m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT02017
|
[1] | |||
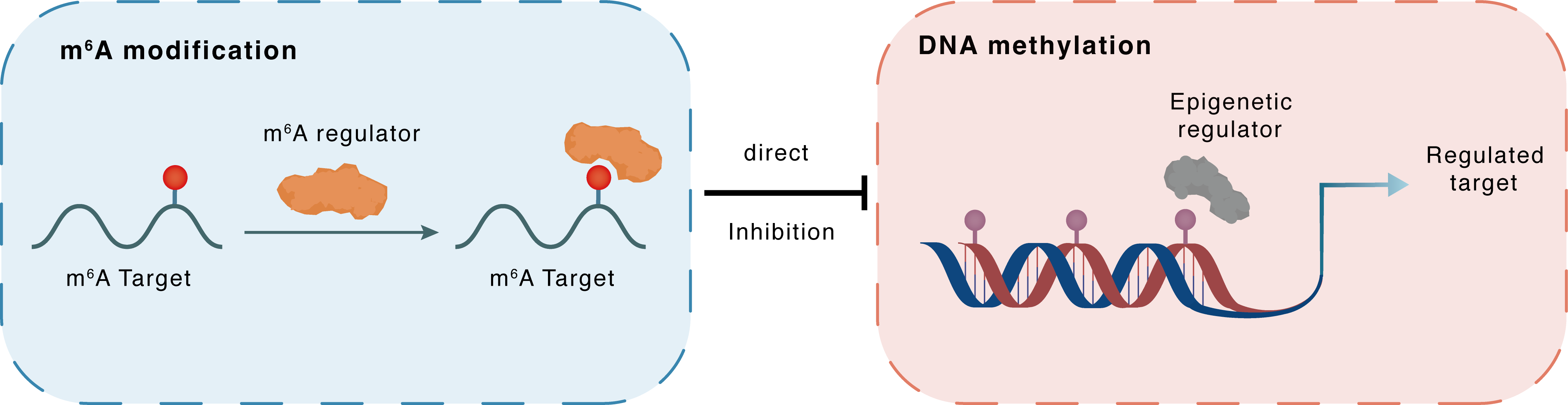 m6A modification
SEMA3F
SEMA3F
IGF2BP3
m6A modification
SEMA3F
SEMA3F
IGF2BP3
 : m6A sites
Direct
Inhibition
DNA methylation
DNMT1
SEMA3F : m6A sites
Direct
Inhibition
DNA methylation
DNMT1
SEMA3F
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3) | READER | |||
| m6A Target | Semaphorin-3F (SEMA3F) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | DNA methylation (DNAMeth) | ||||
| Epigenetic Regulator | DNA (cytosine-5)-methyltransferase 1 (DNMT1) | WRITER | View Details | ||
| Regulated Target | Semaphorin-3F (SEMA3F) | View Details | |||
| Crosstalk Relationship | m6A → DNA methylation | Inhibition | |||
| Crosstalk Mechanism | m6A modification directly impacts DNA methylation through recruiting DNA methyltransferases or demethylases. | ||||
| Crosstalk Summary | Moreover, our findings first emphasized the functional importance of IGF2BPs (IGF2BP2 and IGF2BP3) as epigenetic R-loop readers in transcriptional genetic regulation and cancer biology. In addition, our research provides a novel RBM15/IGF2BPs/DNMT1 trans-omics regulation m6A axis targeting Semaphorin-3F (SEMA3F), indicating the new crosstalk between RNA m6A methylation and DNA methylation in prostate cancer. | ||||
| Responsed Disease | Prostate cancer | ICD-11: 2C82 | |||
| Responsed Drug | Docetaxel | ||||
| Cell Process | Migration inhibition | ||||
| Cell growth retardation | |||||
In-vitro Model |
RWPE-1 | Normal | Homo sapiens | CVCL_3791 | |
| DU145 | Prostate carcinoma | Homo sapiens | CVCL_0105 | ||
| PC-3 | Prostate carcinoma | Homo sapiens | CVCL_0035 | ||
| 22Rv1 | Prostate carcinoma | Homo sapiens | CVCL_1045 | ||
| LNCaP | Prostate carcinoma | Homo sapiens | CVCL_0395 | ||
| LNCaP C4-2B | Prostate carcinoma | Homo sapiens | CVCL_4784 | ||
| VCaP | Prostate carcinoma | Homo sapiens | CVCL_2235 | ||
| HEK293T | Normal | Homo sapiens | CVCL_0063 | ||
| In-vivo Model | All the animal studies and protocols followed the institutional guidelines of the First Affiliated Hospital, School of Medicine, Zhejiang University. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| DNA (cytosine-5)-methyltransferase 1 (DNMT1) | 27 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| SGI110 | Phase 3 | [2] | ||
| MOA | Modulator | |||
| External Link | ||||
| Guadecitabine | Phase 3 | [3] | ||
| Synonyms |
UNII-2KT4YN1DP7; 929901-49-5; 2KT4YN1DP7; SGI-110 free acid; Guadecitabine [USAN:INN]; GuadecitabineSGI-110; Guadecitabine (USAN/INN); CHEMBL3544916; Guanosine, 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxy-; ZINC43203165; AKOS027321496; AKOS030238181; DB11918; CS-3089; HY-13542; D10877; 2'-deoxy-5-azacytidylyl-(3'-5')-2'-deoxyguanosine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CC-486 | Phase 3 | [4] | ||
| Synonyms |
AG-14361; AG14361; 328543-09-5; UNII-48N0U0K50I; AG 14361; CHEMBL65892; 48N0U0K50I; Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-; AG-014361; 1-(4-((dimethylamino)methyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; Imidazo(4,5,1-jk)(1,4)benzodiazepin-7(4H)-one, 2-(4-((dimethylamino)methyl)phenyl)-5,6-dihydro-; 2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one; SMR000486393; MLS006011157; MLS001065917; Nucleoside analogue
