m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05556
|
[1] | |||
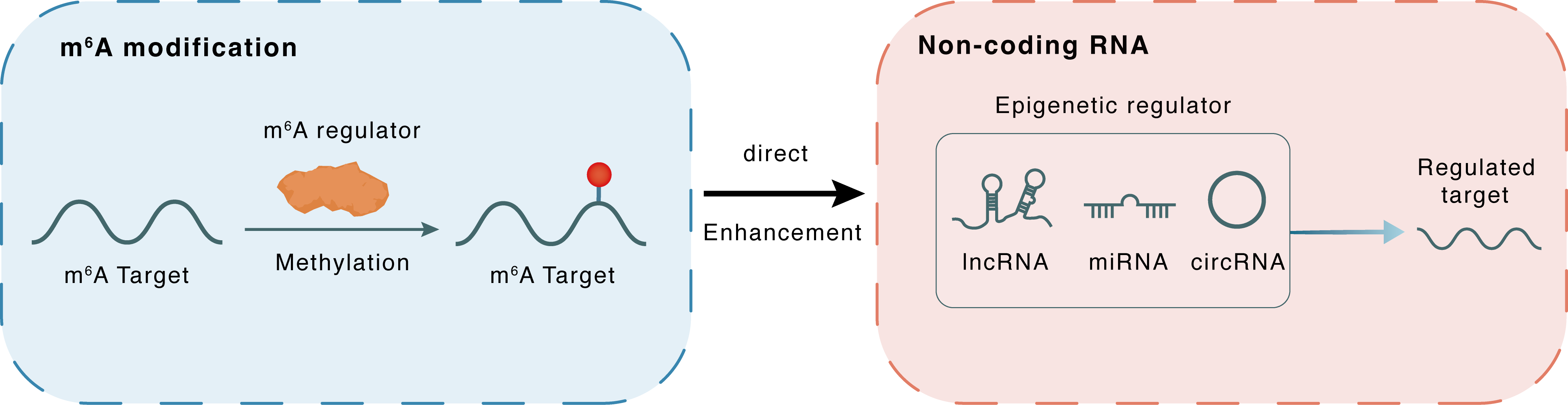 m6A modification
hsa-miR-375-3p
hsa-miR-375-3p
METTL3
Methylation
m6A modification
hsa-miR-375-3p
hsa-miR-375-3p
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR-375-3p
PDK1
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR-375-3p
PDK1
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | hsa-miR-375-3p | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-375-3p | microRNA | View Details | ||
| Regulated Target | PDH kinase 1 (PDK1) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | Silencing METTL3 inhibited m6A level and decreased the binding of DGCR8 to pri-miR-375 and further limited hsa-miR-375-3p expression. METTL3-mediated m6A modification promoted VSMC phenotype transformation and made Atherosclerosis (AS) plaques more vulnerable via the miR-375-3p/PDH kinase 1 (PDK1) axis. | ||||
| Responsed Disease | Atherosclerosis | ICD-11: BD40 | |||
In-vitro Model |
MOVAS (Mouse aortic vascular smooth muscle cell lines) | ||||
| In-vivo Model | The normal group consisted of C57BL/6J mice on normal diet and the HFD group consisted of ApoE C57BL/6J mice on HFD (containing 10% lard oil, 4% milk powder, 2% cholesterol, and 0.5% sodium cholate). Four weeks after HFD feeding, the mice were injected with 200 ul lentivirus containing 1 × 10-/--/-8 TU (lentivirus carrying sh-METTL3 or sh-NC; designed and constructed by GENCHEM (Shanghai, China)) via tail vein. Six weeks after transfection, all mice were euthanized by a tail vein injection of 200 mg/kg pentobarbital sodium. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| PDH kinase 1 (PDK1) | 99 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Dichloroacetate | Phase 4 | [2] | ||
| Synonyms |
2,2-dichloroacetate; Dichloracetate; Dichloroacetate ion; 13425-80-4; Dichloroacetic acid ion(1-); DCA; BRN 3903873; 2q8h; ACETIC ACID, DICHLORO-, ION(1-); 2,2-bis(chloranyl)ethanoate; GTPL4518; CHEBI:28240; DTXSID40158610; STL483470; NCGC00241105-01; 68626-EP2292227A2; 68626-EP2292628A2; 68626-EP2298776A1; 68626-EP2308861A1; 68626-EP2374454A1; A839686; Q27077050
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 4 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 27(6)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Pyrazole derivative 61 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20090111799
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 2000 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2011137219 37(4-6) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 110 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 3 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 26(5)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Pyrimidine derivative 3 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2011076327
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 120 nM | |||
| External Link | ||||
| Heterocyclic derivative 15 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012044157 4(58)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thieno[3,2-c]pyridine-7-carboxamide derivative 2 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2006106326c2
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 160 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 9 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 32(20)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 24 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 18(27)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Heterocyclic derivative 12 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012044157 1(1.5)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20120277229 41(1.3) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 280 nM | |||
| External Link | ||||
| Heterocyclic derivative 17 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012044157 6(360)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 27 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 21(40)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2008079988 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Pyridinone carboxamide derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2010007116
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 1000 nM | |||
| External Link | ||||
| Heterocyclic derivative 4 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20120208819 1(1.5)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thieno[3,2-c]pyridine-7-carboxamide derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2006106326c1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 350 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 9 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 18(27)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2009153313 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20120277229 40(1.2) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 160 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 12 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 21(40)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2004087707 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 320 to 32000 nM | |||
| External Link | ||||
| Benzimidazole and imidazopyridine derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2011006567
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.1 to 10 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 14 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 24(45)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20130053382 38(5-7) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 85 nM | |||
