m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05536
|
[1] | |||
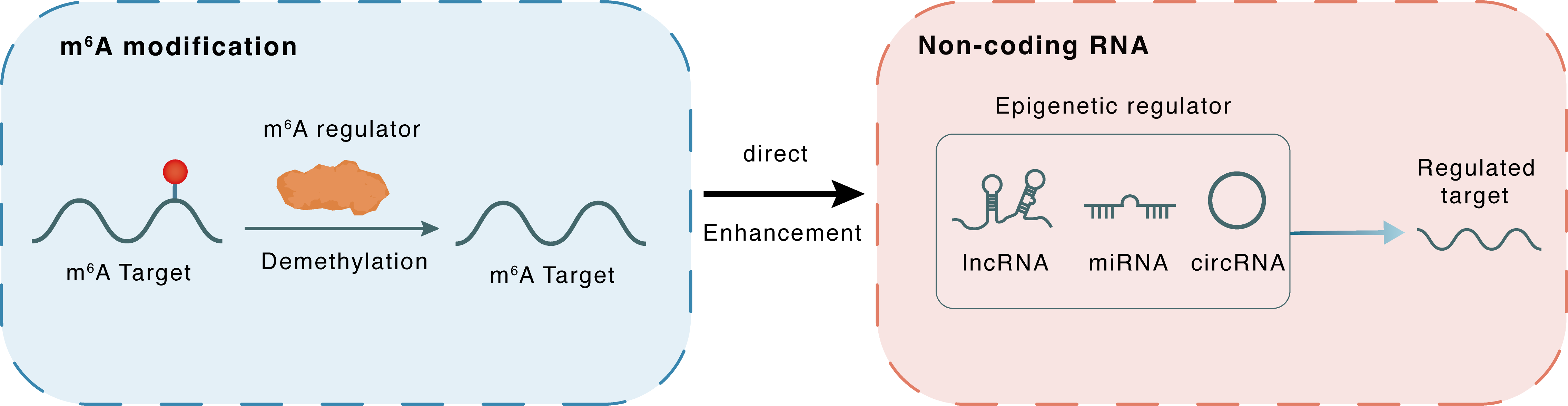 m6A modification
MIR576
MIR576
FTO
Demethylation
m6A modification
MIR576
MIR576
FTO
Demethylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR-576
CDK6
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR-576
CDK6
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | microRNA 576 (MIR576) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | MicroRNA 576 (MIR576) | microRNA | View Details | ||
| Regulated Target | Cyclin-dependent kinase 6 (CDK6) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | FTO promoted bladder cancer cell proliferation, migration and invasion via the FTO/microRNA 576 (MIR576)/Cyclin-dependent kinase 6 (CDK6) pathways in an m6A-dependent manner. | ||||
| Responsed Disease | Bladder cancer | ICD-11: 2C94 | |||
| Cell Process | Cell proliferation | ||||
In-vitro Model |
T24 | Bladder carcinoma | Homo sapiens | CVCL_0554 | |
| 5637 | Bladder carcinoma | Homo sapiens | CVCL_0126 | ||
| UM-UC-3 | Bladder carcinoma | Homo sapiens | CVCL_1783 | ||
| In-vivo Model | Approximately 1 × 107 stably transfected T24 cells were subcutaneously injected into BALB/c nude mice. The length (L) and width (W) of the tumours were measured weekly using callipers, while their volume was calculated using the equation: V = (L × W2)/2. After 4 weeks of injections, the mice were euthanised, and the tumour tissues were removed and weighed. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Cyclin-dependent kinase 6 (CDK6) | 32 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Palbociclib | Approved | [2] | ||
| Synonyms |
571190-30-2; PD0332991; PD-0332991; Ibrance; PD 0332991; UNII-G9ZF61LE7G; Palbociclib(PD0332991); 6-Acetyl-8-cyclopentyl-5-methyl-2-[[5-(piperazin-1-yl)pyridin-2-yl]amino]-8H-pyrido[2,3-d]pyrimidin-7-one; 6-acetyl-8-cyclopentyl-5-methyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)pyrido[2,3-d]pyrimidin-7(8H)-one; G9ZF61LE7G; PD 332991; 6-ACETYL-8-CYCLOPENTYL-5-METHYL-2-[(5-PIPERAZIN-1-YLPYRIDIN-2-YL)AMINO]PYRIDO[2,3-D]PYRIMIDIN-7(8H)-ONE; LQQ; PD 332991, PD 0332991, PD0332991; 6-Acetyl-8-cyclopentyl-5-methyl-2-(5-piperazin-1-ylpyridin-2-ylamino)-8H-pyrido(2,3-d)pyrimidin-7-one; 6-acetyl-8-cyclopentyl-5-methyl-2-[(5-piperazin-1-ylpyridin-2-yl)amino]pyrido[2,3-d]pyrimidin-7-one; HMR-2934
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 15 nM | |||
| External Link | ||||
| Ribociclib Succinate | Approved | [3] | ||
| Synonyms |
1374639-75-4; LEE011 succinate; LEE011 (succinate); UNII-BG7HLX2919; LEE011-BBA; Ribociclib succinate [USAN]; BG7HLX2919; Kisqali (TN); Ribociclib succinate (USAN); LEE-011 succinate; SCHEMBL2684999; EX-A1586; HY-15777B; 1374639-75-4 (succinate); AKOS030526460; CS-2277; ACN-040739
