m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05719
|
[1] | |||
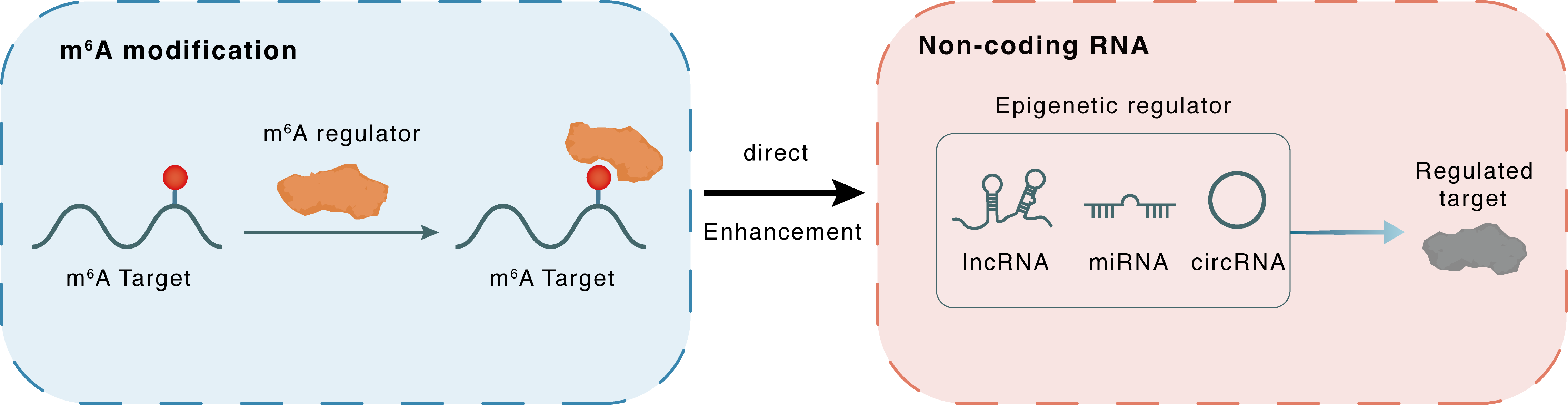 m6A modification
Circ_TET2
Circ_TET2
YTHDC1
m6A modification
Circ_TET2
Circ_TET2
YTHDC1
 : m6A sites
Direct
Enhancement
Non-coding RNA
circTET2
HNRNPC
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
circTET2
HNRNPC
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing protein 1 (YTHDC1) | READER | |||
| m6A Target | Circ_TET2 | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Circ_TET2 | circRNA | View Details | ||
| Regulated Target | Heterogeneous nuclear ribonucleoprotein C (HNRNPC) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | The mTOR inhibitor dactolisib and FAO inhibitor perhexiline exert a synergistic effect on CLL cells. In addition, the biogenesis of Circ_TET2 can be affected by the splicing process and the RBPs RBMX and YTHDC1. CP028, a splicing inhibitor, modulates the expression of circTET2 and shows pronounced inhibitory effects. m6A-Modified circTET2 Interacting with Heterogeneous nuclear ribonucleoprotein C (HNRNPC) Regulates Fatty Acid Oxidation to Promote the Proliferation of Chronic Lymphocytic Leukemia. | ||||
| Responsed Disease | Chronic lymphocytic leukemia | ICD-11: 2A82.0 | |||
| Responsed Drug | CP028 | ||||
| Pathway Response | mTOR signaling pathway | hsa04150 | |||
| Cell Process | Cell proliferation | ||||
In-vitro Model |
PBMCs (Human peripheral blood mononuclear cells (PBMCs) are isolated from peripheral blood and identified as any blood cell with a round nucleus) | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2A82: Leukemogenesis | 1 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| AGS-67E | Phase 1 | [2] | ||
| External Link | ||||
References