| External Link | ||||
| Heterocyclic derivative 9 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20120208819 6(360)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Heterocyclic derivative 10 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20120208819 7(402)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 11 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 20(38)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20130053382 36(3-5) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 170 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20120277229 45(1.5) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 780 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 29 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 24(45)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2008005457 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 500 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 2 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 25(3)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 7 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 16(16)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Heterocyclic derivative 11 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20120208819 8(443)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20120277229 39(1.1) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 140 nM | |||
| External Link | ||||
| Heterocyclic derivative 8 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20120208819 5(355)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 20 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 14(14)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2011137219 36(3-5) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 170 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2010127754 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.5 to 1000 nM | |||
| External Link | ||||
| Indazole derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2003064397
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10 to 140 nM | |||
| External Link | ||||
| Heterocyclic-carboxamide derivative 2 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2011044157
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 1000 nM | |||
| External Link | ||||
| Heterocyclic derivative 6 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20120208819 3(7.6)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Imidazo quinoline derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2008054238
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 34 to 330 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 16 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 10(4)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2012135799 42(1.4) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| Bis-indolylmaleimide derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2005041953
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 750 to 14000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 23 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 17(23)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Heterocyclic derivative 14 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012044157 3(7.6)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 18 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 12(12)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Heterocyclic derivative 18 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012044157 7(402)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 17 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 11(5)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 6 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 29(8)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 19 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 13(13)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 4 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 13(13)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 24(47)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Heterocyclic derivative 13 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012044157 2(2.4)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 2 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 11(5)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Pyrazolopyridines and imidazopyridine derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2010017047
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 30000 nM | |||
| External Link | ||||
| Dibenzo [c,f]-[2,7]naphthyridine derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20070135429
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 60 to 7500 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2011137219 38(5-7) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 85 nM | |||
| External Link | ||||
| Benzonaphthyridine derivative 2 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2008109613
