m6A Target Gene Information
General Information of the m6A Target Gene (ID: M6ATAR00432)
Full List of m6A Methylation Regulator of This Target Gene and Corresponding Disease/Drug Response(s)
TNF
can be regulated by the following regulator(s), and cause disease/drug response(s). You can browse detail information of regulator(s) or disease/drug response(s).
Browse Regulator
Browse Disease
Methyltransferase-like 3 (METTL3) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL3 | ||
| Cell Line | Raw 264.7 cell line | Mus musculus |
|
Treatment: METTL3 knockout Raw 264.7 cells
Control: Wild type Raw 264.7 cells
|
GSE162248 | |
| Regulation |
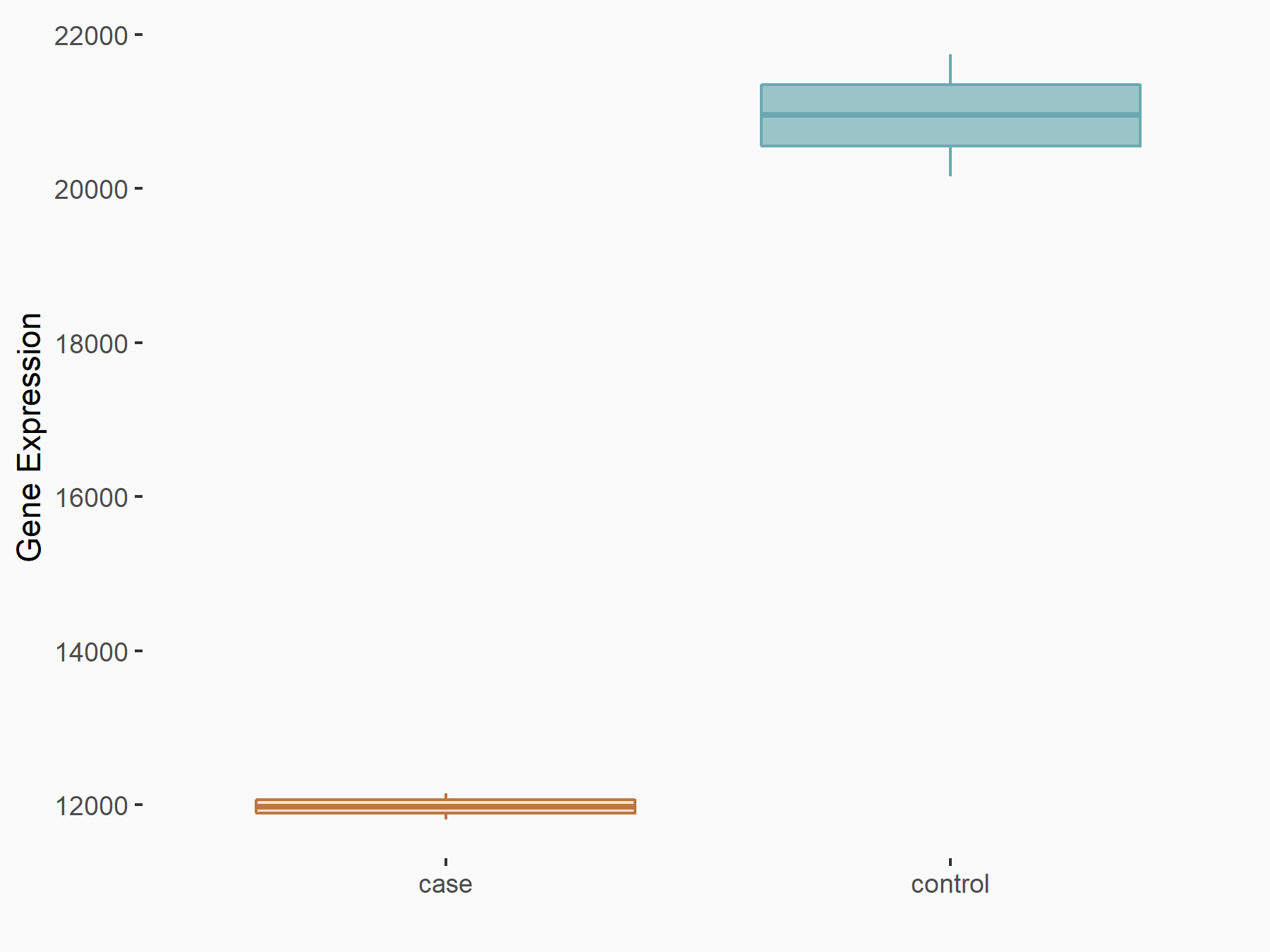  |
logFC: -8.06E-01 p-value: 1.88E-29 |
| More Results | Click to View More RNA-seq Results | |
| Representative RIP-seq result supporting the interaction between TNF and the regulator | ||
| Cell Line | MDA-MB-231 | Homo sapiens |
| Regulation | logFC: 4.17E+00 | GSE60213 |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | Mettl3 inhibitor, S-adenosylhomocysteine promoted the apoptosis and autophagy of chondrocytes with inflammation in vitro and aggravated the degeneration of chondrocytes and subchondral bone in monosodium iodoacetate (MIA) induced temporomandibular joint osteoarthritis mice in vivo. Bcl2 protein interacted with Beclin1 protein in chondrocytes induced by Tumor necrosis factor (TNF/TNF-alpha) stimulation. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through m6A/Ythdf1/Bcl2 signal axis which provides promising therapeutic strategy for temporomandibular joint osteoarthritis. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | B-cell lymphomas | ICD-11: 2A86 | ||
| Pathway Response | Apoptosis | hsa04210 | ||
| Cell Process | Cell proliferation and metastasis | |||
| Cell apoptosis | ||||
| In-vitro Model | ATDC-5 | Mouse teratocarcinoma | Mus musculus | CVCL_3894 |
| In-vivo Model | For MIA + SAH control, S-adenosylhomocysteine (SAH), Mettl3 inhibitor (10 mg/kg) (MCE, NJ, USA) was injected intraperitoneally before MIA injection and maintained twice a week until mice were sacrificed. | |||
Methyltransferase-like 14 (METTL14) [WRITER]
| Representative RNA-seq result indicating the expression of this target gene regulated by METTL14 | ||
| Cell Line | CT26 cell line | Mus musculus |
|
Treatment: METTL14 knockout CT26 cells
Control: CT26 cells
|
GSE142589 | |
| Regulation |
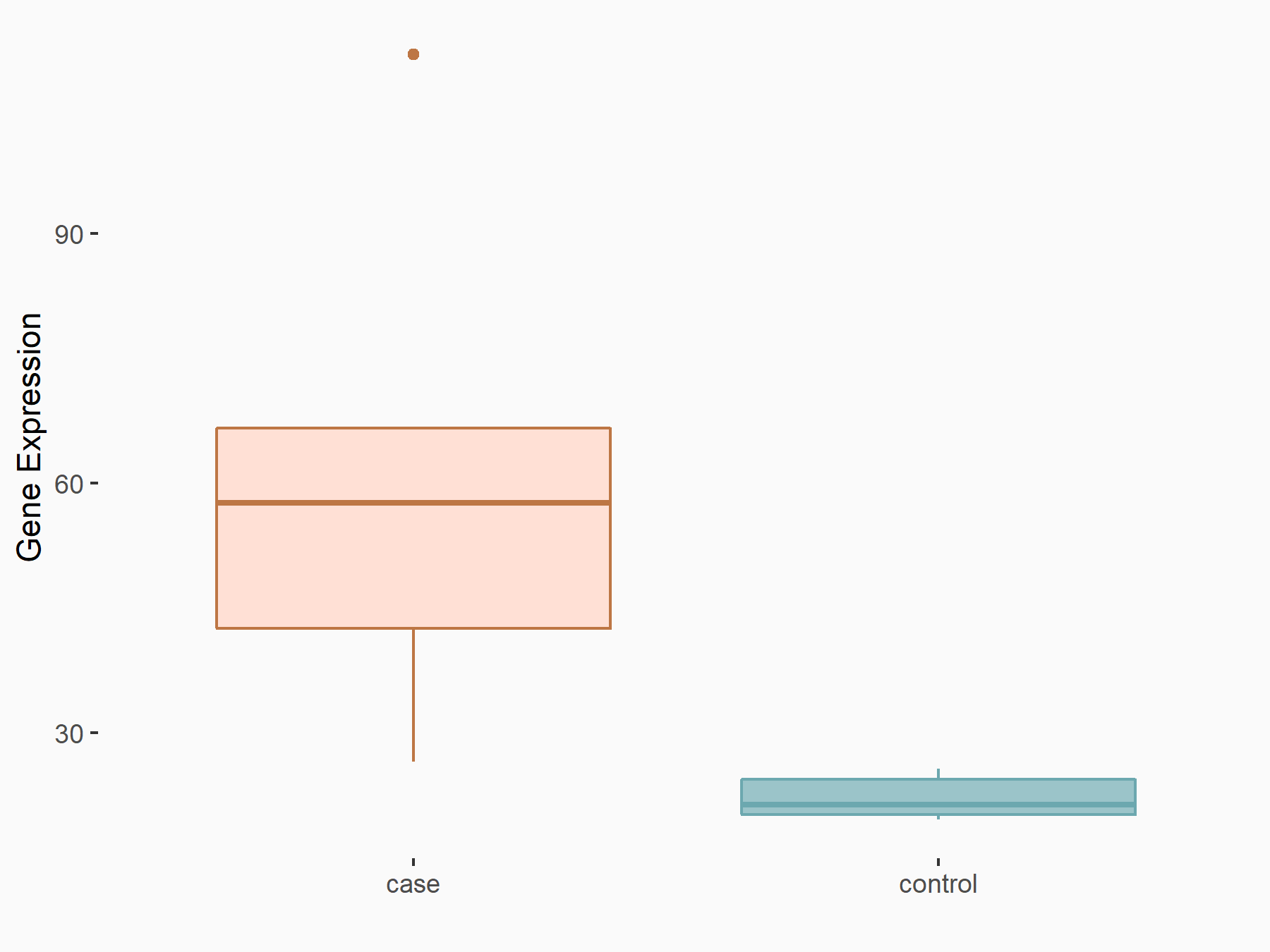  |
logFC: 1.47E+00 p-value: 1.57E-04 |
| More Results | Click to View More RNA-seq Results | |
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [2] | |||
