m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05833
|
[1] | |||
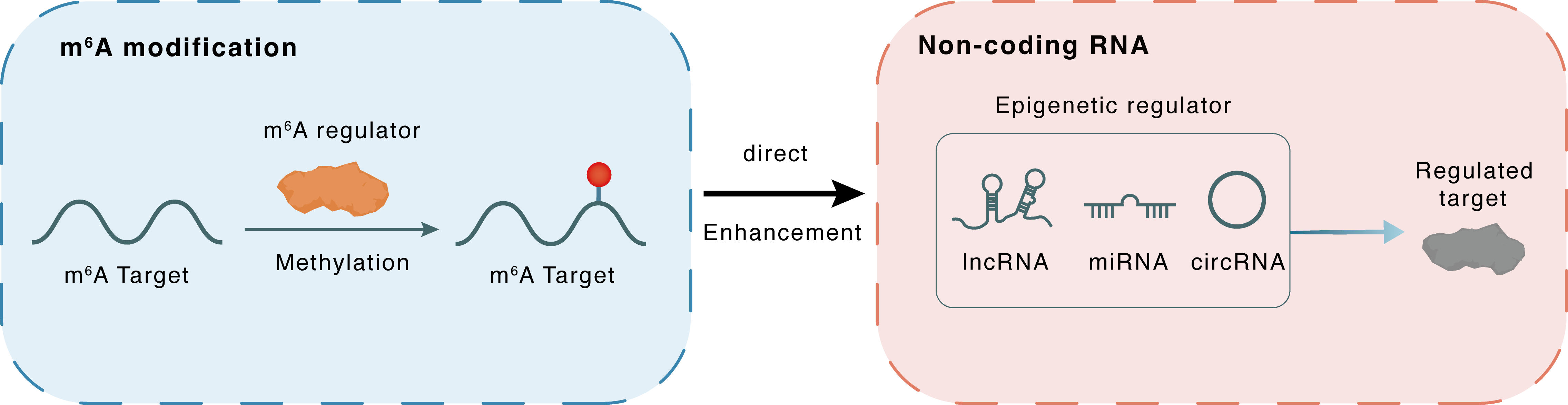 m6A modification
SREBF2-AS1
SREBF2-AS1
METTL3
Methylation
m6A modification
SREBF2-AS1
SREBF2-AS1
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
SREBF2-AS1
FXR1
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
SREBF2-AS1
FXR1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | SREBF2 antisense RNA 1 (SREBF2-AS1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | SREBF2 antisense RNA 1 (SREBF2-AS1) | LncRNA | View Details | ||
| Regulated Target | FMR1 autosomal homolog 1 (FXR1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | METTL3 and METTL14-induced m6A modification upregulated SREBF2 antisense RNA 1 (SREBF2-AS1) expression through increasing SREBF2-AS1 transcript stability. m6A-modified SREBF2-AS1 bound and recruited m6A reader FMR1 autosomal homolog 1 (FXR1) and DNA 5-methylcytosine dioxygenase TET1 to SREBF2 promoter, leading to DNA demethylation at SREBF2 promoter and the upregulation of SREBF2 transcription. | ||||
| Responsed Disease | Acute myeloid leukaemia | ICD-11: 2A60 | |||
| Responsed Drug | Chidamide | ||||
In-vitro Model |
HL-60 | Adult acute myeloid leukemia | Homo sapiens | CVCL_0002 | |
| HEL | Erythroleukemia | Homo sapiens | CVCL_0001 | ||
| MOLM-13 | Adult acute myeloid leukemia | Homo sapiens | CVCL_2119 | ||
| MV4-11 | Childhood acute monocytic leukemia | Homo sapiens | CVCL_0064 | ||
| Kasumi-1 | Myeloid leukemia with maturation | Homo sapiens | CVCL_0589 | ||
|
HS-5
|
N.A. | Homo sapiens | CVCL_3720 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2A60: Acute myeloid leukaemia | 283 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Atezolizumab | Approved | [2] | ||
| External Link | ||||
| ENASIDENIB MESYLATE | Approved | [3] | ||
| Synonyms |
UNII-UF6PC17XAV; AG-221 mesylate; Enasidenib (mesylate); UF6PC17XAV; Enasidenib mesylate [USAN]; Enasidenib mesylate (USAN); Enasidenib mesilate; Enasidenib methanesulfonate; Idhifa (TN); SCHEMBL16448052; HY-18690A; CS-7541
Click to Show/Hide
|
|||
| External Link | ||||
| Lestaurtinib | Approved (orphan drug) | [4] | ||
| Synonyms |
A 1544750; CEP 701; KT 5555; KT5555; SP 924; CEP-701; KT-5555; SPM-924; Lestaurtinib (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| Motixafortide | Approved | [5] | ||
| External Link | ||||
| ARN-509 | Approved | [6] | ||
| Synonyms |
Arn-509 (AR inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| DEOXYCYTIDINE | Approved | [7] | ||
| Synonyms |
Cytosine deoxyribonucleoside; 2'-dC; bmse000323; ACMC-209rv6; CYTIDINE, 2'-DEOXY-; Cytosine deoxy nucleoside hydrochloride; 4-amino-1-[4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2(1H)-one; Desoxycytidine; 4-amino-1-(2-deoxypentofuranosyl)pyrimidin-2(1H)-one; 4-amino-1-[4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl]pyrimidin-2-one; 3h-deoxycytidine; 4-amino-1-(4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one; 40093-94-5; AC1L19OG; TimTec1_003892; NCIOpen2_004589; Oprea1_817993
Click to Show/Hide
|
|||
| External Link | ||||
| Bestatin | Approved | [8] | ||
| Synonyms |
Bestatin (TN); CHEBI:3070; UPCMLD-DP116; CHEMBL476869; SureCN25971; Ubenimex (JP16/INN); Ubenimex (JP17/INN); SCHEMBL25971; GTPL5151; AC1L972Z; UPCMLD-DP116:001; DTXSID4048430; CHEBI:140702; ZINC1532730; ZINC01532730; BDBM50010142; AKOS026750073; NCGC00161660-01; C00732; D00087; ((2S,3R)-3-amino-2-hydroxy-4-phenylbutanoyl)-D-leucine; (2R)-2-[[(2S,3R)-3-amino-2-hydroxy-4-phenylbutanoyl]amino]-4-methylpentanoic acid; (2R)-2-[[(2S,3R)-3-amino-2-hydroxy-4-phenyl-butanoyl]amino]-4-methyl-pentanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Tagraxofusp | Approved | [6] | ||
| Synonyms |
Tagraxofusp [INN]; Molecule 129; Tagraxofusp [USAN]; UNII-8ZHS5657EH; 8ZHS5657EH; Diphtheria toxin-il-3 fusion protein targeting IL-3 receptor
Click to Show/Hide
|
|||
| External Link | ||||
| Uracil mustard | Approved | [9] | ||
| Synonyms |
Chlorethaminacil; Demethyldopan; Desmethyldopan; Nordopan; Uracillost; Uracilmostaza; Uramustin; Uramustina; Uramustine; Uramustinum; Aminouracil mustard; Uracil lost; Uracil lost [German]; Uracil mustard [USAN]; Uracil nitrogen mustard; ENT 50439; U 8344; CB-4835; SK-19849; U-8344; Uracil mustard (TN); Uracil mustard (USAN); Uramustina [INN-Spanish]; Uramustine (INN); Uramustinum [INN-Latin]; URACIL MUSTARD (500 MG) (FOR U.S. SALE ONLY); 2,6-Dihydroxy-5-bis(2-chloroethyl)aminopyrimidine; 2,6-Dihydroxy-5-bis[2-chloroethyl]aminopyrimidine; 5-(Bis(2-chlorethyl)amino)-2,4(1H,3H)pyrimidinedione; 5-(Bis(2-chloroethyl)amino)-2,4(1H,3H)pyrimidinedione; 5-(Bis(2-chloroethyl)amino)-2,4-(1H,3H)pyrimidinedione; 5-(Bis(2-chloroethyl)amino)uracil; 5-(Di-(beta-chloroethyl)amino)uracil; 5-(Di-2-chloroethyl)aminouracil; 5-Aminouracil mustard; 5-N,N-Bis(2-chloroethyl)aminouracil; 5-[Bis(2-chlorethyl)amino]-2,4(1H,3H)pyrimidinedione; 5-[Bis(2-chloroethyl)amino]uracil; 5-[Di(2-chloroethyl)amino]uracil; 5-[Di(beta-chloroethyl)amino]uracil; 5-[Di(beta.-chloroethyl)amino]uracil; 5-[bis(2-chloroethyl)amino]-1H-pyrimidine-2,4-dione; 5-[bis(2-chloroethyl)amino]-2,4(1H,3H)-pyrimidinedione; 5-[bis(2-chloroethyl)amino]pyrimidine-2,4(1H,3H)-dione; 5-[bis(2-chloroethyl)amino]pyrimidine-2,4-diol
Click to Show/Hide
|
|||
| External Link | ||||
| Tisagenlecleucel | Application submitted | [10] | ||
| Synonyms |
Tisagenlecleucel-T
Click to Show/Hide
|
|||
| External Link | ||||
| Olutasidenib | Approved | [2] | ||
| Synonyms |
NEQYWYXGTJDAKR-JTQLQIEISA-N; Olutasidenib; UNII-0T4IMT8S5Z; 0T4IMT8S5Z; (S)-5-((1-(6-chloro-2-oxo-1,2-dihydroquinolin-3-yl)ethyl)amino)-1-methyl-6-oxo-1,6-dihydropyridine-2-carbonitrile; Olutasidenib [USAN]; SCHEMBL17603134; HY-114226; CS-0080183; 5-(((1S)-1-(6-chloro-2-oxo-1,2-dihydroquinolin-3-yl)ethyl)amino)-1-methyl-6-oxo-1,6-dihydropyridine-2-carbonitrile; 2-Pyridinecarbonitrile, 5-(((1S)-1-(6-chloro-1,2-dihydro-2-oxo-3-quinolinyl)ethyl)amino)-1,6-dihydro-1-methyl-6-oxo-; 1887014-12-1
Click to Show/Hide
|
|||
| External Link | ||||
| Midostaurin | Approved | [11] | ||
| Synonyms |
PKC412; 120685-11-2; Cgp 41251; 4'-N-Benzoylstaurosporine; CGP-41251; Benzoylstaurosporine; PKC-412; RYDAPT; PKC 412; UNII-ID912S5VON; N-Benzoylstaurosporine; ID912S5VON; CHEMBL608533; CHEBI:63452; Cgp 41 251; N-[(5S,6R,7R,9R)-6-methoxy-5-methyl-14-oxo-6,7,8,9,15,16-hexahydro-5H,14H-5,9-epoxy-4b,9a,15-triazadibenzo[b,h]cyclonona[1,2,3,4-jkl]cyclopenta[e]-as-indacen-7-yl]-N-methylbenzamide; PKC-412(Midostaurin); Midostaurin (PKC412); Midostaurin (USAN/INN); Midostaurin [USAN:INN]; CGP 41231; Rydapt (TN); CPG 41251
Click to Show/Hide
|
|||
| External Link | ||||
| Quizartinib | Approved | [12] | ||
| Synonyms |
Quizartinib
Click to Show/Hide
|
|||
| External Link | ||||
| Ivosidenib | Approved | [12] | ||
| Synonyms |
UNII-Q2PCN8MAM6; Q2PCN8MAM6; Ivosidenib [INN]; Ivosidenib [USAN]; Ivosidenib [WHO-DD]; GTPL9217; SCHEMBL15122512; EX-A992; MolPort-044-560-317; RG120; s8206; 1448347-49-6 (Ivosidenib); AKOS028113340; ZINC205136523; CS-5122; AS-35058; HY-18767
Click to Show/Hide
|
|||
| External Link | ||||
| Gemtuzumab ozogamicin | Approved | [13] | ||
| Synonyms |
Mylotarg (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Gilteritinib | Approved | [6] | ||
| Synonyms |