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| Heterocyclic derivative 5 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20120208819 2(2.4)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 25 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 19(36)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 10 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 19(36)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 22 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 16(16)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 5 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 28(7)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2008107444 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 <= 1000 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 7 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 30(12)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2011137219 35(1-11) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 33 nM | |||
| External Link | ||||
| Pyrimidinone derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2010007114
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 1000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 21 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 15(15)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 8 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 17(23)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| 1,2-dihydroindazolo[4,3-bc][1,5]benzoxazepine derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2010120854
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7.6 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 10(4)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 15 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 9(2)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 28 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 23(41)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2012135799 43(1.5) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 780 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 3 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 12(12)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20120277229 44(1.4) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 300 nM | |||
| External Link | ||||
| Heterocyclic derivative 16 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012044157 5(355)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| PMID25684022-Compound-WO2006015124 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 1000 to 5000 nM | |||
| External Link | ||||
| Heterocyclic-carboxamide derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2010065384
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 30000 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20130053382 35(1-11) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 33 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 10 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 33(21)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Benzonaphthyridine derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2008109599
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 8 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 5 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 14(14)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 26 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 20(38)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Heterocyclic derivative 7 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20120208819 4(58)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| Heteroaryl-carboxamide derivative 8 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012058176 31(13)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 <= 50 nM | |||
| External Link | ||||
| Heterocyclic derivative 19 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012044157 8(443)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 5000 nM | |||
| External Link | ||||
| PMID25684022-Compound-US20130053382 37(4-6) | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 110 nM | |||
| External Link | ||||
| PMID25684022-Compound-EP20041486488 | Patented | [3] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 20000 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 30 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2012036974 9(2)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 6 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 15(15)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Indolinone derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US200607105563
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 520 nM | |||
| External Link | ||||
| Thiazole carboxamide derivative 13 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-US20130165450 23(41)
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 250 nM | |||
| External Link | ||||
| Pyrido[4,3-d]pyrimidin-5(6H)-one derivative 1 | Patented | [3] | ||
| Synonyms |
PMID25684022-Compound-WO2010019637
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3 to 10000 nM | |||
| External Link | ||||
| BD40: Atherosclerosis | 125 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Acipimox | Approved | [4] | ||
| Synonyms |
Monted; Nedios; Olbemox; Olbetam; K 9321
Click to Show/Hide
|
|||
| External Link | ||||
| LAROPIPRANT | Phase 4 | [5] | ||
| Synonyms |
571170-77-9; MK 0524; Cardaptive; MK-0524; UNII-G7N11T8O78; CHEMBL426559; G7N11T8O78; 2-[(3R)-4-[(4-chlorophenyl)methyl]-7-fluoro-5-methylsulfonyl-2,3-dihydro-1H-cyclopenta[b]indol-3-yl]acetic acid; 2-[(3R)-4-[(4-chlorophenyl)methyl]-7-fluoro-5-methanesulfonyl-1H,2H,3H,4H-cyclopenta[b]indol-3-yl]acetic acid; Tedaptive; Laropiprant [USAN:INN:BAN]; [14C]-Laropiprant; Laropiprant/MK-0524; Laropiprant (INN/USAN); Laropiprant (MK-0524); SCHEMBL991107; AMOT0189; GTPL3356; KS-00000XIE; CTK8F0660; MK-0524B
Click to Show/Hide
|
|||
| External Link | ||||
| Anacetrapib | Phase 3 | [6] | ||
| Synonyms |
