m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05694
|
[1] | |||
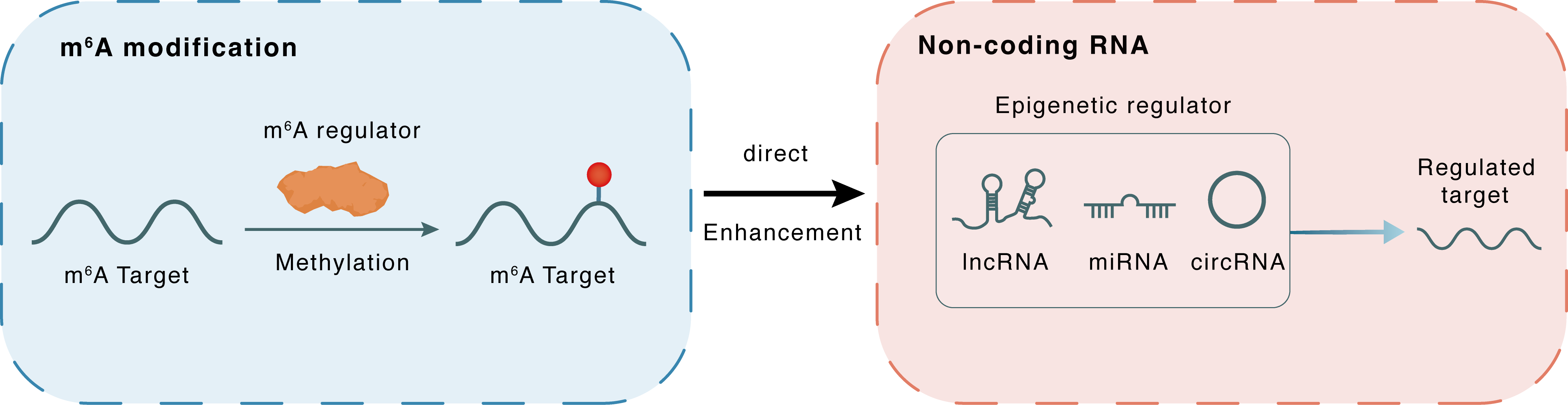 m6A modification
MALAT1
MALAT1
METTL3
Methylation
m6A modification
MALAT1
MALAT1
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
MALAT1
miR-124-3p
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
MALAT1
miR-124-3p
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) | LncRNA | View Details | ||
| Regulated Target | hsa-miR-124-3p | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | ES samples expressed low hsa-miR-124-3p and high METTL3, Metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and CDK4. METTL3 elevated MALAT1 expression by m6A modification. MALAT1 enhanced CDK4 expression by competing with miR-124-3p. In ES cells, METTL3 silencing repressed cell migration and invasion by inhibiting MALAT1. In conclusion, METTL3 promotes tumorigenesis of ES through the MALAT1/miR-124-3p/CDK4 axis. | ||||
| Responsed Disease | Ewing's sarcoma | ICD-11: 2B52 | |||
| Cell Process | Cell migration | ||||
| Cell invasion | |||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2B52: Ewing's sarcoma | 6 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| MK-4827 | Phase 3 | [2] | ||
| Synonyms |
1038915-60-4; (S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-carboxamide; UNII-HMC2H89N35; HMC2H89N35; CHEMBL1094636; 2-{4-[(3s)-piperidin-3-yl]phenyl}-2h-indazole-7-carboxamide; MK4827; MK 4827; Niraparib [USAN:INN]; 2-{4-[(3S)-piperidin-3-yl]phenyl}indazole-7-carboxamide; 2-[4-[(3S)-piperidin-3-yl]phenyl]indazole-7-carboxamide; MK 4827 (Base); Niraparib (USAN); Zejula (TN); MK-4827(Niraparib); SCHEMBL1421875; GTPL8275; CTK8B9123; EX-A290; DTXSID50146129; MolPort-023-219-142; ZINC43206370; BDBM50316226
Click to Show/Hide
|
|||
| External Link | ||||
| FANG vaccine | Phase 3 | [3] | ||
| Synonyms |
FANG (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Seclidemstat | Phase 1 | [4] | ||
| Synonyms |
UNII-TYH386V3WJ; SP-2577; TYH386V3WJ; 1423715-37-0; SP2577; CHEMBL4297641; SCHEMBL14697017; SCHEMBL14697019; EX-A3574; s6722; BS-15371; HY-103713; CS-0039281; Benzoic acid, 3-((4-methyl-1-piperazinyl)sulfonyl)-, (2E)-2-(1-(5-chloro-2-hydroxyphenyl)ethylidene)hydrazide
Click to Show/Hide
|
|||
| External Link | ||||
| pbi-shRNA EWS-FLI-Type 1 | Phase 1 | [5] | ||
| External Link | ||||
| TK216 | Phase 1 | [2] | ||
| External Link | ||||
| Ganitumab | Clinical trial | [6] | ||
| External Link | ||||
References