UNII-66D92MGC8M; 66D92MGC8M; Gilteritinib [USAN:INN]; Gilteritinib(ASP2215); Gilteritinib (USAN/INN); Gilteritinib (ASP2215); Gilteritinib (ASP-2215); SCHEMBL282229; GTPL8708; MolPort-038-934-933; BDBM144315; C29H44N8O3; 3694AH; s7754; AKOS030234455; ZINC113476229; DB12141; CS-3885; KS-0000064E; AS-35199
Click to Show/Hide
|
|||
| External Link | ||||
| Thioguanine | Approved | [14] | ||
| Synonyms |
6-thioguanine; Lanvis; THG; Tabloid; ThioguaninGSK; Tioguanin; Tioguanina; Tioguanine; Tioguaninum; Glaxo Wellcome Brand of Thioguanine; Glaxo Wellcome Brand of Tioguanine; GlaxoSmithKline Brand of Thioguanine; GlaxoSmithKline Brand of Tioguanine; Thioguanin GSK; Thioguanine Hemihydrate; Thioguanine Monosodium Salt; Thioguanine Tabloid; Tioguanina Wellcome; Tioguanine GlaxoSmithKline Brand; Wellcome Brand of Thioguanine; BW 5071; DX4; LT00455187; Wellcome U3B; Lanvis (TN); Thioguanin-GSK; Thioguanine [USAN:BAN]; Tioguanina[INN-Spanish]; Tioguanine (INN); Tioguaninum [INN-Latin]; Purine antimetabolite: antimetabolite: inhibits nucleic acid replication; Guanine, thio-(VAN); 2 Amino 6 Purinethiol; 2-Amino 6MP; 2-Amino-1,7-dihydro-6H-purin-6-thion; 2-Amino-1,7-dihydro-6H-purin-6-thion [Czech]; 2-Amino-1,7-dihydro-6H-purine-6-thione; 2-Amino-6-MP; 2-Amino-6-mercaptopurine; 2-Amino-6-merkaptopurin; 2-Amino-6-merkaptopurin [Czech]; 2-Amino-6-purinethiol; 2-Amino-9H-purine-6-thiol; 2-Aminopurin-6-thiol; 2-Aminopurin-6-thiol [Czech]; 2-Aminopurine-6(1H)-thione; 2-Aminopurine-6-thiol; 2-Thioguanine; 2-amino-3,7-dihydropurine-6-thione; 6 Thioguanine; 6-Mercapto-2-aminopurine; 6-Mercaptoguanine; 6-TG; 6-Thioguanine (6-TG); 6-Thioguanine, Thioguanine; Thioguanine (Guanine analog)
Click to Show/Hide
|
|||
| External Link | ||||
| Daunorubicin | Approved | [15] | ||
| Synonyms |
Daunomycin; 20830-81-3; Rubidomycin; Cerubidine; Daunorubicine; Acetyladriamycin; Leukaemomycin C; Daunorubicinum; Daunarubicinum; Daunorrubicina; Daunamycin; Cerubidin; DaunoXome; Rubomycin C; (+)-Daunomycin; Daunoblastin; Anthracyline; Rubomycin; Daunorubicinum [INN-Latin]; RP 13057; Daunorubicin [INN:BAN]; RCRA waste no U059; FI6339; NSC-82151; DAUNORUBICIN HCL; DaunoXome (TN); UNII-ZS7284E0ZP; CCRIS 914; ZS7284E0ZP; CHEBI:41977; HSDB 5095; C27H29NO10; NCI-C04693; EINECS 244-069-7; Ondena; NSC 83142; Daunoblastine; Antibiotics from Streptomyces coeruleorubidus; DM1; FI 6339; Dauno-Rubidomycine; Daunorubicin (INN); Daunorubicin (liposomal); Daunorubicin, Hydrochloride; VS-103; (1S,3S)-3-acetyl-3,5,12-trihydroxy-10-(methyloxy)-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; (1S,3S)-3-acetyl-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside; (7S,9R)-9-Acetyl-7-[(2S,4S,5S,6S)-4-amino-5-hydroxy-6-methyl-oxan-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-4-methoxy-8,10-dihydro-7H-tetracene-5,12-dione; (8S-cis)-8-Acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyrannosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-napthacenedione; (8S-cis)-8-Acetyl-10-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-5,12-naphthacenedione; Anthracycline
Click to Show/Hide
|
|||
| External Link | ||||
| Idarubicin | Approved | [16] | ||
| Synonyms |
DMDR; Idamycin; Idarubicina; Idarubicine; Idarubicinum; Zavedos; Idarubicin Hcl; Idarubicin Hcl Pfs; Idarubicin hydrochloride; DM5; I 1656; IMI 30; IMI-30; Idamycin (TN); Idarubicin (INN); Idarubicin [INN:BAN]; Idarubicina [INN-Spanish]; Idarubicine [INN-French]; Idarubicinum [INN-Latin]; Zavedos (TN); (1S,3S)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside; (1s,3s)-3-acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydrotetracen-1-yl 3-amino-2,3,6-trideoxy-A-l-lyxo-hexopyranoside; (7S,9S)-9-acetyl-7-[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-8,10-dihydro-7H-tetracene-5,12-dione; (7S-cis)-9-Acetyl-7-[(3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxy-5,12-naphthacenedione; 4-DMD; 4-Demethoxydaunomycin; 4-Demethoxydaunorubicin; 4-Desmethoxydaunorubicin
Click to Show/Hide
|
|||
| External Link | ||||
| Aclarubicin | Approved | [17] | ||
| Synonyms |
Aclacin; Aclacur; Aclarubicine; Aclarubicino; Aclarubicinum; Jaclacin; Aclacinomycin A; Aclucinomycin A; Antibiotic MA 144A; Antibiotic MA 144A1; Antibiotic MA 144G1; MA 144G1; Aclarubicine [INN-French]; Aclarubicino [INN-Spanish]; Aclarubicinum [INN-Latin]; Antibiotic MA144-A1; MA 144-A1; MA-144A1; Acene-1-carboxylate; Aclarubicin (USAN/INN); Aclarubicin [USAN:BAN:INN]; Alpha-L-lyxo-hexopyranosyl]-oxy]-1-naphthacenecarboxalic acid methyl ester; 10-epi-Aclacinomycin A
Click to Show/Hide
|
|||
| External Link | ||||
| Magrolimab | Phase 3 | [18] | ||
| Synonyms |
GS-4721
Click to Show/Hide
|
|||
| External Link | ||||
| DSP-7888 | Phase 2 | [2] | ||
| External Link | ||||
| 131I-labelled aCD45 | Phase 3 | [19] | ||
| External Link | ||||
| ATIR101 | Phase 3 | [20] | ||
| External Link | ||||
| Dociparstat sodium | Phase 3 | [21] | ||
| Synonyms |
DSTAT
Click to Show/Hide
|
|||
| External Link | ||||
| Iomab-B CD45 | Phase 3 | [2] | ||
| External Link | ||||
| Tasisulam | Phase 3 | [22] | ||
| Synonyms |
Tasisulam sodium; LY-573636; LY-573636 sodium; LY-573636Na; Apoptosis stimulator (cancer), Lilly
Click to Show/Hide
|
|||
| External Link | ||||
| Vadastuximab talirine | Phase 3 | [10] | ||
| Synonyms |
Vadastuximab talirine [INN]; Vadastuximab talirine [USAN]; Vadastuximab talirine [WHO-DD]; UNII-T13V17U431; T13V17U431; UNII-X1TW58ZV0U component BNJNAEJASPUJTO-DUOHOMBCSA-N; 1436390-64-5
Click to Show/Hide
|
|||
| External Link | ||||
| Abexinostat | Phase 3 | [2] | ||
| Synonyms |
PCI-24781; 783355-60-2; PCI 24781; CRA-024781; CRA 024781; UNII-IYO470654U; 3-((dimethylamino)methyl)-N-(2-(4-(hydroxycarbamoyl)phenoxy)ethyl)benzofuran-2-carboxamide; CRA-02478; Abexinostat(PCI-24781); PCI-24781 (Abexinostat); Abexinostat (PCI-24781); IYO470654U; 3-[(Dimethylamino)methyl]-N-[2-[4-[(hydroxyamino)carbonyl]phenoxy]ethyl]-2-benzofurancarboxamide; 3-((Dimethylamino)methyl)-N-(2-(4-(hydroxycarbamoyl)-phenoxy)ethyl)benzofuran-2-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Peginterferon lambda-1a | Phase 3 | [23] | ||
| Synonyms |
BMS-914143; IFN lambda, ZymoGenetics/Novo Nordisk; Interferon lambda 1, ZymoGenetics/Novo Nordisk; PEG-IL-29; PEG-rIL-29; Interleukin-29, ZymoGenetics/Novo Nordisk; PEG-IFN lambda, ZymoGenetics/Novo Nordisk; PEG-interferon lambda, ZymoGenetics/Novo Nordisk; PEGylated IL-29 (HCV infection), ZymoGenetics/Novo Nordisk; Peginterferon lambda-1a, ZymoGenetics/Bristol-Myers Squibb; PEG-IFN lambda, ZymoGenetics/Novo Nordisk/Bristol-Myers Squibb; PEG-interferon lambda, ZymoGenetics/Novo Nordisk/Bristol-Myers Squibb; Recombinant PEG-interferon lambda-1, ZymoGenetics/Bristol-Myers Squibb; PEGylated IL-29 (HCV infection), ZymoGenetics/Novo Nordisk/Bristol-Myers Squibb
Click to Show/Hide
|
|||
| External Link | ||||
| CPX-351 | Phase 3 | [12] | ||
| Synonyms |
Vyxeos; Cytarabine / daunonubicin; Daunonubicin / cytarabine; CPX 351; Cytarabine mixture with daunonubicin; Daunonubicin mixture with cytarabine; Daunonubicin and cytarabine liposomal injection; SCHEMBL3959238; 1256639-86-7; 5,12-Naphthacenedione, 8-acetyl-10-((3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranosyl)oxy)-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-, (8S,10S)-, mixt. with 4-amino-1-beta-D-arabinofuranosyl-2(1H)-pyrimidinone