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| LY2835219 | Approved | [4] | ||
| Synonyms |
Abemaciclib; 1231929-97-7; Verzenio; LY-2835219; UNII-60UAB198HK; LY2835219 (free base);
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 1.9 nM | |||
| External Link | ||||
| Trilaciclib | Approved | [5] | ||
| Synonyms |
G1T28; 1374743-00-6; Trilaciclib [USAN]; G1T28(Trilaciclib); GTPL9626; CHEMBL3894860; SCHEMBL10082028; BDBM253928; US9464092, T; HY-101467; CS-0021431; 2'-((5-(4-methylpiperazin-1-yl)pyridin-2-yl)amino)-7',8'-dihydro-6'H-spiro(cyclohexane-1,9'-pyrazino(1',2':1,5)pyrrolo(2,3-d)pyrimidin)-6'-one; Spiro(cyclohexane-1,9'(6'H)-pyrazino(1',2':1,5)pyrrolo(2,3-d)pyrimidin)-6'-one, 7',8'-dihydro-2'-((5-(4-methyl-1-piperazinyl)-2-pyridinyl)amino)-; 2-[[5-(4-methylpiperazin-1-yl)pyridin-2-yl]amino]spiro[7,8-dihydropyra
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Apremilast | Approved | [6] | ||
| Synonyms |
Apremilast (USAN); CC-10004; N-[2-[1-(3-ethoxy-4-methoxy-phenyl)-2-methylsulfonyl-ethyl]-1,3-dioxo-isoindol-4-yl]acetamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LEE011 | Phase 3 | [7] | ||
| Synonyms |
Ribociclib; 1211441-98-3; LEE-011; Kisqali; Ribociclib(LEE011); UNII-TK8ERE8P56; LEE 011; 7-cyclopentyl-N,N-dimethyl-2-((5-(piperazin-1-yl)pyridin-2-yl)amino)-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide; TK8ERE8P56; Ribociclib (LEE011); AK174906; 7-cyclopentyl-N,N-dimethyl-2-{[5-(piperazin-1-yl)pyridin-2-yl]amino}-7H-pyrrolo[2,3-d]pyrimidine-6-carboxamide; 7-cyclopentyl-2-(5-piperazin-1-yl-pyridin-2-ylamino)-7H-pyrrolo [2,3-d]pyrimidine-6-carboxylic acid dimethylamide; Ribociclib [USAN:INN]; LEE011A; Tube013
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| Activity | IC50 = 6 nM | |||
| External Link | ||||
| G1T38 | Phase 2 | [8] | ||
| Synonyms |
YPJRHEKCFKOVRT-UHFFFAOYSA-N; SCHEMBL16036885; CHEMBL3904602; BDBM253941; US9464092, GG; 1628256-23-4
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GLR2007 | Phase 1/2 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| FCN-437 | Phase 1/2 | [10] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| NUV-422 | Phase 1/2 | [11] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| G1T28-1 | Phase 1 | [4] | ||
| MOA | Modulator | |||
| External Link | ||||
| RGT-419B | Phase 1 | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| FN-1501 | Phase 1 | [13] | ||
| Synonyms |