| Response Summary | METTL14 could aggravated high glucose-induced glomerular endothelial cell injury and diabetic nephropathy through m6A modification of alpha-klotho. METTL14 silence decreased the levels of ROS, Tumor necrosis factor (TNF/TNF-alpha) and IL-6 and cell apoptosis. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Chronic kidney disease | ICD-11: GB61.Z | ||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | ||
| AGE-RAGE signaling pathway in diabetic complications | hsa04933 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell apoptosis | |||
| In-vitro Model | HRGECs cell line (Human glomerular microvascular endothelial cells) | |||
| In-vivo Model | After adaptive feeding for 1 week, db/db mice were randomly divided into five groups (n = 6): db/db group, db/db + rAAV group, db/db + rAAV-METTL14 group, db/db + rAAV-klotho group, and db/db + rAAV-METTL14 + rAAV-klotho group. Except db/db group, the other four groups were injected with recombinant adeno-associated virus (rAAV) control, rAAV mediated delivery of METTL14 (rAAV-METTL14), or/and rAAV mediated delivery of klotho (rAAV-klotho) respectively via tail vein. Six db/m mice were chosen as the normal control. | |||
Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) [READER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | N6-methyladenosine (m6A) methylation modification is implicated in the pathogenesis of lung ischemia-reperfusion injury. YTHDF3 or IGF2BP2 knockdown inhibited hypoxia/reoxygenation-activated p38, ERK1/2, AKT, and NF-Kappa-B pathways in BEAS-2B cells, and inhibited p-p65, IL-1-beta and Tumor necrosis factor (TNF/TNF-alpha) secretion. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Gangrene or necrosis of lung | ICD-11: CA43 | ||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Biological regulation | |||
| Cell apoptosis | ||||
| In-vitro Model | BEAS-2B | Normal | Homo sapiens | CVCL_0168 |
| In-vivo Model | After being anesthetized with urethane (i.p.), SD rats were endotracheally intubated and ventilated using an animal ventilator under the conditions: respiratory rate of 70 breaths/min, tidal volume of 20 ml/kg, and inspiratory/expiratory ratio of 1:1. | |||
YTH domain-containing family protein 1 (YTHDF1) [READER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [1] | |||
| Response Summary | Mettl3 inhibitor, S-adenosylhomocysteine promoted the apoptosis and autophagy of chondrocytes with inflammation in vitro and aggravated the degeneration of chondrocytes and subchondral bone in monosodium iodoacetate (MIA) induced temporomandibular joint osteoarthritis mice in vivo. Bcl2 protein interacted with Beclin1 protein in chondrocytes induced by Tumor necrosis factor (TNF/TNF-alpha) stimulation. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through m6A/Ythdf1/Bcl2 signal axis which provides promising therapeutic strategy for temporomandibular joint osteoarthritis. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | B-cell lymphomas | ICD-11: 2A86 | ||