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| S-110 | Phase 3 | [5] | ||
| Synonyms |
DNA demethylating agent (myelodysplastic syndrome), Supergen
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Palifosfamide | Phase 2 | [6] | ||
| Synonyms |
ZIO-201
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RX-3117 | Phase 2 | [7] | ||
| Synonyms |
Antimetabolite (cancer), Rexahn; Antimetabolite (cancer), Rexahn/ Teva
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Antroquinonol | Phase 2 | [8] | ||
| Synonyms |
Hocena; Fungal extract (cancer), Golden Biotechnology
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK4172239 | Phase 1 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-miR-155-5p | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 27.88 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example11 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 39440 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example16 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 22520 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example5 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50(DNMT1) = 3530 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-2HPE | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 135.2 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-1 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 2003 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example4 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 13810 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-2 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 434.1 nM | |||
| External Link | ||||
| PMID27376512-Compound-asCEBP-1HPE | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | Ki = 917.5 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example8 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 6850 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-433 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.22 nM | |||
| External Link | ||||
| PMID27376512-Compound-Table1Example30 | Patented | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID27376512-Compound-MTC-424 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1940 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-427 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 295 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-422 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1430 nM | |||
| External Link | ||||
| PMID27376512-Compound-MTC-423 | Patented | [10] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 363 nM | |||
| External Link | ||||
| PMX-700 | Investigative | [11] | ||
| Synonyms |
SJ-005019; SJ-005059; DC-010-116; Temozolomide analogs (cancer), Pharminox
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| XB-05 | Investigative | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CP-4200 | Investigative | [11] | ||
| Synonyms |
Lipidated azacitidine (cancer, Lipid Vector), Clavis Pharma; 5-azacytidine-5'-elaidate
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C82: Prostate cancer | 1 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| CC-94676 | Phase 1 | [12] | ||
| External Link | ||||
References