m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05838
|
[1] | |||
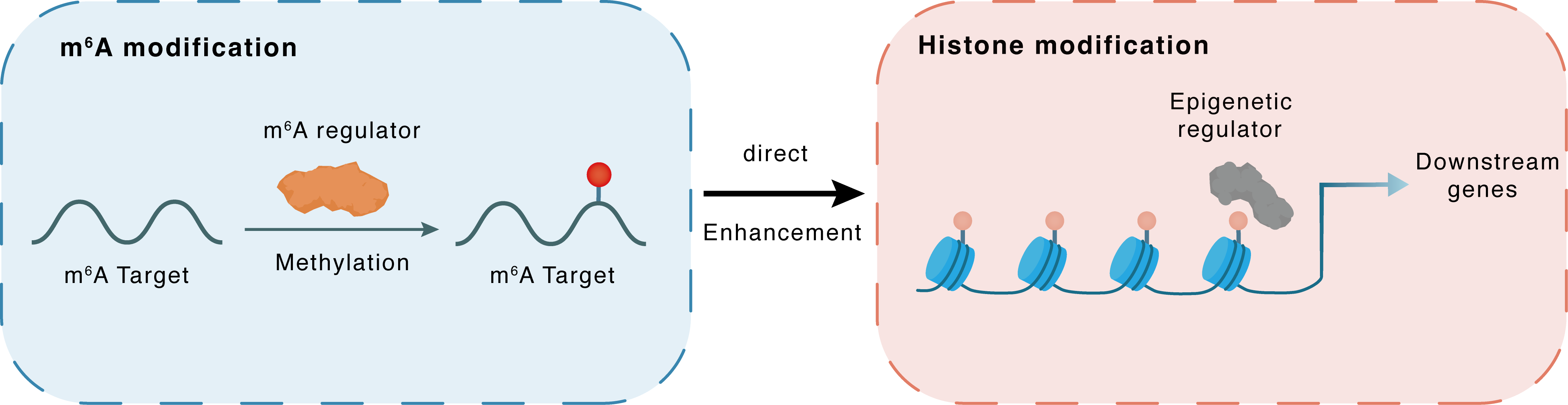 m6A modification
ACSS3
ACSS3
METTL3
Methylation
m6A modification
ACSS3
ACSS3
METTL3
Methylation
 : m6A sites
Indirect
Enhancement
Histone modification
H3K14pr
Regulated Regulator
FASN : m6A sites
Indirect
Enhancement
Histone modification
H3K14pr
Regulated Regulator
FASN
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Acyl-CoA synthetase short-chain family member 3, mitochondrial (ACSS3) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Histone modification (HistMod) | ||||
| Regulated Target | Histone H3 lysine 14 propionylation (H3K14pr) | View Details | |||
| Downstream Gene | FASN | View Details | |||
| Crosstalk Relationship | m6A → Histone modification | Enhancement | |||
| Crosstalk Mechanism | m6A modification indirectly regulates histone modification through downstream signaling pathways | ||||
| Crosstalk Summary | RPN11 deubiquitinates and stabilizes METTL3 to enhance the m6A modification and expression of acyl-coenzyme A (CoA) synthetase short-chain family member 3 (Acyl-CoA synthetase short-chain family member 3, mitochondrial (ACSS3)), which generates propionyl-CoA to upregulate lipid metabolism genes (Acacb, FASN, Me1, Mid1ip1) via Histone H3 lysine 14 propionylation (H3K14pr). The RPN11-METTL3-ACSS3-histone propionylation pathway is activated in the livers of patients with NAFLD. | ||||
| Responsed Disease | Nonalcoholic fatty liver disease | ICD-11: DB92.Z | |||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Fatty acid synthsae (FASN) | 20 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Cerulenin | Approved | [2] | ||
| Synonyms |
Helicocerin; Cerulenin, Cephalosporium caerulens; Oxiranecarboxamide, 3-(1-oxo-4,7-nonadienyl)-, (2R-(2-alpha,3-alpha(4E,7E)))-(9CI); (2R,3S)-2,3-Epoxy-4-oxo-7E,10E-dodecadienamide; (2R,3S)-3-((4E,7E)-Nona-4,7-dienoyl)-oxirane-2-carboxylic acid amide; (2R,3S)-3-((4E,7E)-nona-4,7-dienoyl)oxirane-2-carboxamide; (2R,3S)-3-[(4E,7E)-nona-4,7-dienoyl]oxirane-2-carboxamide; (2R,3S)-3-nona-4,7-dienoyloxirane-2-carboxamide; (2R,3S,E,E)-2,3-Epoxy-4-oxo-7,10-dodecadienamide; (2R-(2alpha,3alpha(4E,7E)))-3-(1-Oxonona-4,7-dienyl)oxirane-2-carboxamide; (2S)(3R)-2,3-Epoxy-4-oxo-7,10-dodecadienoylamide; (2S,3R)-2,3-epoxy-4-oxy-7,10-dodecadienoylamide; (2r,3s)-3-(nona-4,7-dienoyl)oxirane-2-carboxamide; 2,3-Epoxy-4-oxo-7,10-dodecadienamide; 2,3-Epoxy-4-oxo-7,10-dodecadienoylamide; 3-(1-Oxo-4,7-nonadienyl)oxiranecarboxamide; 3-[(4E,7E)-nona-4,7-dienoyl]oxirane-2-carboxamide; 3-nona-4,7-dienoyloxirane-2-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.19 ug.mL-1 | |||
| External Link | ||||
| Epigallocatechin gallate | Phase 3 | [3] | ||
| Synonyms |
