m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05589
|
[1] | |||
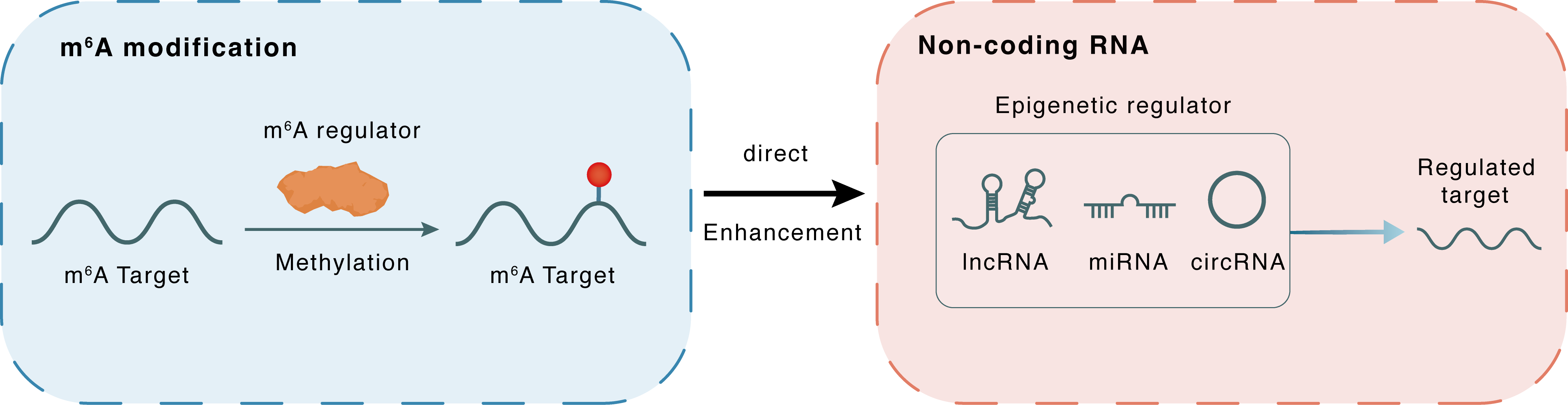 m6A modification
FOXD2-AS1
FOXD2-AS1
WTAP
Methylation
m6A modification
FOXD2-AS1
FOXD2-AS1
WTAP
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
FOXD2-AS1
FOXM1
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
FOXD2-AS1
FOXM1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Wilms tumor 1-associating protein (WTAP) | WRITER | |||
| m6A Target | FOXD2 adjacent opposite strand RNA 1 (FOXD2-AS1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | FOXD2 adjacent opposite strand RNA 1 (FOXD2-AS1) | LncRNA | View Details | ||
| Regulated Target | Forkhead box protein M1 (FOXM1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | A remarkable m6A-modified site was found on the 3'-UTR of FOXD2 adjacent opposite strand RNA 1 (FOXD2-AS1), and m6A methyltransferase WTAP promoted the methylation modification, thus enhancing the stability of FOXD2-AS1 transcripts. m6A-modified FOXD2-AS1 accelerates the osteosarcoma progression through m6A manner, which provides new concepts for osteosarcoma tumorigenesis. FOXD2-AS1 interacted with downstream target Forkhead box protein M1 (FOXM1) mRNA through m6A sites, forming a FOXD2-AS1/m6A/FOXM1 complex to heighten FOXM1 mRNA stability. | ||||
| Responsed Disease | Osteosarcoma | ICD-11: 2B51 | |||
| Cell Process | Cell migration | ||||
| Cell proliferation | |||||
| mRNA stability | |||||
In-vitro Model |
MG-63 | Osteosarcoma | Homo sapiens | CVCL_0426 | |
| SaOS-2 | Osteosarcoma | Homo sapiens | CVCL_0548 | ||
| hFOB 1.19 | Normal | Homo sapiens | CVCL_3708 | ||
| In-vivo Model | In vivo animal assay, FOXD2-AS1 overexpression promoted the tumor growth in mice subcutaneous injection | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Forkhead box protein M1 (FOXM1) | 1 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| (D-Arg)(9)-p19(ARF) 26-44 peptide | Investigative | [2] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B51: Osteosarcoma | 12 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Cisplatin | Approved | [3] | ||
| Synonyms |
Abiplatin; Biocisplatinum; Briplatin; Cismaplat; Cisplatine; Cisplatino; Cisplatinum; Cisplatyl; Citoplationo; Lederplatin; Neoplatin; Plastin; Platamine; Platidiam; Platinoxan; Randa; Cis-DDP; Cis-Diamminedichloroplatinum; Peyrone's chloride; Peyrone's salt; Cis-Dichlorodiammineplatinum(II); Cis-[PtCl2(NH3)2]; Cis-diamminedichloridoplatinum(II); Trans-diamminedichloridoplatinum(II); (SP-4-1)-diamminedichloridoplatinum; (SP-4-1)-diamminedichloroplatinum; (SP-4-2)-diamminedichloridoplatinum; (SP-4-2)-diamminedichloroplatinum; Cisplatin (Chemotherapy)
Click to Show/Hide
|
|||
| External Link | ||||
| Naxitamab | Phase 2 | [3] | ||
| External Link | ||||
| Tideglusib | Phase 2 | [4] | ||
| Synonyms |
NP-031112; NP-12; NP031112; Tideglusib(NP-031112)
Click to Show/Hide
|
|||
| External Link | ||||
| Hu3F8 mAb | Phase 2 | [5] | ||
| External Link | ||||
| Saracatinib | Phase 2 | [6] | ||
| Synonyms |
H8H; AZD-0530; Saracatinib, AZD-0530, AZD0530; N-(5-Chloro-1,3-benzodioxol-4-yl)-7-(2-(4-methylpiperazin-1-yl)ethoxy)-5-(tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine
Click to Show/Hide
|
|||
| External Link | ||||
| ALMB-0168 | Phase 1/2 | [7] | ||
| External Link | ||||
| AU101 | Phase 1/2 | [8] | ||
| External Link | ||||
| [153Sm]CycloSam | Phase 1 | [9] | ||
| External Link | ||||
| GD2 T cells | Phase 1 | [10] | ||
| External Link | ||||
| Anti-GD2-CAR engineered T cells | Phase 1 | [11] | ||
| External Link | ||||
| Robatumumab | Discontinued in Phase 2 | [12] | ||
| External Link | ||||
| DG-8 | Investigative | [13] | ||
| Synonyms |
DH-9; PPAR gamma agonists (osteosarcoma/ADPKD); PPAR gamma agonists (osteosarcoma/ADPKD), Chinese Academy of Sciences
Click to Show/Hide
|
|||
| External Link | ||||
References