MK0859; Anacetrapib (USAN); MK-0859
Click to Show/Hide
|
|||
| External Link | ||||
| LY2157299 | Phase 2/3 | [7] | ||
| Synonyms |
Galunisertib; 700874-72-2; LY 2157299; LY-2157299; UNII-3OKH1W5LZE; 4-(2-(6-methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide; 3OKH1W5LZE; Galunisertib (LY2157299); AK-79916; 4-[5,6-Dihydro-2-(6-methyl-2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-6-quinolinecarboxamide; 4-(2-(6-Methylpyridin-2-yl)-5,6-dihydro-4H-pyrrolo-[1,2-b]pyrazol-3-yl)quinoline-6-carboxamide; 4-[2-(6-methylpyridin-2-yl)-4H,5H,6H-pyrrolo[1,2-b]pyrazol-3-yl]quinoline-6-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| PACTIMIBE | Phase 2/3 | [8] | ||
| Synonyms |
189198-30-9; UNII-D874R9PZ9T; D874R9PZ9T; CHEMBL478858; Pactimibe [INN:BAN]; [7-(2,2-Dimethylpropanamido)-4,6-dimethyl-1-octylindolin-5-yl]acetic acid; (7-(2,2-Dimethylpropanamido)-4,6-dimethyl-1-octylindolin-5-yl)acetic acid; AC1MIXTD; SCHEMBL282098; CTK0H7032; DTXSID80172315; TXIIZHHIOHVWJD-UHFFFAOYSA-N; ZINC1545445; BDBM50263192; DB12971; 2-[7-(2,2-Dimethylpropanoylamino)-4,6-dimethyl-1-octyl-2,3-dihydroindo L-5-yl]acetic acid; AN-30445; 198P309
Click to Show/Hide
|
|||
| External Link | ||||
| LY3012212 | Phase 2 | [9] | ||
| Synonyms |
Icrucumab
Click to Show/Hide
|
|||
| External Link | ||||
| K-134 | Phase 2 | [10] | ||
| Synonyms |
SULBACTAM; 68373-14-8; sulbactam acid; Sulbactamum; Betamaze; Penicillanic Acid Sulfone; (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid 4,4-dioxide; penicillanic acid 1,1-dioxide; UNII-S4TF6I2330; CP 45899; CHEMBL403; CHEBI:9321; S4TF6I2330; Sulbactam;Penicillanic acid sulfone; (2S,5R)-3,3-Dimethyl-7-oxo-4-thia-1-azabicyclo(3.2.0)heptane-2-carboxylic acid 4,4-dioxide; Sulbactam [INN:BAN]; CP-45,899; Sulbactamum [INN-Latin]; DSSTox_CID_3605; DSSTox_RID_77104
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-582949 | Phase 2 | [11] | ||
| Synonyms |
623152-17-0; BMS 582949; UNII-CR743OME9E; BMS582949; CR743OME9E; PS540446; CHEMBL1230065; PS-540446; 4-{[5-(cyclopropylcarbamoyl)-2-methylphenyl]amino}-5-methyl-N-propylpyrrolo[2,1-f][1,2,4]triazine-6-carboxamide; 3mvl; SCHEMBL254996; GTPL7838; DTXSID90211380; MolPort-044-560-326; EX-A1265; BCP14356; ZINC36475284; s8124; BDBM50327009; AKOS030573299; DB12696; Pyrrolo(2,1-f)(1,2,4)triazine-6-carboxamide, 4-((5-((cyclopropylamino)carbonyl)-2-methylphenyl)amino)-5-methyl-n-propyl-; BMS582949 free base; PS 540446
Click to Show/Hide
|
|||
| External Link | ||||
| VB-201 | Phase 2 | [12] | ||
| External Link | ||||
| CSL-112 | Phase 2 | [13] | ||
| Synonyms |
Reconstituted HDL (acute coronary syndrome), CSL
Click to Show/Hide
|
|||
| External Link | ||||
| MK-1903 | Phase 2 | [14] | ||
| Synonyms |
Second-generation niacin receptor agonists (oral, atherosclerosis), Arena/Merck & Co
Click to Show/Hide
|
|||
| External Link | ||||
| DRL-17822 | Phase 2 | [15] | ||
| Synonyms |
CETP inhibitor (dyslipidemia/atherosclerosis/cardiovascular diseases), Dr Reddy's
Click to Show/Hide
|
|||
| External Link | ||||
| PRC-4016 | Investigative | [16] | ||
| External Link | ||||
| K-604 | Phase 2 | [17] | ||
| Synonyms |
BDBM86691
Click to Show/Hide
|
|||
| External Link | ||||
| Revacept | Phase 2 | [18] | ||
| Synonyms |
Arterial thrombosis treatment, Trigen; PR-15; Antiplatelet protein therapy (arterial thrombosis/atherosclerosis), Trigen
Click to Show/Hide
|
|||
| External Link | ||||
| DVC1-0101 | Phase 2 | [19] | ||
| Synonyms |
Ischemia gene therapy, DNAVEC\Kyushu University; FGF2-carrying Sendai virus, DNAVEC\Kyushu University; SeV-FGF2 gene therapy (virus recombinant, ischaemia) DNAVEC\Kyushu University
Click to Show/Hide
|
|||
| External Link | ||||
| ATL-313 | Phase 1/2 | [20] | ||
| Synonyms |
DE-112; ATL-313 (ocular disease), Adenosine Therapeutics; ATL-313 (ocular disease), Clinical Data
Click to Show/Hide
|
|||
| External Link | ||||
| PSI-697 | Phase 1 | [21] | ||
| Synonyms |
UNII-LH1XC916ME; 851546-61-7; LH1XC916ME; CHEMBL219046; 2-(4-Chlorobenzyl)-3-hydroxy-7,8,9,10-tetrahydrobenzo(H)quinoline-4-carboxylic acid; 2-(4-chlorobenzyl)-3-hydroxy-7,8,9,10-tetrahydrobenzo[h]quinoline-4-carboxylic acid; SCHEMBL43527; DIEPFYNZGUUVHD-UHFFFAOYSA-N; ZINC28603970; BDBM50201984; AKOS032945074; CS-5867; DB12211; HY-15526; LS-193902; 2-(4-chloro-benzyl)-3-hydroxy-7,8,9,10-tetrahydro-benzo[h]-quinoline-4-carboxylic acid; 2-(4-Chloro-benzyl)-3-hydroxy-7,8,9,10-tetrahydro-benzo[h]quinoline-4-carboxylic
Click to Show/Hide
|
|||
| External Link | ||||
| SLV-342 | Phase 1 | [22] | ||
| External Link | ||||
| LXR 623 | Phase 1 | [23] | ||
| Synonyms |
LXR-623
Click to Show/Hide
|
|||
| External Link | ||||
| ATR-101 | Phase 1 | [24] | ||
| External Link | ||||
| MDCO-216 | Phase 1 | [25] | ||
| External Link | ||||
| XL-041 | Phase 1 | [26] | ||
| Synonyms |
XCT-0179628; XCT-628; XL-652; XL-652); LXR modulator, X-Ceptor Therapeutics; LXR modulators, Exelixis/Sankyo; Liver X receptormodulator, Exelixis/Sankyo; Liver X receptor modulator, X-Ceptor; LXR agonist (atherosclerosis), BMS/Exelixis; LXR modulators, X-Ceptor Therapeutics/Sankyo; Liver X receptor modulator, X-Ceptor Therapeutics/Sankyo; Liver X receptor modulators (cardiovascular disorders), Exelixis/Bristol-Myers Squibb
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-852927 | Phase 1 | [27] | ||
| External Link | ||||
| CS-8080 | Phase 1 | [28] | ||
| Synonyms |
Liver X receptor modulator (artherosclerosis), Daiichi Sankyo Inc; LXR modulator (anti-arteriosclerotic), Daiichi Sankyo Inc
Click to Show/Hide
|
|||
| External Link | ||||
| ISIS-APOCIII | Phase 1 | [29] | ||