(-)-Epigallocatechin gallate; EGCG; 989-51-5; Epigallocatechin 3-gallate; Epigallocatechin-3-gallate; Tea catechin; (-)-Epigallocatechin-3-o-gallate; Teavigo; Epigallocatechin-3-monogallate; (-)-Epigallocatechol gallate; (2R,3R)-5,7-Dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl 3,4,5-trihydroxybenzoate; Catechin deriv; UNII-BQM438CTEL; Green tea extract; CCRIS 3729; (-)-epigallocatechin 3-gallate; C22H18O11; BQM438CTEL; (2R,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl; EGCG analogs; EGCG, Anagen; Epigallocatechin gallate analogs, Anagen; Epigallocatechin gallate, Anagen; GTPs,Anagen; Green tea polyphenols, Anagen; EPIGALOCATECHIN GALLATE
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 51970 nM | |||
| External Link | ||||
| TVB-2640 | Phase 2 | [4] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| TVB-2640 | Phase 2 | [5] | ||
| Synonyms |
FASN-IN-2; 1399177-37-7; 4-(1-(4-Cyclobutyl-2-methyl-5-(5-methyl-4H-1,2,4-triazol-3- yl)benzoyl)piperidin-4-yl)benzonitrile; US8871790, 480; CHEMBL3661754; SCHEMBL12488853; BDBM137084; BCP30428; EX-A3643; s9714; ZINC150188638; HY-112829; CS-0066310; TVB2640; TVB 2640;FASN-IN-2; US8871790, 152; 4-(1-(4-Cyclobutyl-2-methyl-5-(3-methyl-1H-1,2,4-triazol-5-yl)benzoyl)piperidin-4-yl)benzonitrile; 4-(1-(4-cyclobutyl-2-methyl-5-(5-methyl-4H-1,2,4-triazol-3-yl)benzoyl)piperidin-4-yl)benzonitrile; 4-[1-[4-cyclobutyl-2-methyl-5-(5-methyl-1H-1,2,4-triazol-3-yl)benzoyl]piperidin-4-yl]benzonitrile
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FAS1 | Preclinical | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| FSA2 | Preclinical | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| GSK2194069 | Investigative | [7] | ||
| Synonyms |
GSK-2194069; GSK 2194069
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MT-061 | Investigative | [8] | ||
| Synonyms |
Fatty acid biosynthesis inhibitor (Gram positive bacterial infection), MaxThera; MT-061 lead series, Biota; MT-061 lead series, MaxThera
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Anti-Fas mabs | Investigative | [8] | ||
| Synonyms |
Anti-Fas mAbs (misfolded proteins, cancer); Anticancer monoclonal antibodies (misfolded Fas receptor-targeting), Amorfix/Apexigen; Anticancer monoclonal antibodies (misfolded Fas receptor-targeting), Amorfix/Epitomics; Anti-Fas mAbs (misfolded proteins, cancer), Amorfix/Apexigen
Click to Show/Hide
|
|||
| External Link | ||||
| MG-28 | Investigative | [8] | ||
| Synonyms |
FAS inhibitor (cancer), Institut Catala d'Oncologia; Fatty acid synthase inhibitor (cancer), Institut Catala d'Oncologia
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-hydroxy-8-nitro-3-phenylquinolin-2(1H)-one | Investigative | [9] | ||
| Synonyms |
CHEMBL377646; SCHEMBL4456699; BDBM50189477; 2(1H)-Quinolinone, 4-hydroxy-8-nitro-3-phenyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-hydroxy-6-nitro-3-phenylquinolin-2(1H)-one | Investigative | [9] | ||
| Synonyms |
CHEMBL413773; SCHEMBL4469573; ZINC36185238; BDBM50189455
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1403 nM | |||
| External Link | ||||