1429515-59-2; CHEMBL4077071; UNII-6MC966B505; TQR1001; BDBM50270304; NSC781143; 6MC966B505; NSC-781143; HY-111361; CS-0039834; 4((7HPyrrolo[2,3d]pyrimidin-4-yl)amino)N(4-((4-methylpiperazin-1-yl)methyl)phenyl)1Hpyrazole-3-carboxamide; 4-((7H-Pyrrolo (2,3-d)pyrimidin-4-yl)amino)-N-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-1H-pyrazole-3-carboxamide; 4-((7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)-N-(4-((4-methylpiperazin-1-yl)methyl)phenyl)-1H-pyrazole-5-carboxamide; 4a?(7Ha'Pyrrolo[2,3a'd]pyrimidin-4-yl)amino)a'Na?4-((4-methylpiperazin-1-yl)methyl)phenyl)a?Ha'pyrazole-3-carboxamide; N-[4-[(4-methylpiperazin-1-yl)methyl]phenyl]-4-(7H-pyrrolo[2,3-d]pyrimidin-4-ylamino)-1H-pyrazole-5-carboxamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID25726713-Compound-49 | Patented | [14] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID25726713-Compound-51 | Patented | [14] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| Isoquinoline 1,3-dione derivative 1 | Patented | [15] | ||
| Synonyms |
PMID26161698-Compound-49
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1 nM | |||
| External Link | ||||
| PMID25726713-Compound-48 | Patented | [14] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID25726713-Compound-47 | Patented | [14] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| PMID25726713-Compound-50 | Patented | [14] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 50 nM | |||
| External Link | ||||
| Oxazolyl methylthiothiazole derivative 1 | Patented | [15] | ||
| Synonyms |
PMID26161698-Compound-52
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 100 to 1000 nM | |||
| External Link | ||||
| INOC-005 | Preclinical | [16] | ||
| Synonyms |
Capridine beta (prostate cancer), Prostagenics
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CYC-103 | Terminated | [17] | ||
| Synonyms |
Cyclin groove inhibitors, Cyclacel; CYC-103 (Pimetics series); CYC-103 cyclin groove inhibitors, Cyclacel; CYC-103 program, Cyclacel
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PD-0183812 | Terminated | [18] | ||
| Synonyms |
PETCVZZPKYJZAU-UHFFFAOYSA-N; PD183812; AC1NS8PJ; CHEMBL139653; SCHEMBL5268115; BDBM6280; PD 0183812; N8 Pyrido[2,3-d]pyrimidin-7-one deriv 72; 8-{bicyclo[221]heptan-2-yl}-2-({4-[4-(3-hydroxypropyl)piperidin-1-yl]phenyl}amino)-7H,8H-pyrido[2,3-d]pyrimidin-7-one; 8-(3-bicyclo[221]heptanyl)-2-[4-[4-(3-hydroxypropyl)piperidin-1-yl]anilino]pyrido[2,3-d]pyrimidin-7-one; PD0183813
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Fascaplysin | Investigative | [18] | ||
| Synonyms |
Pyrido[1,2-a:3,4-b']diindol-5-ium,12,13-dihydro-13-oxo-, chloride; GNF-PF-1458; ACMC-20bu3v; AC1L2JLY; AC1Q6JA3; SCHEMBL1728912; CHEMBL602937; GTPL5969; BDBM59087; CTK4A8872; CHEBI:93765; ZINC1616841; pyrido[1,2-a:3,4-b']diindol-5-ium, 12,13-dihydro-13-oxo-; HSCI1_000331; NCGC00346951-01; CJ-26101; BRD-K13287209-003-03-2; BRD-K13287209-311-02-1; BRD-K13287209-311-01-3; BRD-K13287209-003-02-4; BRD-K13287209-003-01-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3400 nM | |||
| External Link | ||||