| External Link | ||||
| Rilapladib | Phase 1 | [30] | ||
| External Link | ||||
| BMS-779788 | Phase 1 | [31] | ||
| External Link | ||||
| BAY-60-5521 | Phase 1 | [32] | ||
| Synonyms |
CETP inhibitors (dyslipidemia), Bayer; Cholesteryl ester transfer protein inhibitors (dyslipidemia), Bayer
Click to Show/Hide
|
|||
| External Link | ||||
| Quinazoline derivative 13 | Patented | [33] | ||
| Synonyms |
PMID26936077-Compound-24
Click to Show/Hide
|
|||
| External Link | ||||
| Darapladib | Discontinued in Phase 3 | [34] | ||
| Synonyms |
SB 480848; Darapladib (USAN)
Click to Show/Hide
|
|||
| External Link | ||||
| Acifran | Discontinued in Phase 3 | [35] | ||
| Synonyms |
Reductol; AY-25712
Click to Show/Hide
|
|||
| External Link | ||||
| ET-642 | Discontinued in Phase 2 | [36] | ||
| Synonyms |
ETC-642; RLT peptide, Esperion; RLT peptide, Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| SQ-32709 | Discontinued in Phase 2 | [37] | ||
| Synonyms |
farnesyl diphosphate; farnesyl pyrophosphate; (2E,6E)-Farnesyl diphosphate; trans,trans-Farnesyl diphosphate; all-trans-Farnesyl pyrophosphate; (all-E)-Farnesyl diphosphate; (E,E)-Farnesyl pyrophosphate; (2E,6E)-Farnesyl pyrophosphate; trans-Farnesyl pyrophosphate; 2-trans,6-trans-Farnesyl pyrophosphate; farnesyl-PP; (E,E)-Farnesyl diphosphate; 2-trans,6-trans-farnesyl diphosphate; trans-trans-farnesyl diphosphate; UNII-79W6B01D07; CHEBI:17407; trans,trans-Farnesyl pyrophosphate; Sq 32709; 13058-04-3; CHEMBL69330
Click to Show/Hide
|
|||
| External Link | ||||
| CETi-1 | Discontinued in Phase 2 | [38] | ||
| Synonyms |
Cholesteryl Ester Transfer Protein Vaccine; CETP vaccine, T Cell Sciences; Vaccine (CETP), AVANT; Vaccine (CETP), T Cell Sciences; Vaccine (atherosclerosis), T Cell Sciences
Click to Show/Hide
|
|||
| External Link | ||||
| Gantofiban | Discontinued in Phase 2 | [39] | ||
| Synonyms |
CHEMBL78871; BDBM50092103; (4-{3-[4-(Imino-methoxycarbonylamino-methyl)-phenyl]-2-oxo-oxazolidin-5-ylmethyl}-piperazin-1-yl)-acetic acid ethyl ester(EMD-122347); (4-{(R)-3-[4-(Imino-methoxycarbonylamino-methyl)-phenyl]-2-oxo-oxazolidin-5-ylmethyl}-piperazin-1-yl)-acetic acid ethyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| FCP-3P1 | Discontinued in Phase 2 | [40] | ||
| Synonyms |
CardioRex; Antihyperlipidemic, Forbes Medi-Tech
Click to Show/Hide
|
|||
| External Link | ||||
| Lifibrol | Discontinued in Phase 2 | [41] | ||
| Synonyms |
K-12148; U-83860; (R)-Lifibrol; (S)-Lifibrol; (S)-Lifibrol methyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| TA-7552 | Discontinued in Phase 2 | [42] | ||
| Synonyms |
TA 7552; 104756-72-1; 1-(3,4-Dimethoxyphenyl)-2,3-bis(methoxycarbonyl)-4-hydroxy-6,7,8-trimethoxynaphthalene; AC1L2U74; SCHEMBL8688163; DTXSID60146695; DTCYXOLBEPGOHV-UHFFFAOYSA-N; HY-100253; CS-0018404; dimethyl 4-(3,4-dimethoxyphenyl)-1-hydroxy-5,6,7-trimethoxynaphthalene-2,3-dicarboxylate; 2,3-Naphthalenedicarboxylic acid, 4-(3,4-dimethoxyphenyl)-1-hydroxy-5,6,7-trimethoxy-, dimethyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| RUS 3108 | Discontinued in Phase 1 | [43] | ||
| External Link | ||||
| GR-328713 | Discontinued in Phase 1 | [44] | ||
| Synonyms |
GW-320449X; GW-328713X
Click to Show/Hide
|
|||
| External Link | ||||
| RG7418 | Discontinued in Phase 1 | [45] | ||
| Synonyms |
BI204
Click to Show/Hide
|
|||
| External Link | ||||
| PF-3052334 | Discontinued in Phase 1 | [46] | ||
| Synonyms |
SCHEMBL3959323
Click to Show/Hide
|
|||
| External Link | ||||
| Goxalapladib | Discontinued in Phase 1 | [47] | ||
| Synonyms |
Goxalapladib < USAN; 2-[2-[2-(2,3-Difluorophenyl)ethyl]-4-oxo-1,4-dihydro-1,8-naphthyridin-1-yl]-N-[1-(2-methoxyethyl)piperidin-4-yl]-N-[4'-(trifluoromethyl)biphenyl-4-ylmethyl]acetamide
Click to Show/Hide
|
|||
| External Link | ||||
| AC3056 | Discontinued in Phase 1 | [48] | ||
| External Link | ||||
| PF-3185043 | Discontinued in Phase 1 | [49] | ||
| External Link | ||||
| GSK568859 | Discontinued in Phase 1 | [50] | ||
| External Link | ||||
| NICANARTINE | Discontinued in Phase 1 | [51] | ||
| Synonyms |
MRZ-3/124; 2,6-Di-tert-butyl-4-[3-(3-pyridylmethoxy)propyl]phenol
Click to Show/Hide
|
|||
| External Link | ||||
| LG-101280 | Discontinued in Phase 1 | [52] | ||
| Synonyms |
LSN-862; LY-WWW; LY-YYY; PPAR modulators, Ligand/Lilly
Click to Show/Hide
|
|||
| External Link | ||||
| PF-3491165 | Discontinued in Phase 1 | [53] | ||
| Synonyms |
PF-03491165
Click to Show/Hide
|
|||
| External Link | ||||
| SB-435495 | Discontinued in Phase 1 | [54] | ||
| External Link | ||||
| P-0654 | Discontinued in Phase 1 | [55] | ||
| Synonyms |
P-0655
Click to Show/Hide
|
|||
| External Link | ||||
| CL-277082 | Discontinued in Phase 1 | [56] | ||
| Synonyms |
UNII-KOF50RA8PQ; KOF50RA8PQ; CHEMBL277986; 3-(2,4-difluorophenyl)-1-[4-(2,2-dimethylpropyl)benzyl]-1-heptylurea; Ddpmhu; AC1Q5MV2; AC1L2PE2; SCHEMBL408973; CTK8D5698; BDBM50022279; ZINC36330844; CL 277082; N'-(2,4-DIFLUOROPHENYL)-N-HEPTYL-N-(4-NEOPENTYLBENZYL)UREA; Urea, N'-(2,4-difluorophenyl)-N-((4-(2,2-dimethylpropyl)phenyl)methyl)-N-heptyl-; 3-(2,4-difluorophenyl)-1-[[4-(2,2-dimethylpropyl)phenyl]methyl]-1-heptylurea; 3-(2,4-Difluoro-phenyl)-1-[4-(2,2-dimethyl-propyl)-benzyl]-1-heptyl-urea
Click to Show/Hide
|
|||
| External Link | ||||
| LF-08-0133 | Discontinued in Phase 1 | [57] | ||
| External Link | ||||
| PF-807925 | Discontinued in Phase 1 | [58] | ||
| External Link | ||||
| F-1394 | Discontinued in Phase 1 | [59] | ||
| Synonyms |