| FAS-031 | Investigative | [8] | ||
| Synonyms |
FASi; FAS inhibitors (cancer); FAS-093; FAS-31; FAS-93; FAS inhibitors (cancer), FASgen/Johns Hopkins; Fatty acid synthase inhibitors (cancer), FASgen/Johns Hopkins; FAS inhibitors (cancer), FASgen/Johns Hopkins/Johnson & Johnson; Fatty acid synthase inhibitors (cancer), FASgen/Johns Hopkins/ Johnson & Johnson
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| C75 | Investigative | [10] | ||
| Synonyms |
C75 trans; C75 (trans); 191282-48-1; 3-Carboxy-4-octyl-2-methylenebutyrolactone; CHEMBL449993; trans-4-Carboxy-5-octyl-3-methylenebutyrolactone; Fatty Acid Synthase Inhibitor, C75; (+)-trans-C75; C75 (racemic); SCHEMBL3007085; CTK8E7727; MolPort-005-933-439; HMS3649D16; ZINC2009913; BCP11074; HY-12364A; BDBM50256128; trans-Tetrahydro-3-methylene-2-oxo-5-n-octyl-4-furancarboxylic acid; AKOS015960616; VC30664; CS-3561; AC-11808; RT-011885; SR-01000946704; SR-01000946704-1; J-012362; UNII-8E9A8CTX2H component VCWLZDVWHQVAJU-NEPJUHHUSA-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-Hexadecynoic acid | Investigative | [11] | ||
| Synonyms |
hexadec-2-ynoic acid; 2-Hexadecynoate; N-2-Hexadecynoic acid; AC1L45K9; SCHEMBL2818253; CTK4G1266; DTXSID00182577; MECFGCCEVOFCNS-UHFFFAOYSA-N; NSC289580; LMFA01030494
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GALLOCATECHIN GALLATE | Investigative | [3] | ||
| Synonyms |
(-)-Gallocatechin gallate; 4233-96-9; (-)-Gallocatechol gallate; UNII-IRW3C4Y31Q; (2S,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)chroman-3-yl 3,4,5-trihydroxybenzoate; Gallocatechin gallate (Gcg); IRW3C4Y31Q; CHEMBL264938; (-)-gallocatechin-3-O-gallate; (2S,3R)-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-(3,4,5-trihydroxybenzoate); [(2S,3R)-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-chromen-3-yl] 3,4,5-trihydroxybenzoate; Benzoic acid, 3,4,5-trihydroxy-, 3,
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3,7,3',4'-TETRAHYDROXYFLAVONE | Investigative | [3] | ||
| Synonyms |
Fisetin; 528-48-3; 2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4-one; 5-Desoxyquercetin; Fustel; Cotinin; Viset; 3,3',4',7-Tetrahydroxyflavone; Fisetholz; Superfustel; Fustet; Fietin; Junger fustik; Ventin sumach; Zante fustic; Young fustic; Superfustel K; Ungarisches gelbholz; CI Natural Brown 1; Young fustic crystals; Bois bleu de Honqrie; BOIS bleude honqrie; CI 75620; NSC 407010; NSC 656275; 5-Deoxyquercetin; 2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-4H-1-benzopyran-4-one; Natural Brown 1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (-)-CATECHINGALLATE | Investigative | [3] | ||
| Synonyms |
(-)-Catechin gallate; 130405-40-2; (2S,3R)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl 3,4,5-trihydroxybenzoate; catechin gallate; UNII-0KT1FO6VO6; ent-Catechin 3-O-gallate; 0KT1FO6VO6; CHEMBL129451; CHEBI:76131; (2S,3R)-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl 3,4,5-trihydroxybenzoate; (2S,3R)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-(3,4,5-trihydroxybenzoate); Catechin gallate, (-)-; CCRIS 9303; Catechin gallateCG