Click to Show/Hide
|
|||
| External Link | ||||
| BYM338 | Phase 2/3 | [24] | ||
| External Link | ||||
| SGI110 | Phase 3 | [25] | ||
| External Link | ||||
| Elacytarabine | Phase 3 | [26] | ||
| Synonyms |
Elacyt (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| RG7388 | Phase 3 | [27] | ||
| Synonyms |
Idasanutlin; 1229705-06-9; Idasanutlin (RG-7388); RG-7388; UNII-QSQ883V35U; QSQ883V35U; CHEMBL2402737; Benzoic acid, 4-((((2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-(2,2-dimethylpropyl)-2-pyrrolidinyl)carbonyl)amino)-3-methoxy-; RO5503781; Idasanutlin [USAN:INN]; Benzoic acid, 4-[[[(2R,3S,4R,5S)-3-(3-chloro-2-fluorophenyl)-4-(4-chloro-2-fluorophenyl)-4-cyano-5-(2,2-dimethylpropyl)-2-pyrrolidinyl]carbonyl]amino]-3-methoxy-; RG-7388;Idasanutlin; RO-5503781; SCHEMBL442856
Click to Show/Hide
|
|||
| External Link | ||||
| Galinpepimut-S | Phase 3 | [28] | ||
| External Link | ||||
| Pracinostat | Phase 3 | [12] | ||
| Synonyms |
929016-96-6; SB939; SB 939; Pracinostat (SB939); SB-939; UNII-GPO2JN4UON; GPO2JN4UON; CHEMBL1851943; (E)-3-(2-butyl-1-(2-(diethylamino)ethyl)-1H-benzo[d]imidazol-5-yl)-N-hydroxyacrylamide; 2-Propenamide, 3-[2-butyl-1-[2-(diethylamino)ethyl]-1H-benzimidazol-5-yl]-N-hydroxy-, (2E)-; Pracinostat [INN]; (2E)-3-(2-Butyl-1-[2-(diethylamino)ethyl]-1h-1,3-benzodiazol-5-yl)-n-hydroxyprop-2-enamide; 2-Propenamide, 3-(2-butyl-1-(2-(diethylamino)ethyl)-1H-benzimidazol-5-yl)-N-hydroxy-, (2E)-
Click to Show/Hide
|
|||
| External Link | ||||
| DFP-10917 | Phase 3 | [2] | ||
| External Link | ||||
| AG-881 | Phase 1 | [2] | ||
| Synonyms |
Vorasidenib; 1644545-52-7; UNII-789Q85GA8P; 789Q85GA8P; AG881; 9UO; 6-(6-chloropyridin-2-yl)-N2,N4-bis[(2R)-1,1,1-trifluoropropan-2-yl]-1,3,5-triazine-2,4-diamine; Vorasidenib [INN]; Vorasidenib (AG-881); SCHEMBL16393139; AG 881 [WHO-DD]; QCZAWDGAVJMPTA-RNFRBKRXSA-N; BDBM279948; EX-A2574; US10028961, Compound 101; AG 881; s8611; CS-8033; HY-104042; 6-(6-chloropyridin-2-yl)-N2,N4-bis((R)-1,1,1-trifluoro propan-2-yl)-1,3,5-triazine-2,4-diamine; 6-(6-chloropyridin-2-yl)-N2,N4-bis((R)-1,1,1-trifluoroprop
Click to Show/Hide
|
|||
| External Link | ||||
| AG-221 | Phase 1/2 | [29] | ||
| Synonyms |
AG-221 (IDH2 inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| CP-868596 | Phase 3 | [2] | ||
| Synonyms |
Crenolanib; 670220-88-9; Crenolanib (CP-868596); ARO-002; UNII-LQF7I567TQ; LQF7I567TQ; CP-868596 (Crenolanib); CP-868,596; [1-[2-[5-(3-Methyloxetan-3-ylmethoxy)benzimidazol-1-yl]quinolin-8-yl]piperidin-4-yl]amine; 1-(2-(5-((3-Methyloxetan-3-yl)methoxy)-1H-benzo-[d]imidazol-1-yl)quinolin-8-yl)piperidin-4-amine; CP868569; 1-[2-[5-[(3-Methyl-3-oxetanyl)methoxy]-1-benzimidazolyl]-8-quinolyl]-4-piperidinamine; J-502712; 1-[2-[5-[(3-methyloxetan-3-yl)methoxy]benzimidazol-1-yl]quinolin-8-yl]piperidin-4-amine
Click to Show/Hide
|
|||
| External Link | ||||
| Rydaptmidostaurin | Phase 3 | [2] | ||
| External Link | ||||
| Sapacitabine | Phase 3 | [30] | ||
| Synonyms |
CYC682
Click to Show/Hide
|
|||
| External Link | ||||
| S-110 | Phase 3 | [25] | ||
| Synonyms |
DNA demethylating agent (myelodysplastic syndrome), Supergen
Click to Show/Hide
|
|||
| External Link | ||||
| VAL-083 | Phase 1/2 | [31] | ||
| Synonyms |
Dianhydrogalactitol; 23261-20-3; UNII-4S465RYF7M; 1,2:5,6-DIANHYDROGALACTITOL; Dulcitoldiepoxide; 4S465RYF7M; Dianhydrogalactitol [USAN:INN]; SCHEMBL4306431; Dianhydrogalactitol (USAN/INN); CHEMBL3137322; AAFJXZWCNVJTMK-GUCUJZIJSA-N; ZINC4963497; CS-1358; DB12873; NSC-1323313; HY-16513; BC657197; D10623; W-5268; J-015059; 457899-38-6
Click to Show/Hide
|
|||
| External Link | ||||
| CC-486 | Phase 3 | [2] | ||
| Synonyms |
AG-14361; AG14361; 328543-09-5; UNII-48N0U0K50I; AG 14361; CHEMBL65892; 48N0U0K50I; Imidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one, 2-[4-[(dimethylamino)methyl]phenyl]-5,6-dihydro-; AG-014361; 1-(4-((dimethylamino)methyl)phenyl)-8,9-dihydro-2,7,9a-triazabenzo[cd]azulen-6(7H)-one; Imidazo(4,5,1-jk)(1,4)benzodiazepin-7(4H)-one, 2-(4-((dimethylamino)methyl)phenyl)-5,6-dihydro-; 2-[4-[(Dimethylamino)methyl]phenyl]-5,6-dihydroimidazo[4,5,1-jk][1,4]benzodiazepin-7(4H)-one; SMR000486393; MLS006011157; MLS001065917; Nucleoside analogue
Click to Show/Hide
|
|||
| External Link | ||||
| Hu5F9-G4 | Phase 3 | [10] | ||
| External Link | ||||
| Iomab-B | Phase 3 | [32] | ||
| Synonyms |
Iomab-B (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| PEG arginine deiminase | Phase 1 | [2] | ||
| Synonyms |
ADI-PEG 20
Click to Show/Hide
|
|||
| External Link | ||||
| CPI-613 | Phase 3 | [12] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Zarnestra | Phase 3 | [33] | ||
| Synonyms |
JAN; Tipifarnib; Tipifarnib [USAN]; R 115777; R115777; R-11577; R-115777; Tipifarnib (USAN/INN); Zarnestra, IND 58359, R115777, Tipifarnib; (R)-6-(Amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl)-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone; (R)-R115777; 2 (1H))-Quinolinone,6-(amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl)-4-(3-chlorophenyl)-1-methyl-, 2(1H)-quinolinone; 6-[(R)-amino(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2(1H)-one; 6-[(R)-amino-(4-chlorophenyl)-(3-methylimidazol-4-yl)methyl]-4-(3-chlorophenyl)-1-methylquinolin-2-one; 6-[(S)-AMINO(4-CHLOROPHENYL)(1-METHYL-1H-IMIDAZOL-5-YL)METHYL]-4-(3-CHLOROPHENYL)-1-METHYLQUINOLIN-2(1H)-ONE
Click to Show/Hide
|
|||
| External Link | ||||
| LMT-X | Phase 3 | [34] | ||
| Synonyms |
Second-generation tau aggregation inhibitor (Alzheimer's disease); Second-generation tau aggregation inhibitor (Alzheimer's disease), TauRx
Click to Show/Hide
|
|||
| External Link | ||||
| Elesclomol | Phase 3 | [35] | ||
| Synonyms |
488832-69-5; Elesclomol (STA-4783); STA-4783; UNII-6UK191M53P; CHEBI:79369; 6UK191M53P; Propanedioic acid, bis[2-methyl-2-(phenylthioxomethyl)hydrazide]; 1,3-Bis[2-methyl-2-(phenylthioxomethyl)hydrazide]propanedioic acid; N'1,N'3-dimethyl-N'1,N'3-di(phenylcarbonothioyl)malonohydrazide; J-503879; C19H20N4O2S2; 1-N'-Benzenecarbothioyl-3-(2-benzenecarbothioyl-2-methylhydrazinyl)-N'-methyl-oxopropanehydrazidide; Elesclomol [USAN:INN]; elesclomolum
Click to Show/Hide
|
|||
| External Link | ||||
| GMI-1271 | Phase 3 | [36] | ||
| Synonyms |
Uproleselan
Click to Show/Hide
|
|||
| External Link | ||||
| AT-406 | Phase 3 | [37] | ||
| Synonyms |
1071992-99-8; SM 406; (5S,8S,10aR)-N-benzhydryl-5-((S)-2-(methylamino)propanamido)-3-(3-methylbutanoyl)-6-oxodecahydropyrrolo[1,2-a][1,5]diazocine-8-carboxamide; CHEMBL2158051; QCR-136; UNII-N65WC8PXDD; N65WC8PXDD; AT406 (SM-406, ARRY-334543); J-501625; (5S,8S,10aR)-N-(Diphenylmethyl)-5-[(N-methyl-L-alanyl)amino]-3-(3-methylbutanoyl)-6-oxodecahydropyrrolo[1,2-a][1,5]diazocine-8-carboxamide; AT-406/Debio-1143
Click to Show/Hide
|
|||
| External Link | ||||
| PR1 peptide antigen vaccine | Phase 3 | [38] | ||
| Synonyms |
PR1 peptide antigen vaccine (leukemia); PR1 peptide antigen vaccine (leukemia), MD Anderson; PR1 peptide antigen vaccine (leukemia), Vaccine Company
Click to Show/Hide
|
|||
| External Link | ||||
| CPI-0610 | Phase 3 | [2] | ||
| External Link | ||||
| SNS-595 | Phase 3 | [39] | ||
| Synonyms |