AC1OCF5R; SCHEMBL4296509; [(1S,2S)-2-[[2,2-dimethylpropyl(nonyl)carbamoyl]amino]cyclohexyl] 3-[(2,2,5,5-tetramethyl-1,3-dioxane-4-carbonyl)amino]propanoate
Click to Show/Hide
|
|||
| External Link | ||||
| CP-800569 | Discontinued in Phase 1 | [60] | ||
| Synonyms |
(2R)-3-[3-(4-chloro-3-ethylphenoxy)-N-[[3-(1,1,2,2-tetrafluoroethoxy)phenyl]methyl]anilino]-1,1,1-trifluoropropan-2-ol
Click to Show/Hide
|
|||
| External Link | ||||
| APP-018 | Discontinued in Phase 1 | [61] | ||
| Synonyms |
D-4F; Apolipoprotein A1 mimetic (atherosclerosis), Bruin Pharma; Apolipoprotein A1 mimetic (atherosclerosis),Novartis
Click to Show/Hide
|
|||
| External Link | ||||
| Nobiletin | Preclinical | [62] | ||
| Synonyms |
478-01-3; Hexamethoxyflavone; 3',4',5,6,7,8-Hexamethoxyflavone; 5,6,7,8,3',4'-Hexamethoxyflavone; UNII-D65ILJ7WLY; 2-(3,4-Dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-1-benzopyran-4-one; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxychromen-4-one; NSC-76751; D65ILJ7WLY; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-chromen-4-one; CHEMBL76447; Nobiletin (Hexamethoxyflavone); CHEBI:7602; NSC76751; MFCD03273560; Flavone, 5,6,7,8,3',4'-hexamethoxy; 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-; SMR000156231; CCRIS 9012; NSC 76751; CPD000156231; Nobiletin, >=97%; Spectrum2_001697; Spectrum3_000921; Spectrum4_001020; KBioGR_001519; MLS000574877; MLS000759462; MLS000877030; MLS001424129; Nobiletin, analytical standard; SCHEMBL244029; SPECTRUM1505268; SPBio_001654; MEGxp0_000930; ACon1_000921; KBio3_001922; DTXSID30197275; 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-chromen-4-one; HMS2051D09; HMS2234A09; HMS3373C14; HMS3393D09; HMS3651G20; HY-N0155; ZINC1531669; 3'4'5,6,7,8-Hexamethoxyflavone; 3,4,5,6,7,8-Hexamethoxyflavone; ANW-42631; BDBM50338976; CCG-38781; CN0043; LMPK12111468; NSC618903; STL565829; AKOS015965334; NOBILETIN, 20% (Technical Grade); AC-1023; CS-5518; MCULE-1015144950; NC00186; NSC-618903; SDCCGMLS-0066776.P001; NCGC00095703-01; NCGC00095703-02; NCGC00169228-01; 5,6,7,8,3'',4''-hexamethoxyflavone; AK168175; AS-17452; NCI60_041691; DB-050181; FT-0686667; N0871; N1311; S2333; SW197566-2; V0181; C10112; SR-01000712262; Q-100511; Q2402963; SR-01000712262-5; BRD-K06753942-001-02-0; 2-(3,4-Dimethoxy-phenyl)-5,6,7,8-tetramethoxy-chromen-4-one; 4H-1-Benzopyran-4-one,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy-; 2-(3,4-Dimethoxyphenyl)-5,6,7,8-tetramethoxy-4H-1-benzopyran-4-one, 9CI; 3 inverted exclamation mark ,4 inverted exclamation mark ,5,6,7,8-HEXAMETHOXYFLAVONE; 4H-1-Benzopyran-4-one, 2-(3,4-dimethoxyphenyl)-5,6,7,8-tetramethoxy- (9CI)
Click to Show/Hide
|
|||
| External Link | ||||
| Quinoxaline | Preclinical | [63] | ||
| Synonyms |
WYE-111672; WYE-672
Click to Show/Hide
|
|||
| External Link | ||||
| HL-004 | Preclinical | [64] | ||
| Synonyms |
TS-962
Click to Show/Hide
|
|||
| External Link | ||||
| ETX-6107 | Preclinical | [65] | ||
| Synonyms |
ETS-6107; ETS-6114; Antihypercholesterolemic agents (atherosclerosis), e-Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-38-1315 | Preclinical | [66] | ||
| Synonyms |
BAY-19-4789
Click to Show/Hide
|
|||
| External Link | ||||
| 2164U90 | Terminated | [67] | ||
| Synonyms |
BW-2164; BW-2164U90
Click to Show/Hide
|
|||
| External Link | ||||
| GT-16-239 | Terminated | [68] | ||
| External Link | ||||
| Cholazol | Terminated | [69] | ||
| Synonyms |
Cholestran
Click to Show/Hide
|
|||
| External Link | ||||
| BB-476 | Terminated | [70] | ||
| External Link | ||||
| CGP-43371 | Terminated | [71] | ||
| Synonyms |
Cgp 43371; UNII-V97741GKF2; V97741GKF2; 123036-23-7; cgp43371; N,15-Didehydro-15-deoxo-1-deoxy-1,15-epoxy-4-O-methyl-3-(4-((2,4,6-trimethyl)methyl)-1-piperazinyl)rifamycin 8-(2,2-dimethylpropanoate); Rifamycin, N,15-didehydro-15-deoxo-1-deoxy-1,15-epoxy-3-(4-((2,4,6-trimethylphenyl)methyl)-1-piperazinyl)-, 8-(2,2-dimethylpropanoate)
Click to Show/Hide
|
|||
| External Link | ||||
| AL-0671 | Terminated | [72] | ||
| Synonyms |
AL-0670
Click to Show/Hide
|
|||
| External Link | ||||
| BIBX-79 | Terminated | [73] | ||
| Synonyms |
175033-26-8; AC1L9UY2; BIBX 79; SCHEMBL8046646; SCHEMBL3363189; CHEMBL2377448; CHEMBL3765649; MolPort-028-951-512; ZINC59696570; ZINC238856506; ZINC100502012; AKOS034800865; MCULE-9414485243; EN300-155171; Z1768160684; 4-chloro-N-[4-[4-(dimethylaminomethyl)phenyl]cyclohexyl]-N-methyl-benzamide; 4-chloro-N-[4-[4-(dimethylaminomethyl)phenyl]cyclohexyl]-N-methylbenzamide; 4-Chloro-N-[4-(4-dimethylaminomethyl-phenyl)-cyclohexyl]-N-methyl-benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| CI-999 | Terminated | [74] | ||
| Synonyms |
PD-138142-15
Click to Show/Hide
|
|||
| External Link | ||||
| Bio-Flow | Terminated | [75] | ||
| External Link | ||||
| CGS-24565 | Terminated | [76] | ||
| Synonyms |
Cgs 24565; 136583-72-7; N,15-Didehydro-11,15-dideoxo-1-deoxy-1,15-epoxy-11-hydroxy-4-O-methyl-3-(4-((2,4,6-trimethylphenyl)methyl)-1-piperazinyl)rifamycin 8-(2,2-dimethylpropanoate); Rifamycin, N,15-didehydro-11,15-dideoxo-1-deoxy-1,15-epoxy-11-hydroxy-4-O-methyl-3-(4-((2,4,6-trimethylphenyl)methyl)-1-piperazinyl)-, 8-(2,2-dimethylpropanoate)
Click to Show/Hide
|
|||
| External Link | ||||
| TEI-6522 | Terminated | [77] | ||
| External Link | ||||
| FR-145237 | Terminated | [78] | ||
| External Link | ||||
| Squalestatin 1 | Terminated | [79] | ||
| Synonyms |