| Deschloroflavopiridol | Investigative | [18] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| RGB-286147 | Investigative | [19] | ||
| Synonyms |
pyrazolopyrimidone analog, RGB-286147
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Chrysin | Investigative | [20] | ||
| Synonyms |
480-40-0; 5,7-Dihydroxyflavone; Chrysine; 5,7-Dihydroxy-2-phenyl-4H-chromen-4-one; Crysin; 5,7-dihydroxy-2-phenylchromen-4-one; 4H-1-Benzopyran-4-one, 5,7-dihydroxy-2-phenyl-; UNII-3CN01F5ZJ5; NSC-407436; FLAVONE, 5,7-DIHYDROXY-; EINECS 207-549-7; NSC407436; 5,7-Dihydroxy-2-phenyl-4H-1-benzopyran-4-one; CHEMBL117; NSC 407436; 5,7-Dihydroxy-2-phenyl-chromen-4-one; BRN 0233276; 3CN01F5ZJ5; CHEBI:75095; RTIXKCRFFJGDFG-UHFFFAOYSA-N; 5,7-Dihydroxy-2-phenyl-4H-benzo(b)pyran-4-one; MFCD00006834; Chrysin, 99+%; CAS-480-40-0
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 6000 nM | |||
| External Link | ||||
| 3,7,3',4'-TETRAHYDROXYFLAVONE | Investigative | [21] | ||
| Synonyms |
Fisetin; 528-48-3; 2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-4H-chromen-4-one; 5-Desoxyquercetin; Fustel; Cotinin; Viset; 3,3',4',7-Tetrahydroxyflavone; Fisetholz; Superfustel; Fustet; Fietin; Junger fustik; Ventin sumach; Zante fustic; Young fustic; Superfustel K; Ungarisches gelbholz; CI Natural Brown 1; Young fustic crystals; Bois bleu de Honqrie; BOIS bleude honqrie; CI 75620; NSC 407010; NSC 656275; 5-Deoxyquercetin; 2-(3,4-Dihydroxyphenyl)-3,7-dihydroxy-4H-1-benzopyran-4-one; Natural Brown 1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 850 nM | |||
| External Link | ||||
| APIGENIN | Investigative | [20] | ||
| Synonyms |
520-36-5; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one; Chamomile; Versulin; Spigenin; Apigenol; 4',5,7-Trihydroxyflavone; Apigenine; C.I. Natural Yellow 1; 5,7,4'-Trihydroxyflavone; Pelargidenon 1449; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone; 2-(p-Hydroxyphenyl)-5,7-dihydroxychromone; UCCF 031; NSC 83244; UNII-7V515PI7F6; 5,7-Dihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one; 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one; CCRIS 3789; CHEBI:18388; CHEMBL28; EINECS 208-292-3; 4H-1-Benzopyran-4-one, 5,7-di
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1700 nM | |||
| External Link | ||||
| 3,4-di-(4-methoxyphenyl)-1H-pyrrole-2,5-dione | Investigative | [22] | ||
| Synonyms |
1H-Pyrrole-2,5-dione, 3,4-bis(4-methoxyphenyl)-; 108774-82-9; ACMC-20mbs9; CHEMBL381099; CTK0G2626; DTXSID90449388
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3,4-diphenyl-1H-pyrrole-2,5-dione | Investigative | [22] | ||
| Synonyms |
2,3-diphenylmaleimide; 1H-Pyrrole-2,5-dione, 3,4-diphenyl-; 31295-36-0; AC1MBL6S; SCHEMBL114611; CHEMBL201949; CTK1B9880; 3,4-diphenylpyrrole-2,5-dione; DTXSID70372903; ZINC3847556