Voreloxin; Vosaroxin; AG 7352; SNS 595; SPC 595; AG 7352 (TN); AG-7352; SNS 595 (TN); SPC 595 (TN); SPC-595; Voreloxin (TN); Voreloxin (USAN); SNS-595 (TN); 1,4-Dihydro-7-(3-methoxy-4-methylamino-1-pyrrolidinyl)-4-oxo-1-(2-thiazolyl)-1,8-naphthyridine-3-carboxylic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Selinexor | Phase 3 | [12] | ||
| Synonyms |
Xpovio; KPT 330; KPT-330; KPT-330(Selinexor); KPT330;Selinexor; 1393477-72-9; 31TZ62FO8F; CHEMBL3545185; SCHEMBL14678327; Selinexor (KPT-330); Selinexor [USAN:INN]; Tube706; UNII-31TZ62FO8F
Click to Show/Hide
|
|||
| External Link | ||||
| Volasertib | Phase 3 | [12] | ||
| Synonyms |
755038-65-4; BI 6727; BI-6727; BI6727 (Volasertib); Volasertib (BI 6727); UNII-6EM57086EA; 6EM57086EA; BI6727; N-{trans-4-[4-(Cyclopropylmethyl)piperazin-1-Yl]cyclohexyl}-4-{[(7r)-7-Ethyl-5-Methyl-8-(1-Methylethyl)-6-Oxo-5,6,7,8-Tetrahydropteridin-2-Yl]amino}-3-Methoxybenzamide; Volasertib [USAN:INN]; IBI; Volasertib (USAN); BI6727,Volasertib; Volasertib(BI6727); BI6727 - Volasertib; Volasertib (BI6727); BI6727 (Volasertib)/; BI 6727 (Volasertib); MLS006011195; SCHEMBL738946; SCHEMBL9888052; SCHEMBL2169101; GTPL7947
Click to Show/Hide
|
|||
| External Link | ||||
| Rivo-cel | Phase 2/3 | [40] | ||
| Synonyms |
BPX-501
Click to Show/Hide
|
|||
| External Link | ||||
| CLL1 CAR-T Cell | Phase 2/3 | [41] | ||
| External Link | ||||
| NLG8189 | Phase 2/3 | [2] | ||
| Synonyms |
1-Methyl-D-tryptophan; Indoximod; 110117-83-4; D-Tryptophan, 1-methyl-; D-1MT; Indoximod (NLG-8189); D-1-methyltryptophan; UNII-TX5CYN1KMZ; D-(+)-1-Methyltryptophan; TX5CYN1KMZ; (R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methylindol-3-yl)propanoic acid; NSC-721782; (2R)-2-amino-3-(1-methyl-3-indolyl)propanoic acid; 1-MT; (2R)-2-azanyl-3-(1-methylindol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; D-l-Methyltryptophan; Indoximod [USAN:INN]; NLG-8189; NLG 8189
Click to Show/Hide
|
|||
| External Link | ||||
| CD123/CLL1 CAR-T Cells | Phase 2/3 | [41] | ||
| External Link | ||||
| K-NK002 | Phase 2 | [42] | ||
| Synonyms |
CSTD-002-NK
Click to Show/Hide
|
|||
| External Link | ||||
| Dilanubicel | Phase 2 | [43] | ||
| Synonyms |
NLA101
Click to Show/Hide
|
|||
| External Link | ||||
| DS-3201b | Phase 2 | [44] | ||
| Synonyms |
Valemetostat; 1809336-39-7; UNII-60RD0234VE; 60RD0234VE; 1809336-39-7 (free base); DS-3201; (2R)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-N-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide; Valemetostat 2HCl; Valemetostat [INN]; Valemetostat (DS-3201); CHEMBL4597193; EZH1/2 inhibitor DS-3201; SCHEMBL18393626; SCHEMBL18393627; SCHEMBL18639210; EX-A3423; DS3201; NSC813381; s8926; NSC-813381; HY-109108; CS-0039740; D11551
Click to Show/Hide
|
|||
| External Link | ||||
| BST-236 | Phase 2 | [45] | ||
| Synonyms |
Aspacytarabine; UNII-JL7V54Z2BR; JL7V54Z2BR; 2098942-53-9; N4-(1-beta-D-arabinofuranosyl-2-oxo-1,2-dihydropyrimidin-4-yl)-; Astarabine; Aspacytarabine [INN]; CHEMBL4297599; L-Asparagine, N-(1-beta-D-arabinofuranosyl-1,2-dihydro-2-oxo-4-pyrimidinyl)-; N4-(1-N2-D-Arabinofuranosyl-2-oxo-1,2-dihydropyrimidin-4-yl)- L-asparagine
Click to Show/Hide
|
|||
| External Link | ||||
| Zelenoleucel | Phase 2 | [46] | ||
| External Link | ||||
| CX-01 | Phase 2 | [2] | ||
| External Link | ||||
| LY2090314 | Phase 2 | [47] | ||
| Synonyms |
603288-22-8; LY-2090314; UNII-822M3GYM67; Kinome_3681; LY 2090314; CHEMBL362558; 822M3GYM67; 3-(9-Fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetrahydro-[1,4]diazepino[6,7,1-hi]indol-7-yl)-4-(imidazo[1,2-a]pyridin-3-yl)-1H-pyrrole-2,5-dione; SCHEMBL633455; GTPL7958; DTXSID90209085; MolPort-035-944-332; EX-A2214; ZINC3817327; BCP07855; s7063; BDBM50150699; AKOS032950045; AKOS026750195; CS-1633; DB11913; SB16558; NCGC00378942-05; NCGC00378942-02; BC600682; QC-11735; HY-16294; KB-78238; FT-0698670; LY2090314, >
Click to Show/Hide
|
|||
| External Link | ||||
| Flotetuzumab | Phase 2 | [48] | ||
| External Link | ||||
| BGB-324 | Phase 1/2 | [12] | ||
| Synonyms |
BGB-001; SiRNA therapeutic (metastasis cancer), BiobergenBio
Click to Show/Hide
|
|||
| External Link | ||||
| PTX-200 | Phase 2 | [2] | ||
| Synonyms |
Plant-derived antiparkinsonian, Phytrix
Click to Show/Hide
|
|||
| External Link | ||||
| LY2523355 | Phase 2 | [49] | ||
| Synonyms |
Litronesib
Click to Show/Hide
|
|||
| External Link | ||||
| Prexigebersen | Phase 2 | [2] | ||
| Synonyms |
Prexigebersen [INN]; Prexigebersen [USAN:INN]; UNII-8W1O4Y961B; 8W1O4Y961B; BP-100-1.01/L-Grb2; DNA, d(A-T-A-T-T-T-G-G-C-G-A-T-G-G-C-T-T-C); 202484-91-1; 1352967-54-4
Click to Show/Hide
|
|||
| External Link | ||||
| ALT-801 | Phase 2 | [50] | ||
| Synonyms |
ALT-801 (donor lymphocyte infusion, cancer); ALT-801 (donor lymphocyte infusion, cancer), Altor; STAR IL-2 conjugate (donor lymphocyte infusion, cancer), Altor; STAR-Ck (donor lymphocyte infusion, cancer), Altor; Soluble T-cell Antigen Receptor IL-2 conjugate (donor lymphocyte infusion, cancer), Altor
Click to Show/Hide
|
|||
| External Link | ||||
| ENMD-2076 | Phase 2 | [51] | ||
| Synonyms |
934353-76-1; ENMD 2076; (E)-N-(5-Methyl-1H-pyrazol-3-yl)-6-(4-methylpiperazin-1-yl)-2-styrylpyrimidin-4-amine; UNII-J6U9WP10T7; ENMD2076; J6U9WP10T7; CHEMBL482968; 6-(4-methylpiperazin-1-yl)-N-(5-methyl-1H-pyrazol-3-yl)-2-[(E)-2-phenylethenyl]pyrimidin-4-amine; 6-(4-METHYL-1-PIPERAZINYL)-N-(5-METHYL-1H-PYRAZOL-3-YL)-2-[(1E)-2-PHENYLETHENYL]-4-PYRIMIDINAMINE; ENMD-981693; MLS006011042; SCHEMBL596481; GTPL7885; SCHEMBL15668060; SCHEMBL10122872; ENND-2076; EX-A235; DTXSID60239430; MolPort-009-679-391; AOB87159
Click to Show/Hide
|
|||
| External Link | ||||
| FPI-01 | Phase 2 | [52] | ||
| Synonyms |
WT-1 therapeutic vaccine (cancer), Formula Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Inecalcitol oral | Phase 2 | [2] | ||
| Synonyms |
Inecalcitol; TX-522; UNII-05FZV98342; TX 522; 163217-09-2; AC1OCD0K; 19-Nor-14-epi-23-yne-1,25 dihydroxyvitamin D3; 05FZV98342; (1R,3R)-5-[(2E)-2-[(1R,3aR,7aR)-1-[(2R)-6-hydroxy-6-methylhept-4-yn-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]cyclohexane-1,3-diol; (7E)-(1R,3R,14R)-19-nor-23-yne-9,10-seco-5,7-cholestadiene-1,3,25-triol; Inecalcitol [INN]; SCHEMBL754593; GTPL7747; CHEMBL2105107; LMST03020649; 8151AH; ZINC12504514; AKOS025312295; AN-7569; DB04796; TX-527; Vitamin D analogs, Catholic University of Leuven; Vitamin D analogs, KULeuven; Inecalcitol (oral, hyperthyroidism), Hybrigenics; Inecalcitol (sc, psoriasis), Hybrigenics; Vitamin D analog (sc, psoriasis), Hybrigenics; Inecalcitol (oral, prostate cancer/psoriasis), Hybrigenics
Click to Show/Hide
|
|||
| External Link | ||||
| Eryaspase | Phase 2 | [2] | ||
| External Link | ||||
| ALXN6000 | Phase 2 | [10] | ||
| Synonyms |
Samalizumab
Click to Show/Hide
|
|||
| External Link | ||||
| BI 2536 | Phase 2 | [53] | ||
| Synonyms |
BI2536, BI 2536
Click to Show/Hide
|
|||
| External Link | ||||
| MK8242 | Phase 2 | [54] | ||
| External Link | ||||
| Leukemia DNA vaccine | Phase 2 | [55] | ||
| External Link | ||||
| Anti-CD45 mAb 131I-BC8 | Phase 2 | [12] | ||
| External Link | ||||
| BREQUINAR | Phase 1/2 | [56] | ||
| Synonyms |
96187-53-0; 6-Fluoro-2-(2'-fluoro-[1,1'-biphenyl]-4-yl)-3-methylquinoline-4-carboxylic acid; brequinarum [Latin]; Brequinar [INN]; Biphenquinate; 6-fluoro-2-(2'-fluorobiphenyl-4-yl)-3-methylquinoline-4-carboxylic acid; Brequinarum [INN-Latin]; UNII-5XL19F49H6; C23H15F2NO2; NSC-368390; NSC 368390; 6-fluoro-2-[4-(2-fluorophenyl)phenyl]-3-methylquinoline-4-carboxylic acid; PHEZJEYUWHETKO-UHFFFAOYSA-N; Dup 785; 5XL19F49H6; 6-FLUORO-2-(2'-FLUORO-1,1'-BIPHENYL-4-YL)-3-METHYLQUINOLINE-4-CARBOXYLIC ACID; brequinarum