| Pathway Response | Apoptosis | hsa04210 | ||
| Cell Process | Cell proliferation and metastasis | |||
| Cell apoptosis | ||||
| In-vitro Model | ATDC-5 | Mouse teratocarcinoma | Mus musculus | CVCL_3894 |
| In-vivo Model | For MIA + SAH control, S-adenosylhomocysteine (SAH), Mettl3 inhibitor (10 mg/kg) (MCE, NJ, USA) was injected intraperitoneally before MIA injection and maintained twice a week until mice were sacrificed. | |||
YTH domain-containing family protein 3 (YTHDF3) [READER]
| In total 1 item(s) under this regulator | ||||
| Experiment 1 Reporting the m6A Methylation Regulator of This Target Gene | [3] | |||
| Response Summary | N6-methyladenosine (m6A) methylation modification is implicated in the pathogenesis of lung ischemia-reperfusion injury. YTHDF3 or IGF2BP2 knockdown inhibited hypoxia/reoxygenation-activated p38, ERK1/2, AKT, and NF-Kappa-B pathways in BEAS-2B cells, and inhibited p-p65, IL-1-beta and Tumor necrosis factor (TNF/TNF-alpha) secretion. | |||
| Target Regulation | Up regulation | |||
| Responsed Disease | Gangrene or necrosis of lung | ICD-11: CA43 | ||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Biological regulation | |||
| Cell apoptosis | ||||
| In-vitro Model | BEAS-2B | Normal | Homo sapiens | CVCL_0168 |
| In-vivo Model | After being anesthetized with urethane (i.p.), SD rats were endotracheally intubated and ventilated using an animal ventilator under the conditions: respiratory rate of 70 breaths/min, tidal volume of 20 ml/kg, and inspiratory/expiratory ratio of 1:1. | |||
B-cell lymphomas [ICD-11: 2A86]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | Mettl3 inhibitor, S-adenosylhomocysteine promoted the apoptosis and autophagy of chondrocytes with inflammation in vitro and aggravated the degeneration of chondrocytes and subchondral bone in monosodium iodoacetate (MIA) induced temporomandibular joint osteoarthritis mice in vivo. Bcl2 protein interacted with Beclin1 protein in chondrocytes induced by Tumor necrosis factor (TNF/TNF-alpha) stimulation. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through m6A/Ythdf1/Bcl2 signal axis which provides promising therapeutic strategy for temporomandibular joint osteoarthritis. | |||
| Responsed Disease | B-cell lymphomas [ICD-11: 2A86] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Apoptosis | hsa04210 | ||
| Cell Process | Cell proliferation and metastasis | |||
| Cell apoptosis | ||||
| In-vitro Model | ATDC-5 | Mouse teratocarcinoma | Mus musculus | CVCL_3894 |