Zaragozic acid A; Squalestatin; 142561-96-4; ZARAGOZIC ACIDS A; UNII-1117HVX02L; CHEMBL280978; CHEBI:75170; 1117HVX02L; 1S-((4S-acetoxy-5R-methyl-3-methylene-6-phenylhexyl)-6-(E)-4S,6S-dimethyloct-2-enoyloxy)-4,7S-dihydroxy-2,8-dioxabicyclo[321]octane-3S,4S,5R-tricarboxylic acid; L-erythro-L-glycero-D-altro-7-Trideculo-7,4-furanosonic acid, 2,7-anhydro-3,4-di-C-carboxy-8,9,10,12,13-pentadeoxy-10-methylene-12-(phenylmethyl)-, 11-acetate 5-(4,6-dimethyl-2-octenoate), (5(2E,4S,6S),7S)-; Squalestatin 1, Glaxo; Zaragozic acid A, Glaxo
Click to Show/Hide
|
|||
| External Link | ||||
| F-12509A | Terminated | [80] | ||
| External Link | ||||
| TA-993 | Terminated | [81] | ||
| Synonyms |
MB-3, Tanabe
Click to Show/Hide
|
|||
| External Link | ||||
| Barixibat | Terminated | [82] | ||
| Synonyms |
BARI-1453; Bile acid resorption inhibitor, Aventis; HMR-1453; 1453, Aventis
Click to Show/Hide
|
|||
| External Link | ||||
| PD-146176 | Terminated | [83] | ||
| Synonyms |
PD 146176; 4079-26-9; 6,11-dihydrothiochromeno[4,3-b]indole; 6,11-Dihydro[1]benzothiopyrano[4,3-b]indole; CHEMBL180917; pd146176; NSC168807; 6,3-b]indole; NSC 168807; AC1L6RUM; 6,11-Dihydro-5-thia-11-aza-benzo(a)fluorene; SCHEMBL1986281; ZINC6892; CTK4I3773; AOB5548; MolPort-003-959-269; BCP24885; BS0260; BDBM50208823; MFCD05664738; AKOS024457313; NSC-168807; [1]Benzothiopyrano[4, 6,11-dihydro-; NCGC00165868-01; B7208; PD 146176, > (1)Benzothiopyrano(4,3-b)indole, 6,11-dihydro-
Click to Show/Hide
|
|||
| External Link | ||||
| BIBB-515 | Terminated | [84] | ||
| Synonyms |
BIBB 515; 156635-05-1; UNII-CG2Q6688S4; CHEMBL417571; CG2Q6688S4; (4-Chloro-phenyl)-{4-[4-(4,5-dihydro-oxazol-2-yl)-benzylidene]-piperidin-1-yl}-methanone; (4-chloro-phenyl)-(4-[4-(4,5-dihydro-oxazol-2-yl)-benzylidene]-piperidin-1-yl)-methanone; AC1L9UY8; SCHEMBL3676833; CTK8E8258; DTXSID00333401; MolPort-009-019-333; ZINC598970; BDBM50128071; RT-011501; J-009325; 1-(4-Chlorobenzoyl)-4-((4-(2-oxazolin-2-yl) benzylidene))piperidine; Piperidine, 1-(4-chlorobenzoyl)-4-((4-(4,5-dihydro-2-oxazolyl)phenyl)methylene)-
Click to Show/Hide
|
|||
| External Link | ||||
| LF-7-0165c | Terminated | [85] | ||
| External Link | ||||
| SDZ-267-489 | Terminated | [86] | ||
| External Link | ||||
| WAY-121898 | Terminated | [87] | ||
| Synonyms |
136100-14-6; 1-Piperidinecarboxylic acid, 4-methyl-, 4-phenoxyphenyl ester; ACMC-20mw0l; SCHEMBL2597837; CHEMBL267052; ZINC14493; CTK0F3948; DTXSID30430876; 4-PHENOXYPHENYL 4-METHYL-1-PIPERIDINECARBOXYLATE
Click to Show/Hide
|
|||
| External Link | ||||
| SR12813 | Terminated | [88] | ||
| Synonyms |
SR 12813; 126411-39-0; SR-12813; CHEBI:77317; GW 485801; CHEMBL458767; [2-(3,5-DI-TERT-BUTYL-4-HYDROXY-PHENYL)-1-(DIETHOXY-PHOSPHORYL)-VINYL]-PHOSPHONIC ACID DIETHLYL ESTER; 4-[2,2-bis(diethoxyphosphoryl)ethenyl]-2,6-ditert-butylphenol; Tetraethyl 2-(3,5-di-tert-butyl-4-hydroxyphenyl)ethenyl-1,1-bisphosphonate; tetraethyl [2-(3,5-di-tert-butyl-4-hydroxyphenyl)ethene-1,1-diyl]bis(phosphonate); [[3,5-Bis(1,1-dimethylethyl)-4-hydroxyphenyl]ethenylidene]bis-phosphonic acid tetraethyl ester; SRL; 1ilh; AC1L9JHO
Click to Show/Hide
|
|||
| External Link | ||||
| CP-230821 | Terminated | [89] | ||
| External Link | ||||
| T-686 | Terminated | [90] | ||
| Synonyms |
PAI-1 inhibitor, Tanabe
Click to Show/Hide
|
|||
| External Link | ||||
| RPR-101821 | Terminated | [91] | ||
| External Link | ||||
| MDL-29311 | Terminated | [92] | ||
| Synonyms |
MDL-27272; MDL-29097
Click to Show/Hide
|
|||
| External Link | ||||
| CP-83101 | Terminated | [93] | ||
| Synonyms |
AC1O5PZP; SCHEMBL1660808; SCHEMBL1660809; 3,5-Dihydroxy-9,9-diphenyl-6,8-nonadienoate; methyl (3R,5S,6E)-3,5-dihydroxy-9,9-diphenylnona-6,8-dienoate; 6,8-Nonadienoic acid, 3,5-dihydroxy-9,9-diphenyl-, methyl ester, (R*,S*-(E))-(+-)-
Click to Show/Hide
|
|||
| External Link | ||||
| INCB3344 | Terminated | [94] | ||
| Synonyms |
INCB-3268; INCB-3344; PF-4254196; CCR2 antagonists, Incyte/Pfizer
Click to Show/Hide
|
|||
| External Link | ||||
| CGS-23425 | Terminated | [95] | ||
| Synonyms |
CHEMBL46882; N-[3,5-dimethyl-4-(4'-hydroxy-3'-isopropylphenoxy)-phenyl]-oxamic acid; cgs23425; SCHEMBL281877; DTXSID7040998; NOCAS_40998; CGS 23425; BDBM50036402; AKOS027327083; N-[4-(4-Hydroxy-3-isopropyl-phenoxy)-3,5-dimethyl-phenyl]-oxalamic acid; N-[3,5-dimethyl-4-(4''-hydroxy-3''-isopropylphenoxy)phenyl]oxamic acid; ({4-[4-hydroxy-3-(propan-2-yl)phenoxy]-3,5-dimethylphenyl}amino)(oxo)acetic acid
Click to Show/Hide
|
|||
| External Link | ||||
| RP-73163 | Terminated | [96] | ||
| Synonyms |
CHEMBL434418; BDBM50106700
Click to Show/Hide
|
|||
| External Link | ||||
| Astenose | Terminated | [97] | ||
| Synonyms |
GM-1077; GMI-077
Click to Show/Hide
|
|||
| External Link | ||||
| S-12340 | Terminated | [98] | ||
| Synonyms |
S12340; S 12340; 144754-35-8; SCHEMBL4782175; 8-(3-(3,5-Diterbutyl-4-hydroxyphenylthio)propyl)-1-oxa-2-oxo-3,8-diazaspiro(4.5)decane; 8-(3-(3,5-Di-tert-butyl-4-hydroxyphenylthio)propyl)-1-oxa-2-oxo-3,8-diazaspiro(4.5)decane; 1-Oxa-3,8-diazaspiro(4.5)decan-2-one, 8-(3-((3,5-bis(1,1-dimethylethyl)-4-hydroxyphenyl)thio)propyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| ZD-9720 | Terminated | [99] | ||
| Synonyms |
ZD-7851
Click to Show/Hide
|
|||
| External Link | ||||
| IL1aQb | Terminated | [100] | ||
| Synonyms |
IL1aQb therapeutic vaccines (atherosclerosis); CYT-018-IL1aQb; IL1aQb therapeuticvaccines (atherosclerosis), Cytos; Immunodrug vaccines (atherosclerosis), Cytos
Click to Show/Hide
|
|||
| External Link | ||||
| NTE-122 | Terminated | [101] | ||
| Synonyms |
ACAT inhibitor, Nissin Food
Click to Show/Hide
|
|||
| External Link | ||||
| B-5354a | Terminated | [102] | ||
| Synonyms |
B-5354b; B-5354c; Sphingosine kinase inhibitors, Sankyo
Click to Show/Hide