Click to Show/Hide
|
|||
| External Link | ||||
| Leukemia cancer vaccine | Phase 2 | [57] | ||
| External Link | ||||
| ADCT-301 | Phase 1 | [2] | ||
| Synonyms |
camidanlumab tesirine
Click to Show/Hide
|
|||
| External Link | ||||
| Flavopiridol | Phase 2 | [12] | ||
| Synonyms |
FLAVO; Alvocidib [INN]; Flavopiridol hydrochloride; L 868275; HMR-1275; L-868275; L86-8275; HMR-1275, Alvocidib, L868275, Flavopiridol; (-)-cis-5,7-Dihydroxy-2-(2-chlorophenyl)-8-(4-(3-hydroxy-1-methyl)piperidinyl)-4H-1-benzopyran-4-one; 2-(2-CHLORO-PHENYL)-5,7-DIHYDROXY-8-(3-HYDROXY-1-METHYL-PIPERIDIN-4-YL)-4H-BENZOPYRAN-4-ONE; 2-(2-chlorophenyl)-5,7-dihydroxy-8-[(3S,4R)-3-hydroxy-1-methylpiperidin-4-yl]chromen-4-one
Click to Show/Hide
|
|||
| External Link | ||||
| BP-100-1-01 | Phase 2 | [58] | ||
| External Link | ||||
| IRX-2 | Phase 2 | [59] | ||
| External Link | ||||
| Cenersen | Phase 2 | [60] | ||
| External Link | ||||
| Lirilumab | Phase 2 | [61] | ||
| External Link | ||||
| LOR-2040 | Phase 2 | [62] | ||
| External Link | ||||
| Sodium butyrate | Phase 2 | [63] | ||
| Synonyms |
Butyrate sodium; Sodium butanoate; Sodium propanecarboxylate; OR8783; Butanoic acid, sodium salt; Butyric Acid, Na; Sodium butyrate (USP); Sodium n-butyrate; TPA/BA; Tetradecanoyl phorbol acetate/ sodium butyrate; Butanoic acid, sodium salt (1:1); Tetradecanoyl phorbol acetate (TPA)/ sodium butyrate (BA)
Click to Show/Hide
|
|||
| External Link | ||||
| GRNVAC1 | Phase 2 | [64] | ||
| External Link | ||||
| H3B-8800 | Phase 1 | [2] | ||
| External Link | ||||
| GS-9973 | Phase 2 | [2] | ||
| Synonyms |
Entospletinib; 1229208-44-9; Entospletinib (GS-9973); gs9973; UNII-6I3O3W6O3B; 6-(1H-Indazol-6-yl)-N-(4-morpholinophenyl)imidazo[1,2-a]pyrazin-8-amine; GS 9973; 6I3O3W6O3B; CHEMBL3265032; C23H21N7O; 6-(1H-Indazol-6-yl)-N-(4-(morpholin-4-yl)phenyl)imidazo(1,2-a)pyrazin-8-amine; Entospletinib [INN]; Entospletinib [USAN:INN]; 4puz; 6-(1h-Indazol-6-Yl)-N-[4-(Morpholin-4-Yl)phenyl]imidazo[1,2-A]pyrazin-8-Amine; SCHEMBL2483776; GTPL7889; MolPort-035-395-879; HMS3653D13; EX-A1120; BCP09582; AOB87385
Click to Show/Hide
|
|||
| External Link | ||||
| ALT-803 | Phase 2 | [65] | ||
| Synonyms |
IL-15 agonist/ IL-15R alpha-Fc fusion complex (cancer), Altor BioScience
Click to Show/Hide
|
|||
| External Link | ||||
| CHR-2797 | Phase 2 | [66] | ||
| Synonyms |
Tosedostat; Tosedostat, CHR2797, CHR-2797
Click to Show/Hide
|
|||
| External Link | ||||
| HTERT RNA vaccine | Phase 2 | [67] | ||
| External Link | ||||
| MB-102 | Phase 2 | [10] | ||
| Synonyms |
Relmapirazin; UNII-Q3UQB8PQ6H; Q3UQB8PQ6H; Relmapirazin [INN]; CHEMBL1949708; SCHEMBL16738795; N,N'-((3,6-Diamino-2,5-pyrazinediyl)dicarbonyl)bis(D-serine); D-Serine, N,N'-((3,6-diamino-2,5-pyrazinediyl)dicarbonyl)bis-; 1313706-17-0
Click to Show/Hide
|
|||
| External Link | ||||
| phorbol 12-myristate 13-acetate | Phase 2 | [2] | ||
| Synonyms |
TPA; 12-O-tetradecanoylphorbol-13-acetate; tetradecanoyl-beta-phorbol acetate
Click to Show/Hide
|
|||
| External Link | ||||
| AEB1102 | Phase 1 | [2] | ||
| External Link | ||||
| Ifabotuzumab | Phase 2 | [10] | ||
| External Link | ||||
| BP1001 | Phase 2 | [12] | ||
| External Link | ||||
| AS-101 | Phase 2 | [68] | ||
| Synonyms |
Ossirene; Tellaxium trichloride glycol; IVX-Q-101
Click to Show/Hide
|
|||
| External Link | ||||
| ALRN-6924 | Phase 2 | [2] | ||
| External Link | ||||
| GO-203-2c | Phase 2 | [2] | ||
| External Link | ||||
| Talacotuzumab | Phase 2 | [10] | ||
| Synonyms |
JNJ 473; JNJ 56022473; TAL
Click to Show/Hide
|
|||
| External Link | ||||
| AST-VAC1 | Phase 2 | [69] | ||
| External Link | ||||
| Lomab B | Phase 2 | [70] | ||
| Synonyms |
BCS-I-131 construct
Click to Show/Hide
|
|||
| External Link | ||||
| Fipamezole | Phase 2 | [71] | ||
| Synonyms |
150586-58-6; JP-1730; 1H-Imidazole,5-(2-ethyl-5-fluoro-2,3-dihydro-1H-inden-2-yl)-; Fipamezole [INN]; ACMC-1BZQD; AC1Q4NYS; SCHEMBL935549; AC1L4U51; SCHEMBL18826053; CHEMBL1255582; CTK4C6705; 5-(2-ethyl-5-fluoro-1,3-dihydroinden-2-yl)-1H-imidazole; KXSUAWAUCNFBQJ-UHFFFAOYSA-N; 5-(2-ethyl-5-fluoro-2,3-dihydro-1h-inden-2-yl)-1h-imidazole; BDBM50417007; SB17014; 4-(2-ethyl-5-fluoro-indan-2-yl)-1H-imidazole; L001472; 4-((2RS)-2-Ethyl-5-fluoroindan-2-yl)-1H-imidazole
Click to Show/Hide
|
|||
| External Link | ||||
| PR104 | Phase 2 | [72] | ||
| Synonyms |
851627-62-8; PR-104; UNII-V16D2ZT7DT; V16D2ZT7DT; 2-((2-Bromoethyl)(2,4-dinitro-6-((2-(phosphonooxy)ethyl)carbamoyl)phenyl)amino)ethyl methanesulfonate; 2-[(2-bromoethyl)(2,4-dinitro-6-{[2-(phosphonooxy)ethyl]carbamoyl}phenyl)amino]ethyl methanesulfonate; PR 104; SCHEMBL367963; ZINC43131754; AKOS022174966; RL05275; KB-80083; 4CA-1041; AX8282408; J-507569; Benzamide, 2-((2-bromoethyl)(2-((methylsulfonyl)oxy)ethyl)amino)-3,5- dinitro-N-(2-(phosphonooxy)ethyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| BI-836858 | Phase 1/2 | [2] | ||
| External Link | ||||
| Ficlatuzumab | Phase 2 | [2] | ||
| Synonyms |
AV-299
Click to Show/Hide
|
|||
| External Link | ||||
| K562/GM-CSF | Phase 2 | [73] | ||
| Synonyms |
GM-CSF-secreting cells+tumor cell vaccine, Cell Genesys/Johns Hopkins
Click to Show/Hide
|
|||
| External Link | ||||
| PCM-075 | Phase 2 | [2] | ||
| Synonyms |
Onvansertib
Click to Show/Hide
|
|||
| External Link | ||||
| Actimab-A | Phase 2 | [10] | ||
| External Link | ||||
| IMG-7289 | Phase 2 | [2] | ||
| Synonyms |
KQKBMHGOHXOHTD-KKUQBAQOSA-N; UNII-Y2T4ALDEAT; Y2T4ALDEAT; SCHEMBL17984236; Benzamide, N-((1S)-4-(((1R,2S)-2-(4-fluorophenyl)cyclopropyl)amino)-1-((4-methyl-1-piperazinyl)carbonyl)butyl)-4-(1H-1,2,3-triazol-1-yl)-; 1990504-34-1; N-[(2S)-1-(4-(methyl)piperazin-1-yl)-5-[[(1R,2S)-2-(4-fluorophenyl)-cyclopropyl]amino]-1-oxopentan-2-yl]-4-(1H-1,2,3-triazol-1-yl)benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| WT1-targeted autologous dendritic cell vaccine | Phase 2 | [74] | ||
| Synonyms |
WT1-targeted autologous dendritic cell vaccine (cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| AZD-4877 | Phase 2 | [75] | ||
| Synonyms |
Anticancer therapeutic (solid tumors), AstraZeneca
Click to Show/Hide
|
|||
| External Link | ||||
| IPH-2102 | Phase 2 | [76] | ||
| External Link | ||||
| ASP7517 | Phase 1/2 | [77] | ||
| External Link | ||||
| CG-806 | Phase 1/2 | [78] | ||
| Synonyms |
1370466-81-1; UNII-7W3FGR71NN; 7W3FGR71NN; 1-(3-fluoro-4-(7-(4-methyl-1H-imidazol-2-yl)-1-oxoisoindolin-4-yl)phenyl)-3-(3-(trifluoromethyl)phenyl)urea; SCHEMBL1292507; EX-A2932; NSC810717; CG'806; NSC-810717; CG-026806; HY-112646; CS-0058852; 1-(3-Fluoro-4-(7-(5-methyl-1H-imidazol-2-yl)-1-oxo-2,3-dihydro-1H-isoindo-1-4-yl)-phenyl)-3-(3-trifluoromethyl-phenyl)urea; Urea, N-(4-(2,3-dihydro-7-(5-methyl-1H-imidazol-2-yl)-1-oxo-1H-isoindol-4-yl)-3-fluorophenyl)-N'-(3-(trifluoromethyl)phenyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| KO-539 | Phase 1/2 | [79] | ||
| Synonyms |
UNII-4MOD1F4ENC; 4MOD1F4ENC; SCHEMBL20846943; KO539; Menin-mll interaction inhibitor KO 539; (S)-4-Methyl-5-((4-((2-(methylamino)-6-(2,2,2-trifluoroethyl)thieno(2,3-d)pyrimidin-4-yl)amino)piperidin-1-yl)methyl)-1-(2-(4-(methylsulfonyl)piperazin-1-yl)propyl)-1H-indole-2-carbonitrile; 2134675-36-6