| In-vivo Model | For MIA + SAH control, S-adenosylhomocysteine (SAH), Mettl3 inhibitor (10 mg/kg) (MCE, NJ, USA) was injected intraperitoneally before MIA injection and maintained twice a week until mice were sacrificed. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | Mettl3 inhibitor, S-adenosylhomocysteine promoted the apoptosis and autophagy of chondrocytes with inflammation in vitro and aggravated the degeneration of chondrocytes and subchondral bone in monosodium iodoacetate (MIA) induced temporomandibular joint osteoarthritis mice in vivo. Bcl2 protein interacted with Beclin1 protein in chondrocytes induced by Tumor necrosis factor (TNF/TNF-alpha) stimulation. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through m6A/Ythdf1/Bcl2 signal axis which provides promising therapeutic strategy for temporomandibular joint osteoarthritis. | |||
| Responsed Disease | B-cell lymphomas [ICD-11: 2A86] | |||
| Target Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Apoptosis | hsa04210 | ||
| Cell Process | Cell proliferation and metastasis | |||
| Cell apoptosis | ||||
| In-vitro Model | ATDC-5 | Mouse teratocarcinoma | Mus musculus | CVCL_3894 |
| In-vivo Model | For MIA + SAH control, S-adenosylhomocysteine (SAH), Mettl3 inhibitor (10 mg/kg) (MCE, NJ, USA) was injected intraperitoneally before MIA injection and maintained twice a week until mice were sacrificed. | |||
Gangrene or necrosis of lung [ICD-11: CA43]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [3] | |||
| Response Summary | N6-methyladenosine (m6A) methylation modification is implicated in the pathogenesis of lung ischemia-reperfusion injury. YTHDF3 or IGF2BP2 knockdown inhibited hypoxia/reoxygenation-activated p38, ERK1/2, AKT, and NF-Kappa-B pathways in BEAS-2B cells, and inhibited p-p65, IL-1-beta and Tumor necrosis factor (TNF/TNF-alpha) secretion. | |||
| Responsed Disease | Gangrene or necrosis of lung [ICD-11: CA43] | |||
| Target Regulator | Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Biological regulation | |||
| Cell apoptosis | ||||
| In-vitro Model | BEAS-2B | Normal | Homo sapiens | CVCL_0168 |
| In-vivo Model | After being anesthetized with urethane (i.p.), SD rats were endotracheally intubated and ventilated using an animal ventilator under the conditions: respiratory rate of 70 breaths/min, tidal volume of 20 ml/kg, and inspiratory/expiratory ratio of 1:1. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [3] | |||
| Response Summary | N6-methyladenosine (m6A) methylation modification is implicated in the pathogenesis of lung ischemia-reperfusion injury. YTHDF3 or IGF2BP2 knockdown inhibited hypoxia/reoxygenation-activated p38, ERK1/2, AKT, and NF-Kappa-B pathways in BEAS-2B cells, and inhibited p-p65, IL-1-beta and Tumor necrosis factor (TNF/TNF-alpha) secretion. | |||