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(4-methoxyphenyl)-4-phenyl-1H-pyrrole-2,5-dione | Investigative | [22] | ||
| Synonyms |
CHEMBL372076; SCHEMBL3822337
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C94: Bladder cancer | 83 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Enfortumab vedotin | Phase 3 | [23] | ||
| Synonyms |
Padcev
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [8] | ||
| External Link | ||||
| Halaven | Phase 1/2 | [8] | ||
| Synonyms |
Eribulin mesylate; Eribulin (mesylate); Eribulin mesilate; UNII-AV9U0660CW; Eribulin mesylate [USAN]; 441045-17-6; AV9U0660CW; CHEBI:70710; E 7389; E7389; Eribulin mesylate (USAN); B-1939; NSC-707389; Eribulin mesilate (JAN); CHEMBL1683544; QAMYWGZHLCQOOJ-WRNBYXCMSA-N; HY-13442A; AKOS030238218; CS-2803; D08914; 2-(3-amino-2-hydroxypropyl)hexacosahydro-3-methoxy-26-methyl-20,27-bis(methylene)11,15-18,21-24,28-triepoxy-7,9-ethano-12,15-methano-9H,15H-furo(3,2-i)furo(2',3'-5,6)pyrano(4,3-b)(1,4)dioxacyclopent
Click to Show/Hide
|
|||
| External Link | ||||
| RG-7446 | Approved | [24] | ||
| External Link | ||||
| Pemigatinib | Approved | [8] | ||
| Synonyms |
Unii-Y6BX7BL23K; Y6BX7BL23K; GTPL9767; SCHEMBL15556271; HCDMJFOHIXMBOV-UHFFFAOYSA-N; example 126 [WO2014007951]; 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(morpholin-4-ylmethyl)-4,7-dihydropyrrolo[4,5]pyrido[1,2-d]pyrimidin-2-one; 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(morpholin-4-ylmethyl)-1,3,4,7-tetrahydro-2H-pyrrolo[3',2':5,6]pyrido[4,3-d]pyrimidin-2-one; INCB54828
Click to Show/Hide
|
|||
| External Link | ||||
| Erdafitinib | Approved | [25] | ||
| Synonyms |
1346242-81-6; UNII-890E37NHMV; 890E37NHMV; Erdafitinib [USAN:INN]; Erdafitinib (USAN/INN); GTPL9039; SCHEMBL2583760; CHEMBL3545376; MolPort-044-560-398; JNJ-42756493 (Erdafitinib); s8401; compound 4 [WO2011135376]; ZINC168520308; AKOS030526429; CS-4988; DB12147; AC-30222; 1,2-Ethanediamine, N1-(3,5-dimethoxyphenyl)-N2-(1-methylethyl)-N1-(3-(1-methyl-1H-pyrazol-4-yl)-6-quinoxalinyl)-; HY-18708; AS-35040; KB-333716; D10927; N'-(3,5-dimethoxyphenyl)-N'-[3-(1-methylpyrazol-4-yl)quino
Click to Show/Hide
|
|||
| External Link | ||||
| Hexyl aminolevulinate | Approved | [26] | ||
| Synonyms |
Hexvix (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| BCG vaccine | Approved | [26] | ||
| Synonyms |
OncoTice; TiceBCG; BCG vaccine, Organon
Click to Show/Hide
|
|||
| External Link | ||||
| Valrubicin | Approved | [27] | ||
| Synonyms |