Click to Show/Hide
|
|||
| External Link | ||||
| SAR440234 | Phase 1/2 | [80] | ||
| External Link | ||||
| HM43239 | Phase 1/2 | [81] | ||
| External Link | ||||
| Annamycin | Phase 1/2 | [2] | ||
| Synonyms |
92689-49-1; UNII-SNU299M83Q; 2'-Iodo-3'-hydroxy-4'-epi-4-demethoxydoxorubicin; SNU299M83Q; (7S,9S)-7-(((2R,3R,4R,5R,6S)-4,5-Dihydroxy-3-iodo-6-methyltetrahydro-2H-pyran-2-yl)oxy)-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-7,8,9,10-tetrahydrotetracene-5,12-dione; AR-522; SCHEMBL19368; (7S,9S)-7-[(2R,3R,4R,5R,6S)-4,5-dihydroxy-3-iodo-6-methyloxan-2-yl]oxy-6,9,11-trihydroxy-9-(2-hydroxyacetyl)-8,10-dihydro-7H-tetracene-5,12-dione; ZINC3918134; DB06420; 5,12-Naphthacenedione, 7-((2,6-dideoxy-2-iodo-alpha-L-mannopyranosyl)oxy)-7,8,9,10-tetrahydro-6,9,11-trihydroxy-9-(hydroxyacetyl)-, (7S-cis)-; 689A491; Q4767903
Click to Show/Hide
|
|||
| External Link | ||||
| ONO-7475 | Phase 1/2 | [82] | ||
| Synonyms |
1646839-59-9; UNII-0VCB95RHRV; N-(5-((6,7-Dimethoxyquinolin-4-yl)oxy)pyridin-2-yl)-2,5-dioxo-1-phenyl-1,2,5,6,7,8-hexahydroquinoline-3-carboxamide; N-[5-[(6,7-Dimethoxy-4-quinolinyl)oxy]-2-pyridinyl]-1,2,5,6,7,8-hexahydro-2,5-dioxo-1-phenyl-3-quinolinecarboxamide; SCHEMBL16426362; ONO7475; BCP33232; MFCD32689448; s8933; ONO 7475; HY-114358; CS-0083699; 3-Quinolinecarboxamide, N-(5-((6,7-dimethoxy-4-quinolinyl)oxy)-2-pyridinyl)-1,2,5,6,7,8-hexahydro-2,5-dioxo-1-phenyl-; N-[5-(6,7-dimethoxyquinolin-4-yl)oxypyridin-2-yl]-2,5-dioxo-1-phenyl-7,8-dihydro-6H-quinoline-3-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| FF-10101 | Phase 1/2 | [83] | ||
| Synonyms |
1472797-69-5; UNII-7V7IHI0SYG; 7V7IHI0SYG; (S,E)-N-(1-((5-(2-((4-Cyanophenyl)amino)-4-(propylamino)pyrimidin-5-yl)pent-4-yn-1-yl)amino)-1-oxopropan-2-yl)-4-(dimethylamino)-N-methylbut-2-enamide; SCHEMBL15584726; SCHEMBL16443760; BDBM397428; BCP23613; s8899; US9987278, Compound Reference 38; HY-109584; CS-0032038; FF 10101; FF10101; (E)-N-[(2S)-1-[5-[2-(4-cyanoanilino)-4-(propylamino)pyrimidin-5-yl]pent-4-ynylamino]-1-oxopropan-2-yl]-4-(dimethylamino)-N-methylbut-2-enamide; 2-Butenamide, N-((1S)-2-((5-(2-((4-cyanophenyl)amino)-4-(propylamino)-5-pyrimidinyl)-4-pentyn-1-yl)amino)-1-methyl-2-oxoethyl)-4-(dimethylamino)-N-methyl-, (2E)-
Click to Show/Hide
|
|||
| External Link | ||||
| SL-401 | Phase 1/2 | [84] | ||
| External Link | ||||
| SEL-24 | Phase 1/2 | [2] | ||
| Synonyms |
Multikinase inhibitor (cancer), Selvita; SEL-24-1; SEL-24-11; SEL-24-20; Pim-1 kinase inhibitors (cancer), Selvita
Click to Show/Hide
|
|||
| External Link | ||||
| SEL24 | Phase 1/2 | [85] | ||
| Synonyms |
MEN1703
Click to Show/Hide
|
|||
| External Link | ||||
| CD38-specific gene-engineered T cells | Phase 1/2 | [86] | ||
| External Link | ||||
| HuM-195-Ac-225 | Phase 1/2 | [87] | ||
| Synonyms |
HuM-195 [225Ac]; SMART 225Ac-M195; Actinium-225-M195; SMART actinium-225-M195; Actinium-225-HuM-195; 225Ac-HuM-195; 225Ac-lintuzumab
Click to Show/Hide
|
|||
| External Link | ||||
| NEXI-001 | Phase 1/2 | [88] | ||
| External Link | ||||
| INCB59872 | Phase 1/2 | [2] | ||
| External Link | ||||
| MG7-CART | Phase 1/2 | [89] | ||
| External Link | ||||
| PRI-724 | Phase 1/2 | [90] | ||
| External Link | ||||
| CART-123 cells | Phase 1/2 | [91] | ||
| External Link | ||||
| OXi4503 | Phase 1/2 | [2] | ||
| Synonyms |
Combretastatin A-1 phosphate; 288847-34-7; Combretastatin A1 disodium phosphate; 1,2-Benzenediol, 3-methoxy-6-((1Z)-2-(3,4,5-trimethoxyphenyl)ethenyl)-, bis(dihydrogen phosphate), tetrasodium salt
Click to Show/Hide
|
|||
| External Link | ||||
| CIK-CAR.CD19 | Phase 1/2 | [2] | ||
| External Link | ||||
| RhH1.3 | Phase 1/2 | [92] | ||
| Synonyms |
Oncohist; Histone H1, SymbioTec; Recombinant human histone H1.3, SymbioTec; RhH1.3, SymbioTec
Click to Show/Hide
|
|||
| External Link | ||||
| JTCR016 | Phase 1/2 | [10] | ||
| External Link | ||||
| Muc1-specific gene-engineered T cells | Phase 1/2 | [86] | ||
| External Link | ||||
| Lintuzumab Ac-225 | Phase 1/2 | [93] | ||
| Synonyms |
Actimab-A (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| CD33-specific gene-engineered T cells | Phase 1/2 | [86] | ||
| External Link | ||||
| CD19 specific CAR T cells | Phase 1/2 | [94] | ||
| External Link | ||||
| EPZ-5676 | Phase 1/2 | [95] | ||
| Synonyms |
pinometostat; 1380288-87-8; EPZ5676; UNII-8V9YR09EF3; UNII-F66X4M38G5; 8V9YR09EF3; CHEMBL3087499; CHEMBL3414626; F66X4M38G5; 1380288-88-9; (2R,3R,4S,5R)-2-(6-amino-9H-purin-9-yl)-5-((((1r,3S)-3-(2-(5-(tert-butyl)-1H-benzo[d]imidazol-2-yl)ethyl)cyclobutyl)(isopropyl)amino)methyl)tetrahydrofuran-3,4-diol; Pinometostat, trans-; 5'-[{cis-3-[2-(5-Tert-Butyl-1h-Benzimidazol-2-Yl)ethyl]cyclobutyl}(Propan-2-Yl)amino]-5'-Deoxyadenosine
Click to Show/Hide
|
|||
| External Link | ||||
| BSK01 | Phase 1/2 | [10] | ||
| External Link | ||||
| CNDO-109 | Phase 1/2 | [12] | ||
| External Link | ||||
| DT388IL-3 | Phase 1/2 | [96] | ||
| External Link | ||||
| PLX2853 | Phase 1/2 | [97] | ||
| External Link | ||||
| CWP232291 | Phase 1/2 | [98] | ||
| External Link | ||||
| BPX-501 | Phase 1/2 | [2] | ||
| External Link | ||||
| RTX-240 | Phase 1/2 | [99] | ||
| External Link | ||||
| G0-203-2c | Phase 1/2 | [100] | ||
| External Link | ||||
| CD123-specific gene-engineered T cells | Phase 1/2 | [86] | ||
| External Link | ||||
| CD56-specific gene-engineered T cells | Phase 1/2 | [86] | ||
| External Link | ||||
| OTX-015 | Phase 1/2 | [101] | ||
| Synonyms |
MK 8628
Click to Show/Hide
|
|||
| External Link | ||||
| FF-10501-01 | Phase 1/2 | [2] | ||
| External Link | ||||
| SGN-CD33A | Phase 1/2 | [12] | ||
| External Link | ||||
| CLL1-specific gene-engineered T cells | Phase 1/2 | [86] | ||
| External Link | ||||
| CLT030 | Phase 1 | [102] | ||
| External Link | ||||
| FT500 | Phase 1 | [103] | ||
| External Link | ||||
| 225Ac-labelled aCD33 | Phase 1 | [104] | ||
| External Link | ||||
| PRGN-3006 | Phase 1 | [105] | ||
| External Link | ||||
| GEM333 | Phase 1 | [106] | ||
| External Link | ||||
| VOB560 | Phase 1 | [107] | ||
| Synonyms |
S 65487
Click to Show/Hide
|
|||
| External Link | ||||
| SEL120 | Phase 1 | [108] | ||
| Synonyms |
SEL120-34A HCl; UNII-SDM3M518PJ; SDM3M518PJ; 1609452-30-3; SEL-120; SEL120-34A (monohydrochloride); SE-120-34A; 1609452-30-3 (HCl); 7,8-Dibromo-9-methyl-2-piperazin-1-yl-5,6-dihydro-4H-imidazo[4,5,1-ij]quinoline Hydrochloride; CHEMBL4578881; SCHEMBL17106021; s8840; EX-A2929-1; HY-111388A; BS-16042; CS-0041061; A17086; SEL120(SEL120-34,SEL120-34A); 4H-Imidazo(4,5,1-ij)quinoline, 7,8-dibromo-5,6-dihydro-9-methyl-2-(1-piperazinyl)-, hydrochloride (1:1); 7,8-Dibromo-5,6-dihydro-9-methyl-2-(1-piperazinyl)-4himidazo(4,5,1-ij)quinoline hydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| NKX101 | Phase 1 | [109] | ||
| External Link | ||||
| AMG 673 | Phase 1 | [2] | ||
| External Link | ||||
| SAR443579 | Phase 1 | [110] | ||
| External Link | ||||
| IO-202 | Phase 1 | [111] | ||
| External Link | ||||
| CYC140 | Phase 1 | [112] | ||
| Synonyms |