| Responsed Disease | Gangrene or necrosis of lung [ICD-11: CA43] | |||
| Target Regulator | YTH domain-containing family protein 3 (YTHDF3) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | MAPK signaling pathway | hsa04010 | ||
| PI3K-Akt signaling pathway | hsa04151 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Biological regulation | |||
| Cell apoptosis | ||||
| In-vitro Model | BEAS-2B | Normal | Homo sapiens | CVCL_0168 |
| In-vivo Model | After being anesthetized with urethane (i.p.), SD rats were endotracheally intubated and ventilated using an animal ventilator under the conditions: respiratory rate of 70 breaths/min, tidal volume of 20 ml/kg, and inspiratory/expiratory ratio of 1:1. | |||
Dentofacial anomalies [ICD-11: DA0E]
| In total 2 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | Mettl3 inhibitor, S-adenosylhomocysteine promoted the apoptosis and autophagy of chondrocytes with inflammation in vitro and aggravated the degeneration of chondrocytes and subchondral bone in monosodium iodoacetate (MIA) induced temporomandibular joint osteoarthritis mice in vivo. Bcl2 protein interacted with Beclin1 protein in chondrocytes induced by Tumor necrosis factor (TNF/TNF-alpha) stimulation. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through m6A/Ythdf1/Bcl2 signal axis which provides promising therapeutic strategy for temporomandibular joint osteoarthritis. | |||
| Responsed Disease | Temporomandibular joint disorders [ICD-11: DA0E.8] | |||
| Target Regulator | Methyltransferase-like 3 (METTL3) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Apoptosis | hsa04210 | ||
| Cell Process | Cell proliferation and metastasis | |||
| Cell apoptosis | ||||
| In-vitro Model | ATDC-5 | Mouse teratocarcinoma | Mus musculus | CVCL_3894 |
| In-vivo Model | For MIA + SAH control, S-adenosylhomocysteine (SAH), Mettl3 inhibitor (10 mg/kg) (MCE, NJ, USA) was injected intraperitoneally before MIA injection and maintained twice a week until mice were sacrificed. | |||
| Experiment 2 Reporting the m6A-centered Disease Response | [1] | |||
| Response Summary | Mettl3 inhibitor, S-adenosylhomocysteine promoted the apoptosis and autophagy of chondrocytes with inflammation in vitro and aggravated the degeneration of chondrocytes and subchondral bone in monosodium iodoacetate (MIA) induced temporomandibular joint osteoarthritis mice in vivo. Bcl2 protein interacted with Beclin1 protein in chondrocytes induced by Tumor necrosis factor (TNF/TNF-alpha) stimulation. Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through m6A/Ythdf1/Bcl2 signal axis which provides promising therapeutic strategy for temporomandibular joint osteoarthritis. | |||