Valstar; Valrubicin [USAN]; Valstar Preservative Free; AD 32; Antibiotic AD 32; Valstar (TN); N-Trifluoroacetyladriamycin 14-valerate; N-Trifluoroacetyldoxorubicin 14-valerate; Trifluoroacetyladriamycin-14-valerate; Valrubicin (USP/INN); N-Trifluoroacetyladriamycin-14-valerate; Adriamycin, trifluoroacetyl-, 14-valerate; [2-oxo-2-[(2S,4S)-2,5,12-trihydroxy-4-[5-hydroxy-6-methyl-4-[(2,2,2-trifluoroacetyl)amino]oxan-2-yl]oxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]ethyl] pentanoate; (2S-cis)-2-(1,2,3,4,6,11-Hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetyl)amino)-alpha-L-lyxo-hexopyranosyl)oxy)-2-naphthacenyl)-2-oxoethyl pentanoate; (2S-cis)-Pentanoic acid, 2-(1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetyl)amino)-alpha-L-lyxo-hexopyranosyl)oxy)-2-naphth acenyl)-2-oxoethyl ester; (8S,10S)-8-Glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-((2,3,6-trideoxy-3-(2,2,2-trifluoroacetamido)-alpha-L-lyxo-hexopyranosyl)oxy)-5,12-naphthacenedione 8(sup 2)-valerate; Pentanoic acid, 2-((2S,4S)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetylamino)-, alpha-L-lysohexopyranoxyl)oxy)-2-naphthacenyl)-2-oxoethyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| Vicineum | Phase 3 | [28] | ||
| Synonyms |
Oportuzumab monatox
Click to Show/Hide
|
|||
| External Link | ||||
| Sasanlimab | Phase 3 | [29] | ||
| Synonyms |
PF-06801591
Click to Show/Hide
|
|||
| External Link | ||||
| CG0070 | Phase 3 | [30] | ||
| Synonyms |
CG-5757; Oncolytic virus therapy, Cell Genesys; Oncolytic virus therapy, Cold Genesys
Click to Show/Hide
|
|||
| External Link | ||||
| Nadofaragene firadenovec | Phase 3 | [8] | ||
| External Link | ||||
| Oportuzumab monatox | Phase 3 | [30] | ||
| External Link | ||||
| Apaziquone | Phase 3 | [31] | ||
| Synonyms |
EOquin; 114560-48-4; Apaziquonum; NOR-701; EO 9 (pharmaceutical); EO-9; Apaziquone [USAN:INN]; NSC-382459; Apaziquonum [INN-Latin]; E 09; NSC 382459; UNII-H464ZO600O; E-85/053; E-09; EO9; NSC 382456; H464ZO600O; 5-(Azridin-1-yl)-3-(hydroxymethyl)-2-((1E)-3-hydroxyprop-1-enyl)-methyl-1H-indole-4,7-dione; (E)-5-(1-Azirinyl)-3-(hydroxymethyl)-2-(3-hydroxy-1-propenyl)-1-methyl-1H-indole-4,7-dione; E09; 1H-Indole-4,7-dione, 5-(1-aziridinyl)-3-(hydroxymethyl)-2-(3-hydroxy-1-propenyl)-1-methyl-, (E)-; Neoquin; Qapzola; EO 9; Eoquin (TN); Apaziquone (USAN/INN); E-85/050; 3-hydroxymethyl-5-aziridinyl-1-methyl-2-(1H-indole-4,7-dione)prop-beta-en-alpha-ol; 5-(aziridin-1-yl)-3-(hydroxymethyl)-2-[(E)-3-hydroxyprop-1-enyl]-1-methylindole-4,7-dione; Apaziquone/EOquin
Click to Show/Hide
|
|||
| External Link | ||||
| Tesetaxel | Phase 2 | [32] | ||
| Synonyms |
DJ-927; 333754-36-2; UNII-UG97LO5M8Y; UG97LO5M8Y; Tesetaxel [INN]; DJ927; DJ 927; CHEMBL2107787; SCHEMBL12060837; DB12019; Z-3104; (2AS,2BR,3S,4S,6S,8AR,10R,11AS,11BR,13AR)-2A-ACETOXY-6-(((2R,3S)-3-((TERT-BUTOXYCARBONYL)AMINO)-3-(3-FLUOROPYRIDIN-2-YL)-2-HYDROXYPROPANOYL)OXY)-10-((DIMETHYLAMINO)METHYL)-4-HYDROXY-7,11B,14,14-TETRAMETHYL-2A,2B,3,4,5,6,8A,11A,11B,12,13,13A-DODECAHYDRO-2H-4,8-METHANOOXETO[3'',2'':3',4']BENZO[1',2':3,4]CYCLODECA[1,2-D][1,3]DIOXOL-3-YL BENZOATE