Telparevir; Telaprevir - VX-950; SCHEMBL6468440; CS-M3592; ZINC164377440; (1S,3aR,6aS)-2-((R)-2-((S)-2-cyclohexyl-2-(pyrazine-2-carboxamido)acetamido)-3,3-dimethylbutanoyl)-N-((S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl)octahydrocyclopenta[c]pyrrole-1-carboxamide; (3S,3aS,6aR)-2-[(2R)-2-[[(2S)-2-cyclohexyl-2-(pyrazine-2-carbonylamino)acetyl]amino]-3,3-dimethylbutanoyl]-N-[(3S)-1-(cyclopropylamino)-1,2-dioxohexan-3-yl]-3,3a,4,5,6,6a-hexahydro-1H-cyclopenta[c]pyrrole-3-carboxamide; Cyclopenta[c]pyrrole-1-carboxamide, (2S)-2-cyclohexyl-N-(2-pyrazinylcarbonyl)glycyl-3-methyl-L-valyl-N-[(1S)-1-[2-(cyclopropylamino)-2-oxoacetyl]butyl]octahydro-, (1S,3aR,6aS)-
Click to Show/Hide
|
|||
| External Link | ||||
| SKI-G-801 | Phase 1 | [113] | ||
| External Link | ||||
| CC-90002 | Phase 1 | [10] | ||
| External Link | ||||
| TAS-1440 | Phase 1 | [114] | ||
| External Link | ||||
| RG6007 | Phase 1 | [115] | ||
| External Link | ||||
| CB-5339 | Phase 1 | [116] | ||
| External Link | ||||
| SAR445419 | Phase 1 | [117] | ||
| External Link | ||||
| AB-110 | Phase 1 | [118] | ||
| External Link | ||||
| LP-108 | Phase 1 | [119] | ||
| External Link | ||||
| H3B-8800 | Phase 1 | [120] | ||
| Synonyms |
(2S,3S,6S,7R,10R,E)-7,10-Dihydroxy-3,7-dimethyl-12-oxo-2-((R,2E,4E)-6-(pyridin-2-yl)hepta-2,4-dien-2-yl)oxacyclododec-4-en-6-yl 4-methylpiperazine-1-carboxylate; 1825302-42-8; SCHEMBL17255784; DB14017
Click to Show/Hide
|
|||
| External Link | ||||
| CD123-CD33 Ccar | Phase 1 | [121] | ||
| External Link | ||||
| JNJ-63709178 | Phase 1 | [2] | ||
| External Link | ||||
| JNJ-67571244 | Phase 1 | [122] | ||
| External Link | ||||
| AMG 427 | Phase 1 | [123] | ||
| External Link | ||||
| AMG 330 | Phase 1 | [2] | ||
| External Link | ||||
| ABBV-184 | Phase 1 | [124] | ||
| External Link | ||||
| MIK665 | Phase 1 | [125] | ||
| Synonyms |
1799631-75-6; (2~{R})-2-[5-[3-chloranyl-2-methyl-4-[2-(4-methylpiperazin-1-yl)ethoxy]phenyl]-6-(4-fluorophenyl)thieno[2,3-d]pyrimidin-4-yl]oxy-3-[2-[[2-(2-methoxyphenyl)pyrimidin-4-yl]methoxy]phenyl]propanoic acid; (R)-2-((5-(3-chloro-2-methyl-4-(2-(4-methylpiperazin-1-yl)ethoxy)phenyl)-6-(4-fluorophenyl)thieno[2,3-d]pyrimidin-4-yl)oxy)-3-(2-((2-(2-methoxyphenyl)pyrimidin-4-yl)methoxy)phenyl)propanoic acid; MIK-665; S-64315; SCHEMBL16815710; EX-A2912; NSC809971; NSC-809971; HY-112218; CS-0044179; (5R)-(R)-2-((5-(3-Chloro-2-methyl-4-(2-(4-methylpiperazin-1-yl)ethoxy)phenyl)-6-(4-fluorophenyl)thieno[2,3-d]pyrimidin-4-yl)oxy)-3-(2-((2-(2-methoxyphenyl)pyrimidin-4-yl)methoxy)phenyl)propanoic acid; 1799831-02-9; OK5
Click to Show/Hide
|
|||
| External Link | ||||
| FT538 | Phase 1 | [126] | ||
| External Link | ||||
| BGB-11417 | Phase 1 | [127] | ||
| External Link | ||||
| SEA-CD70 | Phase 1 | [128] | ||
| External Link | ||||
| CLL1-CD33 cCART cell therapy | Phase 1 | [129] | ||
| External Link | ||||
| KITE-222 | Phase 1 | [130] | ||
| External Link | ||||
| CYAD-02 | Phase 1 | [131] | ||
| External Link | ||||
| GDX012 | Phase 1 | [132] | ||
| External Link | ||||
| APVO436 | Phase 1 | [133] | ||
| External Link | ||||
| LAM-003 | Phase 1 | [2] | ||
| External Link | ||||
| IMGN779 | Phase 1 | [2] | ||
| External Link | ||||
| BPX-701 | Phase 1 | [10] | ||
| External Link | ||||
| BMS-936564 | Phase 1 | [134] | ||
| External Link | ||||
| UCART123 | Phase 1 | [2] | ||
| External Link | ||||
| FF-10101-01 | Phase 1 | [2] | ||
| External Link | ||||
| CART-56 cells | Phase 1 | [135] | ||
| External Link | ||||
| AVB-S6-500 | Phase 1 | [2] | ||
| External Link | ||||
| BB-MPI-03 | Phase 1 | [69] | ||
| External Link | ||||
| ONO-7475 | Phase 1 | [2] | ||
| External Link | ||||
| LC-1 | Phase 1 | [136] | ||
| Synonyms |
Dimethylamino-parthenolide; Parthenolide analog (leukemia), Leuchemix
Click to Show/Hide
|
|||
| External Link | ||||
| CART123 cells | Phase 1 | [137] | ||
| External Link | ||||
| NKR-2 cells | Phase 1 | [138] | ||
| External Link | ||||
| CART-33 cells | Phase 1 | [135] | ||
| External Link | ||||
| BI-811283 | Phase 1 | [139] | ||
| External Link | ||||
| INNO-305 | Phase 1 | [140] | ||
| External Link | ||||
| IGN523 | Phase 1 | [141] | ||
| External Link | ||||
| CART-34 cells | Phase 1 | [135] | ||
| External Link | ||||
| CM-CS1 T-cell | Phase 1 | [142] | ||
| External Link | ||||
| Mivebresib | Phase 1 | [2] | ||
| Synonyms |
ABBV-075; 1445993-26-9; UNII-VR86R11J7J; VR86R11J7J; N-[4-(2,4-Difluorophenoxy)-3-(6-Methyl-7-Oxo-6,7-Dihydro-1h-Pyrrolo[2,3-C]pyridin-4-Yl)phenyl]ethanesulfonamide; N-(4-(2,4-difluorophenoxy)-3-(6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridin-4-yl)phenyl)ethanesulfonamide; 8NG; Mivebresib [INN]; ABBV-075 (Mivebresib); ABBV075; GTPL9117; SCHEMBL15068241; CHEMBL3987016; Mivebresib(ABBV-075 pound(c); MolPort-044-561-801; RDONXGFGWSSFMY-UHFFFAOYSA-N; BDBM220447; EX-A1082; s8400; ZINC146486516; AKOS030628486; CS-5815
Click to Show/Hide
|
|||
| External Link | ||||
| AMV564 | Phase 1 | [2] | ||
| External Link | ||||
| SAR-103168 | Phase 1 | [143] | ||
| External Link | ||||
| DS-3201 | Phase 1 | [2] | ||
| Synonyms |
QTGYNKYZRZATJB-UHFFFAOYSA-N; 701899-63-0; 2-(2-(2-Methyl-1H-imidazol-5-yl)ethyl)isoindoline-1,3-dione; 2-(2-(2-Methyl-1H-imidazol-5-yl)-ethyl)isoindoline-1,3-dione; SCHEMBL7743461; MolPort-035-945-474; MolPort-035-690-353; ZINC98086069; AKOS024459002; AKOS024262663; MCULE-2708350770; FCH4077443; AK158834; AX8292467; ST2403812; 2-[2-(2-methyl-1H-imidazol-5-yl)ethyl]-2,3-dihydro-1H-isoindole-1,3-dione
Click to Show/Hide
|
|||
| External Link | ||||
| PF-06747143 | Phase 1 | [10] | ||
| External Link | ||||
| DCLL9718S | Phase 1 | [2] | ||
| External Link | ||||
| CD123CAR-41BB-CD3zeta-EGFRt-expressing T cells | Phase 1 | [144] | ||
| External Link | ||||
| CSL-362 | Phase 1 | [145] | ||
| Synonyms |
CSL-360; Monoclonal antibody (acute myelogenous leukemia), CSL; Therapeutic leukaemia antibody (AML), CSL; 7G3
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 176 | Phase 1 | [146] | ||
| Synonyms |
JQNINBDKGLWYMU-GEAQBIRJSA-N; AMG-176; 1883727-34-1; AMG176; SCHEMBL17550216; EX-A2666; HY-101565; CS-0021721; Spiro[5,7-etheno-1H,11H-cyclobut[i][1,4]oxazepino[3,4-f][1,2,7]thiadiazacyclohexadecine-2(3H),1'(2'H)-naphthalen]-8(9H)-one, 6'-chloro-3',4',12,13,16,16a,17,18,18a,19-decahydro-16-methoxy-11,12-dimethyl-,10,10-dioxide, (1'S,11R,12S,14E,16S,16aR,18aR)-
Click to Show/Hide
|
|||
| External Link | ||||
| XmAb14045 | Phase 1 | [2] | ||
| External Link | ||||
| SY-1425 | Phase 1 | [2] | ||
| External Link | ||||
| AGS62P1 | Phase 1 | [2] | ||
| Synonyms |
AGS-62P1
Click to Show/Hide
|
|||
| External Link | ||||
| CART-117 cells | Phase 1 | [135] | ||
| External Link | ||||
| CART-Muc1 cells | Phase 1 | [135] | ||
| External Link | ||||
| BAY1436032 | Phase 1 | [2] | ||
| Synonyms |
RNMAUIMMNAHKQR-QFBILLFUSA-N; BAY-1436032; 1803274-65-8; BAY 1436032; SCHEMBL17009632; EX-A1606; AKOS032946249; SB19763; HY-100020; CS-0017982; 3-(2-((4-(trifluoromethoxy)phenyl)amino)-1-((1R,5R)-3,3,5-trimethylcyclohexyl)-1H-benzo[d]imidazol-5-yl)propanoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| CD33-CAR-T Cell | Phase 1 | [147] | ||
| External Link | ||||
| CART-123 cells | Phase 1 | [135] | ||
| External Link | ||||
| FATE-NK100 | Phase 1 | [2] | ||
| External Link | ||||
| CC-90009 | Phase 1 | [2] | ||
| External Link | ||||
| NKR-2 CAR-T Cells | Phase 1 | [148] | ||
| External Link | ||||
| Anti-CEA CAR-T cells | Phase 1 | [149] | ||
| External Link | ||||