| Responsed Disease | Temporomandibular joint disorders [ICD-11: DA0E.8] | |||
| Target Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | Apoptosis | hsa04210 | ||
| Cell Process | Cell proliferation and metastasis | |||
| Cell apoptosis | ||||
| In-vitro Model | ATDC-5 | Mouse teratocarcinoma | Mus musculus | CVCL_3894 |
| In-vivo Model | For MIA + SAH control, S-adenosylhomocysteine (SAH), Mettl3 inhibitor (10 mg/kg) (MCE, NJ, USA) was injected intraperitoneally before MIA injection and maintained twice a week until mice were sacrificed. | |||
Chronic kidney disease [ICD-11: GB61]
| In total 1 item(s) under this disease | ||||
| Experiment 1 Reporting the m6A-centered Disease Response | [2] | |||
| Response Summary | METTL14 could aggravated high glucose-induced glomerular endothelial cell injury and diabetic nephropathy through m6A modification of alpha-klotho. METTL14 silence decreased the levels of ROS, Tumor necrosis factor (TNF/TNF-alpha) and IL-6 and cell apoptosis. | |||
| Responsed Disease | Chronic kidney disease [ICD-11: GB61.Z] | |||
| Target Regulator | Methyltransferase-like 14 (METTL14) | WRITER | ||
| Target Regulation | Up regulation | |||
| Pathway Response | NF-kappa B signaling pathway | hsa04064 | ||
| AGE-RAGE signaling pathway in diabetic complications | hsa04933 | |||
| Apoptosis | hsa04210 | |||
| Cell Process | Cell apoptosis | |||
| In-vitro Model | HRGECs cell line (Human glomerular microvascular endothelial cells) | |||
| In-vivo Model | After adaptive feeding for 1 week, db/db mice were randomly divided into five groups (n = 6): db/db group, db/db + rAAV group, db/db + rAAV-METTL14 group, db/db + rAAV-klotho group, and db/db + rAAV-METTL14 + rAAV-klotho group. Except db/db group, the other four groups were injected with recombinant adeno-associated virus (rAAV) control, rAAV mediated delivery of METTL14 (rAAV-METTL14), or/and rAAV mediated delivery of klotho (rAAV-klotho) respectively via tail vein. Six db/m mice were chosen as the normal control. | |||
RNA Modification Sequencing Data Associated with the Target (ID: M6ATAR00432)
| In total 11 m6A sequence/site(s) in this target gene | |||
| mod ID: M6ASITE074347 | Click to Show/Hide the Full List | ||
| mod site | chr6:31575586-31575587:+ | [4] | |
| Sequence | CAGACGCTCCCTCAGCAAGGACAGCAGAGGACCAGCTAAGA | ||
| Motif Score | 3.643047619 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712103 | ||
| mod ID: M6ASITE074348 | Click to Show/Hide the Full List | ||
| mod site | chr6:31575639-31575640:+ | [4] | |
| Sequence | ACTACAGACCCCCCCTGAAAACAACCCTCAGACGCCACATC | ||
