m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02084
|
[1] | |||
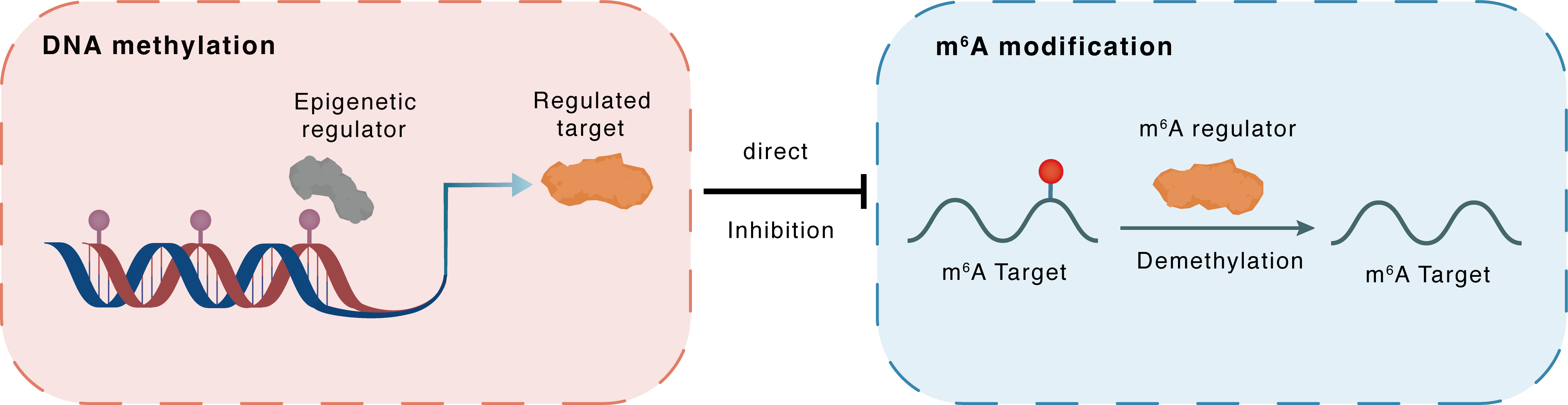 DNA methylation
DNMT3A
FTO
Direct
Inhibition
m6A modification
NTRK2
NTRK2
FTO
Demethylation
DNA methylation
DNMT3A
FTO
Direct
Inhibition
m6A modification
NTRK2
NTRK2
FTO
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | BDNF/NT-3 growth factors receptor (NTRK2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | Cysteine methyltransferase DNMT3A (DNMT3A) | WRITER | View Details | ||
| Regulated Target | FTO alpha-ketoglutarate dependent dioxygenase (FTO) | View Details | |||
| Crosstalk Relationship | DNA methylation → m6A | Inhibition | |||
| Crosstalk Mechanism | DNA methylation directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | A downregulation in demethylating enzyme Fto and upregulation in methylating enzyme Mettl3 were also noted. The FTO promoter was hypomethylated due to the lower expression of DNMT1 and DNMT3A and Nr3c1, Creb1, BDNF/NT-3 growth factors receptor (NTRK2), Bdnf are downstream target genes of both METTL3 and FTO. | ||||
| Responsed Disease | Major depressive disorder | ICD-11: 6A70.3 | |||
In-vitro Model |
SH-SY5Y | Neuroblastoma | Homo sapiens | CVCL_0019 | |
| In-vivo Model | Rats were housed at 23 ° C and 55% humidity and were given ad libitum food and water. During acclimatization (1 week), rats were placed randomly (3/cage); however, after initial behavioral testing, they were grouped according to their behavioral phenotypes. All experiments were performed under a light cycle (8:00 AM and 10:00 AM). The protocol to induce LH behavior was approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. The animal study also adhered to the international guidelines for the use and care of laboratory animals. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| BDNF/NT-3 growth factors receptor (NTRK2) | 18 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Larotrectinib | Approved | [2] | ||
| Synonyms |
UNII-PF9462I9HX; PF9462I9HX; ARRY 470; Larotrectinib [USAN:INN]; GTPL8909; SCHEMBL2241012; NYNZQNWKBKUAII-KBXCAEBGSA-N; BDBM136597; ZINC118399834; AKOS027338709; example 14 [US8865698 B2]; CS-5722; HY-12866; AS-35231; J3.628.138C; US8865698, 14; ARRY470; ARRY-470
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5.21 nM | |||
| External Link | ||||
| Entrectinib | Approved | [3] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| MK-2461 | Phase 1/2 | [4] | ||
| Synonyms |
MK 2461
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 61 nM | |||
| External Link | ||||
| Macrocycle derivative 13 | Patented | [5] | ||
| Synonyms |
PMID28270010-Compound-Figure21-a
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Cyclopenta[d]pyrimidine derivative 1 | Patented | [5] | ||
| Synonyms |
PMID28270010-Compound-Figure28
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 12.12 nM | |||
| External Link | ||||
| PMID28270021-Compound-WO2016054807Example1 | Patented | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 81000 nM | |||
| External Link | ||||
| PMID28270010-Compound-Figure24-b | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.1 nM | |||
| External Link | ||||
| Pyrrolo[2,3-d]pyrimidine derivative 4 | Patented | [6] | ||
| Synonyms |
PMID28270021-Compound-WO2012137089PF-06278121 (Example 9)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7 nM | |||
| External Link | ||||
| 3-amino-5-benzyl-substituted indazole derivative 1 | Patented | [6] | ||
| Synonyms |
PMID28270021-Compound-WO2010106028Entrectinib (RXDX-101)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 nM | |||
| External Link | ||||
| PMID28270010-Compound-Figure5-1 | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.79 nM | |||
| External Link | ||||
| PMID28270021-Compound-WO2015042088Example4 | Patented | [6] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 81000 nM | |||
| External Link | ||||
| PMID28270010-Compound-Figure5-2 | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.3 nM | |||
| External Link | ||||
| PMID28270010-Compound-Figure5-3 | Patented | [5] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1.6 nM | |||
| External Link | ||||
| Azaindazole amide derivative 1 | Patented | [6] | ||
| Synonyms |
PMID28270021-Compound-WO2014016434Example30
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 31 nM | |||
| External Link | ||||
| TrkB NAM | Investigative | [7] | ||
| Synonyms |
TrkB negative allosteric modulators (neurodegenerative diseases); TrkB NAM program (neurodegenerative diseases), Addex; TrkB negative allosteric modulators (neurodegenerative diseases), Addex
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| GNF-5837 | Investigative | [8] | ||
| Synonyms |
GNF 5837; GNF5837
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 12 nM | |||
| External Link | ||||
| AZD1332 | Investigative | [7] | ||
| Synonyms |
AZD 1332; AZD-1332
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID24432909C8e | Investigative | [9] | ||
| Synonyms |
4cd0; GTPL8137; ZINC98050687; BDBM50448785; NCGC00485046-01; P-355; AWJ
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4 nM | |||
| External Link | ||||
| Cysteine methyltransferase DNMT3A (DNMT3A) | 8 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PMID27376512-Compound-Figure3CN | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 1100 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure3CG | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 2400 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure3CM | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | EC50 = 1100 nM | |||
| External Link | ||||
| PMID27376512-Compound-Figure2aExample1 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 3000 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
References