| RG7775 | Phase 1 | [150] | ||
| External Link | ||||
| OTSSP167 | Phase 1 | [2] | ||
| Synonyms |
OTSSP 167; OTSSP-167; AMX10201
Click to Show/Hide
|
|||
| External Link | ||||
| IMC-EB10 | Phase 1 | [151] | ||
| Synonyms |
Anti-Flt-3 Mab
Click to Show/Hide
|
|||
| External Link | ||||
| TCN-P | Phase 1 | [152] | ||
| Synonyms |
TRICIRIBINE PHOSPHATE; 61966-08-3; UNII-5L5GE3DV88; Triciribine phosphate [USAN]; Tricirbine Phosphate; NSC-280594; C13H17N6O7P; NSC 280594; Pentaazaacenaphthylene-5' phosphate ester; 5L5GE3DV88; PHOSPHATE SALT OF TRICYCLIC NUCLEOSIDE; 3-Amino-1,5-dihydro-5-methyl-1-beta-D-ribofuranosyl-1,4,5,6,8-pentaazaacenaphthylene 5'-(dihydrogen phosphate); Triciribine phosphate (USAN); VQD-002; TCN-monophosphate; 1,4,5,6,8-Pentaazaacenaphthylen-3-amine, 1,5-dihydro-5-methyl-1-(5-O-phosphono-beta-D-ribofuranosyl)-; NSC280594
Click to Show/Hide
|
|||
| External Link | ||||
| CART-38 cells | Phase 1 | [135] | ||
| External Link | ||||
| ABBV-744 | Phase 1 | [2] | ||
| Synonyms |
OEDSFMUSNZDJFD-UHFFFAOYSA-N; 2138861-99-9; N-ethyl-4-(2-(4-fluoro-2,6-dimethylphenoxy)-5-(2-hydroxypropan-2-yl)phenyl)-6-methyl-7-oxo-6,7-dihydro-1H-pyrrolo[2,3-c]pyridine-2-carboxamide; ABBV 744; SCHEMBL19463409; EX-A2713; ACN-054460; HY-112090; CS-0043318
Click to Show/Hide
|
|||
| External Link | ||||
| AGS-67E | Phase 1 | [10] | ||
| External Link | ||||
| PNK-007 | Phase 1 | [10] | ||
| External Link | ||||
| GSK-2130579A | Phase 1 | [153] | ||
| Synonyms |
Leukemia vaccine, GlaxoSmithKline; WT1 ASCI, GlaxoSmithKline; WT1 antigen specific cancer immunotherapeutic, GlaxoSmithKline; WT1 vaccine, GlaxoSmithKline
Click to Show/Hide
|
|||
| External Link | ||||
| SGN-CD123A | Phase 1 | [2] | ||
| External Link | ||||
| IM23 | Phase 1 | [154] | ||
| External Link | ||||
| Anti-CD33 | Phase 1 | [2] | ||
| External Link | ||||
| KW-2449 | Phase 1 | [155] | ||
| Synonyms |
Tumor antigen-specific mAb (cancer), Kyowa; Tumor antigen-specific monoclonal antibody (cancer), Kyowa Hakko Kirin; Tumor antigen-specific monoclonal antibody (cancer), Kyowa Hakko Kogyo
Click to Show/Hide
|
|||
| External Link | ||||
| CAR-T cells targeting CD56 | Clinical trial | [156] | ||
| External Link | ||||
| CAR-T cells targeting CD38 | Clinical trial | [156] | ||
| External Link | ||||
| Valspodar | Discontinued in Phase 3 | [157] | ||
| External Link | ||||
| LY335979 | Discontinued in Phase 3 | [158] | ||
| Synonyms |
Zosuquidar HCl; Zosuquidar Trihydrochloride; LY 335979; LY-335979; Zosuquidar (TN); Zosuquidar trihydrochloride (USAN); RS-33295-198; Zosuquidar trihydrochloride, RS-33295-198, LY335979; (R)-4-((1aR,6R,10bS)-1,2-Difluoro-1,1a,6,10b-tetrahydrodibenzo(a,e)cyclopropa(c)cycloheptan-6-yl)-alpha-((5-quinoloyloxy)methyl)-1-piperazineethanol, trihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| Lintuzumab Bi-213 | Discontinued in Phase 1/2 | [159] | ||
| Synonyms |
Bismab-A (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| HuM-195-Bi-213 | Discontinued in Phase 1/2 | [159] | ||
| Synonyms |
SMART 213Bi-M195; SMART Y90-M195; Y90-HuM195; Bismuth-213-HuM195; SMART bismuth-213-M195; Yttrium-90-HuM195; Alpha-particle-emitting radioisotope-linked lintuzumab; 213Bi-HuM195
Click to Show/Hide
|
|||
| External Link | ||||
| CER-227185 | Discontinued in Phase 1/2 | [160] | ||
| Synonyms |
TCTP inhibitor (cancer), Cerenis; Translationally controlled tumor protein inhibitor (acute myeloid leukemia), Cerenis
Click to Show/Hide
|
|||
| External Link | ||||
| Autologous Anti-CD 123 CAR TCR/4-1BB-expressing T-lymphocytes | Phase 0 | [161] | ||
| External Link | ||||
| ATA2321 | Preclinical | [162] | ||
| External Link | ||||
| 2-D08 | Preclinical | [163] | ||
| Synonyms |
144707-18-6; 2',3',4'-trihydroxyflavone; 2-(2,3,4-trihydroxyphenyl)-4H-1-benzopyran-4-one; 2-(2,3,4-Trihydroxyphenyl)-4H-chromen-4-one; SCHEMBL1772778; CHEMBL3115475; AOB4275; SYN5001; AMY31051; BCP18314; EX-A3562; MFCD27995567; s8696; ZINC97439024; CCG-267148; AC-31428; AK312791; AS-16368; HY-114166; 2D08;2 D08; CS-0077855; A16852; 2-(2,3,4-TRIHYDROXYPHENYL)CHROMEN-4-ONE
Click to Show/Hide
|
|||
| External Link | ||||
| CAR-T cells targeting CD117 | Preclinical | [156] | ||
| External Link | ||||
| CAR-T cells targeting CD123 | Preclinical | [156] | ||
| External Link | ||||
| CAR-T cells targeting Mucl | Preclinical | [156] | ||
| External Link | ||||
| CAR-T cells targeting CD33 | Preclinical | [156] | ||
| External Link | ||||
| CAR-T cells targeting CD34 | Preclinical | [156] | ||
| External Link | ||||
| CAR-T cells targeting CD133 | Preclinical | [156] | ||
| External Link | ||||
| CEP-4186 | Terminated | [164] | ||
| Synonyms |
CB-1093; CEP-3265; GS-1590; Neurotrophic compounds, Cephalon/LEO Pharma; Neurotrophic factors, Cephalon/LEO Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| BI 7273 | Investigative | [165] | ||
| Synonyms |
BI-7273; 1883429-21-7; CHEMBL3823478; 4-(4-((Dimethylamino)methyl)-3,5-dimethoxyphenyl)-2-methyl-2,7-naphthyridin-1(2H)-one; 4-[4-[(Dimethylamino)methyl]-3,5-Dimethoxy-Phenyl]-2-Methyl-2,7-Naphthyridin-1-One; 4-[4-[(dimethylamino)methyl]-3,5-dimethoxyphenyl]-2-methyl-2,7-naphthyridin-1-one; 4-{4-[(dimethylamino)methyl]-2,6-dimethoxyphenyl}-2-methyl-1,2-dihydro-2,7-naphthyridin-1-one; 4-[4-[(dimethylamino)methyl]-3,5-dimethoxyphenyl]-2-methyl-2,7-naphthyridin-1(2H)-one; 5SW; BI7273; GTPL9146; SCHEMBL19869878; EX-A990; BCP17545; BDBM50183448; MFCD30489736; s8179; ZINC575448880; CCG-268068; CS-5887; HY-100351; J3.533.936A; A16068; Q27075221
Click to Show/Hide
|
|||
| External Link | ||||
| G3139 + cytarabine (ARA-C) | Investigative | [166] | ||
| External Link | ||||
| G3139 + Fludarabine | Investigative | [166] | ||
| External Link | ||||
| CG-1255 | Investigative | [167] | ||
| Synonyms |
CG-1552; Histone deacetylase inhibitors, CircaGen
Click to Show/Hide
|
|||
| External Link | ||||
| AC-501 | Investigative | [167] | ||
| External Link | ||||
| AKN-028 | Investigative | [168] | ||
| Synonyms |
Flt3 tyrosine kinase inhibitor (AML), Akinion/Swedish Orphan Biovitrum/Karolinska
Click to Show/Hide
|
|||
| External Link | ||||
| VLIM-88 | Investigative | [167] | ||
| External Link | ||||
| G3139 + G-CSF | Investigative | [166] | ||
| External Link | ||||
| Anti-CD44 mab | Investigative | [167] | ||
| Synonyms |
Hyaloxan; MAT-102; AML therapy (chimerized monoclonal antibody), MAT Biopharma; Acute myeloid leukemia (chimerized monoclonal antibody), MAT Biopharma; Acute myeloid leukemia therapy (chimerized monoclonal antibody), MAT Biopharma; Anti-CD44 mAb (AML); Anti-CD44 mAb (AML), MAT Biopharma; Anti-CD44 monoclonal antibody (acute myeloid leukemia), MAT Biopharma
Click to Show/Hide
|
|||
| External Link | ||||
| SB-559457 | Investigative | [169] | ||
| External Link | ||||
| COTI-001 | Investigative | [167] | ||
| Synonyms |
COTI-002; COTI-003E; Protein tyrosine kinase inhibitors (AML); Protein tyrosine kinase inhibitors (AML), Critical Outcome Technologies
Click to Show/Hide
|
|||
| External Link | ||||
| Rebelex | Investigative | [167] | ||
| Synonyms |
Spleen tyrosine kinase inhibitor (siRNA/oligonucleotide, acute myelogenous leukemia), Biothorpe Pharmaceuticals; Syk kinase inhibitor (siRNA/oligonucleotide, acute myelogenous leukemia), Biothorpe Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
References