|
|||
| External Link | ||||
| F-2833 | Terminated | [103] | ||
| Synonyms |
107430-45-5; F 2833; 2-[4-(2-chlorophenyl)phenyl]propan-2-ol; AC1L3UIQ; (2-Chloro-2'-(1,1')-4-biphenyl)-2-propanol; SCHEMBL10802061; DTXSID80148050; f2833; (1,1'-Biphenyl)-4-methanol, 2'-chloro-alpha,alpha-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| LF-13-0491c | Terminated | [104] | ||
| External Link | ||||
| FR-129169 | Terminated | [105] | ||
| External Link | ||||
| AM-1101 | Investigative | [16] | ||
| Synonyms |
Lactosylceramide synthase inhibitors (hyperproliferative disorders), Amalyte Pharmaceuticals; GAL T-2 inhibitors (hyperproliferative disorders), Amalyte Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| Apovasc | Investigative | [16] | ||
| Synonyms |
CM-121; CM-125; CM-121 + CM-125
Click to Show/Hide
|
|||
| External Link | ||||
| KY-382 | Investigative | [106] | ||
| External Link | ||||
| Adiponectin mimetics | Investigative | [16] | ||
| Synonyms |
Adiponectin mimetics (oral, type 2 diabetes/atherosclerosis/muscle metabolic diseases)
Click to Show/Hide
|
|||
| External Link | ||||
| HL-135 | Investigative | [16] | ||
| External Link | ||||
| KM-011 | Investigative | [16] | ||
| Synonyms |
Apo-A1 mimetic peptide (atherosclerosis), KineMed; Apolipoprotein-A1 mimetic peptide (atherosclerosis), KineMed
Click to Show/Hide
|
|||
| External Link | ||||
| VULM-1457 | Investigative | [106] | ||
| Synonyms |
Acyl CoA cholesterol acyltransferase inhibitor (dyslipidemia/atherosclerosis), Univerzity Komenskeho v Bratislave
Click to Show/Hide
|
|||
| External Link | ||||
| ARI-1778 | Investigative | [16] | ||
| Synonyms |
KH-01503; Rev-D-4F; Reverse-D-4F; Apo-A1 agonist (atherosclerosis), Kos Pharmaceuticals; Apolipoprotein-A1 agonist (atherosclerosis), Abbott; Apolipoprotein-A1 agonist (atherosclerosis), Kos Pharmaceuticals; Apolipoprotein-A1 mimetic peptide (atherosclerosis), Abbott; Apolipoprotein-A1 mimetic peptide (atherosclerosis), Kos Pharmaceuticals; Apo-A1 agonist (atherosclerosis), Abbott/Arisaph
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-1344386B | Investigative | [107] | ||
| Synonyms |
CCR2 antagonist (atherosclerosis); CCR2 antagonist (atherosclerosis), GlaxoSmithKline
Click to Show/Hide
|
|||
| External Link | ||||
| SCH-602539 | Investigative | [108] | ||
| Synonyms |
UNII-7467O90MW3; CHEMBL1270738; 7467O90MW3; SCH602539; 618385-42-5; SCHEMBL8058894; BDBM50329618; Carbamic acid, N-((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((1E)-2-(2,3'-bipyridin)-6'-ylethenyl)dodecahydro-1-methyl-3-oxonaphtho(2,3-C)furan-6-yl)-, ethyl ester; Ethyl ((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((E)-2-(2,3'-bipyridin-6'-yl)vinyl)-1-methyl-3-oxododecahydronaphtho(2,3-C)furan-6-yl}carbamate; Carbamic acid, ((1R,3aR,4aR,6R,8aR,9S,9aS)-9-((1E)-2-(2,3'-bipyridin)-6'-ylethenyl)dodecahydro-1-methyl-3-oxonaphtho(2,3-C)fura
Click to Show/Hide
|
|||
| External Link | ||||
| Radiolabeled VEGF | Investigative | [16] | ||
| Synonyms |
Lu-scVEGF; Radiolabeled VEGF (cancer); Radiolabeled VEGF (cancer), Sibtech/Stanford; Technetium-99m-HYNIC-VEGF; Technetium-99m-single chain-VEGF; Lu-177-DOTA-PEG-scVEGF; 99mTc-HYNIC-VEGF; 99mTc-singlechain-VEGF
Click to Show/Hide
|
|||
| External Link | ||||
| AG-4070 | Investigative | [16] | ||
| Synonyms |
LXR agonists (atherosclerosis); LXR agonists (atherosclerosis), F.Hoffmann-La Roche
Click to Show/Hide
|
|||
| External Link | ||||
| Integrin alpha-V/beta-3 receptor mab | Investigative | [16] | ||
| Synonyms |
Integrin alpha-V/beta-3 receptor mAb (atherosclerosis); Integrin alpha-V/beta-3 antagonist (atherosclerosis), Vascular Pharmaceuticals; Integrin alpha-V/beta-3 receptor mAb (atherosclerosis), Vascular Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| ICI-245991 | Investigative | [16] | ||
| External Link | ||||
| MK-6892 | Investigative | [109] | ||
| Synonyms |
Nicotinic acid 1 receptor (GPR109A) agonists, Merck
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-753951 | Investigative | [16] | ||
| Synonyms |
Gadolinium chelate MR imaging agent (atherosclerosis), BMS
Click to Show/Hide
|
|||
| External Link | ||||
| TGFTX-1 | Investigative | [16] | ||
| External Link | ||||
| INV-400 | Investigative | [16] | ||
| Synonyms |
INV-403; INV-404; INV-405; Invasc-400 series; SREBP transcription factor 2 stimulator (atherosclerosis), InVasc; Sterol regulatory element-binding protein 2 stimulator (atherosclerosis), InVasc
Click to Show/Hide
|
|||
| External Link | ||||
| GX-401 | Investigative | [16] | ||
| External Link | ||||
| RP-805 | Investigative | [16] | ||
| Synonyms |
Matrix metalloproteinase inhibitor (atherosclerosis); Matrix metalloproteinase inhibitor (atherosclerosis), Lantheus; RP-782-111In; Indium-111-RP-782; Technetium-99m-RP-805; Matrix metalloproteinase (MMP) inhibitor (tumor imaging), Bristol-Myers Squibb; 111In-RP-782; 99mTc-RP-805
Click to Show/Hide
|
|||
| External Link | ||||
| RXP-470 | Investigative | [110] | ||
| Synonyms |
MMP-12 inhibitors (atherosclerosis); MMP-12 inhibitors (atherosclerosis), CEA; RXP-470 derivatives (atherosclerosis), CEA
Click to Show/Hide
|
|||
| External Link | ||||
| 2NTX-99 | Investigative | [16] | ||
| Synonyms |
Thromboxane A2 synthesis inhibitor (thrombosis/atherosclerosis), Nile; Thromboxane A2 synthesis inhibitor (thrombosis/atherosclerosis), University of Milano
Click to Show/Hide
|
|||
| External Link | ||||
| BAY-1006451 | Investigative | [16] | ||
| Synonyms |
[18F]-BAY-1006451
Click to Show/Hide
|
|||
| External Link | ||||
References