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| biochanin A | Investigative | [3] | ||
| Synonyms |
491-80-5; Biochanin; 4'-Methylgenistein; 5,7-Dihydroxy-4'-methoxyisoflavone; 5,7-Dihydroxy-3-(4-methoxyphenyl)-4H-chromen-4-one; Genistein 4-methyl ether; 5,7-Dihydrox -4'-methoxyisoflavone; Biochanine A; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-3-(4-methoxyphenyl)-; olmelin; Pratensol; NSC 123538; Biochanin-A; 5,7-dihydroxy-3-(4-methoxyphenyl)chromen-4-one; 4-Methylgenistein; C16H12O5; UNII-U13J6U390T; CCRIS 5449; 5,7-Dihydroxy-3-p-methoxyphenyl-4H-chromen-4-one; EINECS 207-744-7; NSC123538; Genistein 4'-methyl ether
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MORIN | Investigative | [3] | ||
| Synonyms |
480-16-0; Aurantica; Calico Yellow; Al-Morin; Toxylon Pomiferum; Morin hydrate; 2',3,4',5,7-Pentahydroxyflavone; 2-(2,4-Dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one; Bois d,Arc; Osage Orange; Osage Orange Extract; Bois d'arc; C.I. Natural Yellow 11; C.I. Natural Yellow 8; Osage Orange Crystals; 3,5,7,2',4'-Pentahydroxyflavone; 3,5,7,2',4'-Pentahydroxyflavonol; 2'-Hydroxypelargidenolon 1522; 2',4',3,5,7-Pentahydroxyflavone; C.I. 75660; Zlut prirodni 11; 2',4',5,7-Tetrahydroxyflavan-3-ol; Bois d'arc [French]
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| DB92: Non-alcoholic fatty liver disease | 12 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Epeleuton | Phase 2 | [12] | ||
| Synonyms |
(S,5Z,8Z,11Z,13E,17Z)-Ethyl 15-hydroxyicosa-5,8,11,13,17-pentaenoate; 15(S)-HEPE-EE; 15(S)-HYDROXY-(5Z,8Z,11Z,13E,17Z)-EICOSAPENTAENOIC ACID ETHYL ESTER; 1667760-39-5; 5,8,11,13,17-Eicosapentaenoic acid, 15-hydroxy-, ethyl ester, (5Z,8Z,11Z,13E,15S,17Z)-; AKOS040748327; CHEMBL5095178; Epeleuton; Epeleuton [INN]; FA9BPX1T6V; UNII-FA9BPX1T6V
Click to Show/Hide
|
|||
| External Link | ||||
| IMM-124E | Phase 2 | [13] | ||
| External Link | ||||
| TVB-2640 | Phase 2 | [14] | ||
| Synonyms |
FASN-IN-2; 1399177-37-7; 4-(1-(4-Cyclobutyl-2-methyl-5-(5-methyl-4H-1,2,4-triazol-3- yl)benzoyl)piperidin-4-yl)benzonitrile; US8871790, 480; CHEMBL3661754; SCHEMBL12488853; BDBM137084; BCP30428; EX-A3643; s9714; ZINC150188638; HY-112829; CS-0066310; TVB2640; TVB 2640;FASN-IN-2; US8871790, 152; 4-(1-(4-Cyclobutyl-2-methyl-5-(3-methyl-1H-1,2,4-triazol-5-yl)benzoyl)piperidin-4-yl)benzonitrile; 4-(1-(4-cyclobutyl-2-methyl-5-(5-methyl-4H-1,2,4-triazol-3-yl)benzoyl)piperidin-4-yl)benzonitrile; 4-[1-[4-cyclobutyl-2-methyl-5-(5-methyl-1H-1,2,4-triazol-3-yl)benzoyl]piperidin-4-yl]benzonitrile
Click to Show/Hide
|
|||
| External Link | ||||
| Vupanorsen | Phase 2 | [15] | ||
| Synonyms |
IONIS-ANGPTL3-LRx; AKCEA-ANGPTL3-LRx
Click to Show/Hide
|
|||
| External Link | ||||
| ZED1227 | Phase 2 | [16] | ||