Click to Show/Hide
|
|||
| External Link | ||||
| EN3488 | Phase 3 | [33] | ||
| External Link | ||||
| NKTR 214 | Phase 3 | [30] | ||
| External Link | ||||
| Ramucirumab | Phase 3 | [8] | ||
| External Link | ||||
| Vicinium | Phase 3 | [8] | ||
| External Link | ||||
| ICP-192 | Phase 2 | [34] | ||
| External Link | ||||
| Inodiftagene vixteplasmid | Phase 2 | [35] | ||
| Synonyms |
BC-819
Click to Show/Hide
|
|||
| External Link | ||||
| CPX-POM | Phase 2 | [36] | ||
| External Link | ||||
| LY3012212 | Phase 2 | [37] | ||
| Synonyms |
Icrucumab
Click to Show/Hide
|
|||
| External Link | ||||
| CV-301 | Phase 2 | [8] | ||
| External Link | ||||
| ABI-009 | Phase 2 | [8] | ||
| External Link | ||||
| ALT-801 | Phase 2 | [30] | ||
| Synonyms |
ALT-801 (donor lymphocyte infusion, cancer); ALT-801 (donor lymphocyte infusion, cancer), Altor; STAR IL-2 conjugate (donor lymphocyte infusion, cancer), Altor; STAR-Ck (donor lymphocyte infusion, cancer), Altor; Soluble T-cell Antigen Receptor IL-2 conjugate (donor lymphocyte infusion, cancer), Altor
Click to Show/Hide
|
|||
| External Link | ||||
| INO-5401 | Phase 2 | [8] | ||
| External Link | ||||
| RX-3117 | Phase 2 | [8] | ||
| Synonyms |
Antimetabolite (cancer), Rexahn; Antimetabolite (cancer), Rexahn/ Teva
Click to Show/Hide
|
|||
| External Link | ||||
| BC-819 | Phase 2 | [38] | ||
| External Link | ||||
| IPI-549 | Phase 2 | [39] | ||
| Synonyms |
XUMALORDVCFWKV-IBGZPJMESA-N; IPI549; 1693758-51-8; CHEMBL3984425; GTPL9563; SCHEMBL16629991; IPI 549; MolPort-044-756-207; EX-A1057; s8330; BDBM50192880; ZINC584906867; AKOS030627132; CS-6106; compound 26 [PMID: 27660692]; AC-29898; HY-100716; Pyrazolo[1,5-a]pyrimidine-3-carboxamide, 2-amino-N-[(1S)-1-[1,2-dihydro-8-[2-(1-methyl-1H-pyrazol-4-yl)ethynyl]-1-oxo-2-phenyl-3-isoquinolinyl]ethyl]-; 2-amino-N-[(1S)-1-[8-[2-(1-methylpyrazol-4-yl)ethynyl]-1-oxo-2-phenylisoquinolin-3-yl]ethyl]pyrazolo[1,5-a]pyrimidine-3-carboxamide; (S)-2-amino-N-(1-(8-((
Click to Show/Hide
|
|||
| External Link | ||||
| B-701 | Phase 2 | [8] | ||
| Synonyms |
VKRFJPYJBOIVPD-UHFFFAOYSA-N; B 701; NSC 46406; 78218-88-9; Phosphorodiamidic acid, N,N-bis(2-chloroethyl)-N'-(3-hydroxypropyl)-, (3-chloropropyl) ester; AC1L3VIX; AC1Q6T2K; NSC46406; NSC-46406; 3-chloropropyl n,n-bis(2-chloroethyl)-n'-(3-hydroxypropyl)phosphorodiamidate; LS-107974; 3-[[bis(2-chloroethyl)amino-(3-chloropropoxy)phosphoryl]amin; 3-[[bis(2-chloroethyl)amino-(3-chloropropoxy)phosphoryl]amino]propan-1-ol; Phosphorodiamidic acid,N-bis(2-chloroethyl)-N'-(3-hydroxypropyl)-, 3-chloropropyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| NC-6004 | Phase 2 | [8] | ||
| External Link | ||||
| ALT-803 | Phase 2 | [8] | ||
| Synonyms |
