms |
Bozitinib; PLB-1001; 1440964-89-5; Vebreltinib; Vebreltinib [USAN]; UNII-2WZP8A9VFN; 2WZP8A9VFN; Bozitinib (PLB-1001); SCHEMBL15594471; BDBM107096; CBI-3103; s6762; WHO 11677; HY-125017; CS-0088607; US9695175, 44; 1,2,4-Triazolo(4,3-b)pyridazine, 6-(1-cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6- fluoro-2-methyl-2H-indazol-5-yl)methyl)-; 6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5- yl)methyl)-1,2,4-triazolo(4,3-b)pyridazine
Click to Show/Hide
|
|||
| External Link | ||||
| SGN-LIV1A | Phase 2 | [50] | ||
| Synonyms |
Ladiratuzumab Vedotin
Click to Show/Hide
|
|||
| External Link | ||||
| BNT116 | Phase 2 | [51] | ||
| External Link | ||||
| AZD7789 | Phase 2 | [52] | ||
| External Link | ||||
| L-DOS47 | Phase 1/2 | [53] | ||
| External Link | ||||
| NC318 | Phase 2 | [54] | ||
| External Link | ||||
| Vorolanib | Phase 2 | [55] | ||
| Synonyms |
UNII-YP8G3I74EL; YP8G3I74EL; 1013920-15-4; (S,Z)-N-(1-(Dimethylcarbamoyl)pyrrolidin-3-yl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; Vorolanib [INN]; SCHEMBL2439528; CHEMBL4297587; N-((3S)-1-(dimethylcarbamoyl)pyrrolidin-3-yl)-5-((Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; s6843; DB15247; HY-109019; CS-0030517; Q27294638; 1H-Pyrrole-3-carboxamide, N-((3S)-1-((dimethylamino)carbonyl)-3-pyrrolidinyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| Xofigo | Phase 2 | [56] | ||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [57] | ||
| External Link | ||||
| Voyager-V1 | Phase 2 | [58] | ||
| Synonyms |
VSV-IFNBeta-NIS
Click to Show/Hide
|
|||
| External Link | ||||
| AB-106 | Phase 2 | [59] | ||
| Synonyms |
DS6051b; GTPL11198; AB106
Click to Show/Hide
|
|||
| External Link | ||||
| RO-5126766 | Phase 2 | [60] | ||
| Synonyms |
VS-6766; CH-5126766; Dual Raf/MEK protein kinase inhibitor (cancer), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| TC-210 | Phase 1/2 | [61] | ||
| External Link | ||||
| EMB-01 | Phase 1/2 | [62] | ||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [63] | ||
| External Link | ||||
| MRx0518 | Phase 1/2 | [64] | ||
| External Link | ||||
| DZD9008 | Phase 1/2 | [65] | ||
| External Link | ||||
| BGB-A425 | Phase 1/2 | [66] | ||
| External Link | ||||
| Rilvegostomig | Phase 1/2 | [67] | ||
| Synonyms |
AZD2936
Click to Show/Hide
|
|||
| External Link | ||||
| IK-007 | Phase 1/2 | [68] | ||
| Synonyms |
grapiprant
Click to Show/Hide
|
|||
| External Link | ||||
| IBI318 | Phase 1 | [69] | ||
| External Link | ||||
| GEN-011 | Phase 1 | [70] | ||
| External Link | ||||
| ENV-105 | Phase 1 | [71] | ||
| Synonyms |
Carotuximab
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 160 | Phase 1 | [72] | ||
| External Link | ||||
| ADP-A2M10 | Phase 1 | [73] | ||
| External Link | ||||
| MEDI5752 | Phase 1 | [74] | ||
| External Link | ||||
| PF-07104091 | Phase 1 | [75] | ||
| External Link | ||||
| PF-06936308 | Phase 1 | [76] | ||
| External Link | ||||
| GEM3PSCA | Phase 1 | [77] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [78] | ||
| External Link | ||||
| Cosibelimab | Phase 1 | [79] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| Gemcitabine | Approved | [80] | ||
| External Link | ||||
| SMI-4a | Investigative | [81] | ||
| Synonyms |
438190-29-5; SMI 4a; TCS PIM-1 4a; (Z)-SMI-4a; (Z)-5-(3-(trifluoromethyl)benzylidene)thiazolidine-2,4-dione; (5Z)-5-[3-(trifluoromethyl)benzylidene]-1,3-thiazolidine-2,4-dione; CHEMBL183906; (5Z)-5-[[3-(TRIFLUOROMETHYL)PHENYL]METHYLENE]-2,4-THIAZOLIDINEDIONE; (5Z)-5-[[3-(trifluoromethyl)phenyl]methylidene]-1,3-thiazolidine-2,4-dione; 327033-36-3; C11H6F3NO2S; (Z)-5-(3-(Trifluoromethyl)benzylidene)-thiazolidine-2,4-dione; (5Z)-5-{[3-(trifluoromethyl)phenyl]methylidene}-1,3-thiazolidine-2,4-dione; Pim inhibitor 4a; 3vc4; SMI-4q; TCS PIM-1-4a; 5-(3-(Trifluoromethyl)benzylidene)thiazolidine-2,4-dione; 5-[3-(Trifluoromethyl)benzylidene]thiazolidine-2,4-dione; cc-717; thiazolidine-2,4-dione, 4a; SCHEMBL2541382; SCHEMBL2541388; BDBM26626; AOB6260; EX-A111; SYN1113; BDBM138364; HMS3229J21; 2720AH; HY-16576A; MFCD01152003; s8005; ZINC12576047; AKOS001314163; SMI-4a, >=98% (HPLC); CCG-265027; NCGC00345836-02; NCGC00345836-14; AC-32861; HY-15474; AB0165836; EC-000.2291; J3.561.866J; A11945; W-5256; US8877795, 12; Q27451064; 5-[[3-(trifluoromethyl)phenyl]methylene]-2,4-thiazolidinedione
Click to Show/Hide
|
|||
| External Link | ||||
References