| Synonyms |
(2E,6S)-7-((1-(2-((2-Ethylbutyl)amino)-2-oxoethyl)-1,2-dihydro-2-oxo-3-pyridinyl)amino)-6-(((1-methyl-1H-imidazol-5-yl)carbonyl)amino)-7-oxo-2-heptenoic acid methyl ester; (2E,6S)-7-[[1-[2-[(2-Ethylbutyl)amino]-2-oxoethyl]-1,2-dihydro-2-oxo-3-pyridinyl]amino]-6-[[(1-methyl-1H-imidazol-5-yl)carbonyl]amino]-7-oxo-2-heptenoic Acid Methyl Ester; 1542132-88-6; 2-Heptenoic acid, 7-((1-(2-((2-ethylbutyl)amino)-2-oxoethyl)-1,2-dihydro-2-oxo-3-pyridinyl)amino)-6-(((1-methyl-1H-imidazol-5-yl)carbonyl)amino)-7-oxo-, methyl ester, (2E,6S)-; 2-Heptenoic acid, 7-[[1-[2-[(2-ethylbutyl)amino]-2-oxoethyl]-1,2-dihydro-2-oxo-3-pyridinyl]amino]-6-[[(1-methyl-1H-imidazol-5-yl)carbonyl]amino]-7-oxo-, methyl ester, (2E,6S)-; AKOS040742843; BDBM50245478; CHEMBL4081588; CS-0015432; EX-A7845R; GLUTAMINASE; GTPL12802; HY-19359; Methyl (2E,6S)-7-((1-(2-((2-ethylbutyl)amino)-2-oxoethyl)-1,2-dihydro-2-oxo-3-pyridinyl)amino)-6-(((1-methyl-1H-imidazol-5-yl)carbonyl)amino)-7-oxo-2-heptenoate; Methyl (2E,6S)-7-[[1-[2-[(2-ethylbutyl)amino]-2-oxoethyl]-1,2-dihydro-2-oxo-3-pyridinyl]amino]-6-[[(1-methyl-1H-imidazol-5-yl)carbonyl]amino]-7-oxo-2-heptenoate; Methyl (E,6S)-7-((1-(2-(2-ethylbutylamino)-2-oxo-ethyl)-2-oxo-3-pyridyl)amino)-6-((3-methylimidazole-4-carbonyl)amino)-7-oxo-hept-2-enoate; methyl (E,6S)-7-[[1-[2-(2-ethylbutylamino)-2-oxoethyl]-2-oxopyridin-3-yl]amino]-6-[(3-methylimidazole-4-carbonyl)amino]-7-oxohept-2-enoate; methyl (S,E)-7-((1-(2-((2-ethylbutyl)amino)-2-oxoethyl)-2-oxo-1,2-dihydropyridin-3-yl)amino)-6-(1-methyl-1H-imidazole-5-carboxamido)-7-oxohept-2-enoate; MS-29784; SCHEMBL16735736; SCHEMBL16751074; T4SR539YKF; TAK-227; UNII-T4SR539YKF; ZED1227; ZED-1227
Click to Show/Hide
|
|||
| External Link | ||||
| PXL-770 | Phase 2 | [17] | ||
| External Link | ||||
| ASP9831 | Phase 2 | [18] | ||
| External Link | ||||
| Netoglitazone | Phase 2 | [19] | ||
| Synonyms |
Isaglitazone; Netoglitazone [USAN]; MCC 555; MCC-555; RWJ-241947; Netoglitazone (USAN/INN); 5-((6-((2-fluorophenyl)methoxy)-2-naphthalenyl)methyl)-2,4-thiazolidinedione; 5-({6-[(2-fluorobenzyl)oxy]naphthalen-2-yl}methyl)-1,3-thiazolidine-2,4-dione; 5-[[6-[(2-fluorophenyl)methoxy]naphthalen-2-yl]methyl]-1,3-thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| RG-125 | Phase 1 | [20] | ||
| Synonyms |
AZD4076
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-677954 | Discontinued in Phase 2 | [21] | ||
| Synonyms |
SCHEMBL2065429
Click to Show/Hide
|
|||
| External Link | ||||
| KD-3020 | Preclinical | [21] | ||
| External Link | ||||
| RIPA-56 | Investigative | [22] | ||
| Synonyms |
1956370-21-0; N-benzyl-N-hydroxy-2,2-dimethylbutanamide; CHEMBL4092421; GTPL9643; SCHEMBL17874088; EX-A4338; BDBM50229025; MFCD30738006; s6511; ZINC616570725; CS-6266; compound 92 [WO2016101885]; compound 56 [PMID: 27992216]; HY-101032; C(C1=CC=CC=C1)N(C(C(CC)(C)C)=O)O; A1-28956
Click to Show/Hide
|
|||
| External Link | ||||
References