| Motif Score | 2.20572619 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712104 | ||
| mod ID: M6ASITE074349 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577542-31577543:+ | [4] | |
| Sequence | ATCATTGCCCTGTGAGGAGGACGAACATCCAACCTTCCCAA | ||
| Motif Score | 3.616982143 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712105 | ||
| mod ID: M6ASITE074350 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577546-31577547:+ | [5] | |
| Sequence | TTGCCCTGTGAGGAGGACGAACATCCAACCTTCCCAAACGC | ||
| Motif Score | 2.951386905 | ||
| Cell/Tissue List | hESC-HEK293T; CD8T | ||
| Seq Type List | MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712106 | ||
| mod ID: M6ASITE074351 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577553-31577554:+ | [4] | |
| Sequence | GTGAGGAGGACGAACATCCAACCTTCCCAAACGCCTCCCCT | ||
| Motif Score | 2.153267857 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712107 | ||
| mod ID: M6ASITE074352 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577670-31577671:+ | [4] | |
| Sequence | GGTCGGAACCCAAGCTTAGAACTTTAAGCAACAAGACCACC | ||
| Motif Score | 3.373380952 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712108 | ||
| mod ID: M6ASITE074353 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577744-31577745:+ | [4] | |
| Sequence | TGCACAGTGAAGTGCTGGCAACCACTAAGAATTCAAACTGG | ||
| Motif Score | 2.153267857 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712109 | ||
| mod ID: M6ASITE074354 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577859-31577860:+ | [4] | |
| Sequence | TCTGGCCAGAATGCTGCAGGACTTGAGAAGACCTCACCTAG | ||
| Motif Score | 4.065041667 | ||
| Cell/Tissue List | CD8T; AML | ||
| Seq Type List | m6A-CLIP/IP; miCLIP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712110 | ||
| mod ID: M6ASITE074355 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577869-31577870:+ | [4] | |
| Sequence | ATGCTGCAGGACTTGAGAAGACCTCACCTAGAAATTGACAC | ||
| Motif Score | 2.876744048 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712111 | ||
| mod ID: M6ASITE074356 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577886-31577887:+ | [5] | |
| Sequence | AAGACCTCACCTAGAAATTGACACAAGTGGACCTTAGGCCT | ||
| Motif Score | 2.859755952 | ||
| Cell/Tissue List | hESC-HEK293T; CD8T | ||
| Seq Type List | MAZTER-seq; m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712112 | ||
| mod ID: M6ASITE074357 | Click to Show/Hide the Full List | ||
| mod site | chr6:31577896-31577897:+ | [4] | |
| Sequence | CTAGAAATTGACACAAGTGGACCTTAGGCCTTCCTCTCTCC | ||
| Motif Score | 3.622404762 | ||
| Cell/Tissue List | CD8T | ||
| Seq Type List | m6A-CLIP/IP | ||
| Transcript ID List | ENST00000449264.3 | ||
| External Link | RMBase: m6A_site_712113 | ||
References