IL-15 agonist/ IL-15R alpha-Fc fusion complex (cancer), Altor BioScience
Click to Show/Hide
|
|||
| External Link | ||||
| Vesigenurtacel-L | Phase 2 | [40] | ||
| External Link | ||||
| BAY1163877 | Phase 2 | [41] | ||
| Synonyms |
Rogaratinib
Click to Show/Hide
|
|||
| External Link | ||||
| Coxsackievirus A21 | Phase 1/2 | [30] | ||
| Synonyms |
Cavatak (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| OGX-427 | Phase 2 | [42] | ||
| External Link | ||||
| PIRITREXIM | Phase 2 | [43] | ||
| Synonyms |
72732-56-0; Piritrexim [INN]; Piritreximum [Latin]; Piritrexime [French]; 6-(2,5-dimethoxybenzyl)-5-methylpyrido[2,3-d]pyrimidine-2,4-diamine; Piritrexima [Spanish]; BW 301U; UNII-MK2A783ZUT; BW-301U; TCMDC-137235; BRN 5768301; MK2A783ZUT; CHEMBL7492; 2,4-Diamino-5-methyl-6-(2,5-dimethoxybenzyl)pyrido(2,3-d)pyrimidine; 6-(2,5-DIMETHOXY-BENZYL)-5-METHYL-PYRIDO[2,3-D]PYRIMIDINE-2,4-DIAMINE; 6-((2,5-Dimethoxyphenyl)methyl)-5-methylpyrido(2,3-d)pyrimidine-2,4-diamine
Click to Show/Hide
|
|||
| External Link | ||||
| VesiGel | Phase 2 | [8] | ||
| External Link | ||||
| Instiladrin | Phase 2 | [44] | ||
| External Link | ||||
| CDX-1307 | Phase 2 | [45] | ||
| Synonyms |
BHCG-VAC; BetaHCG-VAC; MDX-1307; Dendritic cell targeted hCG-beta vaccine, Medarex; B11-hCG-beta, Medarex; Antigen-presenting cell-targeted vaccine (injectable, cancer), Celldex Therapeutics; Antigen-presenting cell-targeted vaccine (intradermal, cancer), Celldex Therapeutics; Antigen-presenting cell-targeted vaccine (iv, cancer), Celldex Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [46] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| MV-NIS | Phase 1 | [8] | ||
| Synonyms |
MV-NIS (intratumoral, SCCHN); Measles virus encoding thyroidal sodium iodide symporter (intratumoral, Head and neck tumor), Nisco International Inc; MV-NIS (intratumoral, SCCHN), Nisco International Inc
Click to Show/Hide
|
|||
| External Link | ||||
| 4SCAR-PSMA | Phase 1/2 | [47] | ||
| External Link | ||||
| MAGE-A10 TCR | Phase 1/2 | [8] | ||
| External Link | ||||
| Lx-TB-PstS1 | Phase 1/2 | [48] | ||
| Synonyms |
Lx-Bladder
Click to Show/Hide
|
|||
| External Link | ||||
| 4SCAR-FRa | Phase 1/2 | [47] | ||
| External Link | ||||
| ABY-025 | Phase 1/2 | [49] | ||
| External Link | ||||
| ADP-A2M10 | Phase 1 | [50] | ||
| External Link | ||||
| TAR-200 | Phase 1 | [8] | ||
| External Link | ||||
| ASG-15ME | Phase 1 | [51] | ||
| External Link | ||||
| Vesimune | Phase 2 | [52] | ||
| External Link | ||||
| NEO-PV-01 | Phase 1 | [8] | ||
| External Link | ||||
| Neo-Urinary Conduit | Phase 1 | [53] | ||
| External Link | ||||
| FPA144 | Phase 1 | [30] | ||
| External Link | ||||
| Ad-IFN-alpha | Phase 1 | [54] | ||
| Synonyms |
Ad-IFN-alpha (cancer); Ad-IFN-alpha (cancer), MD Anderson Cancer Center/NCI; Adenoviral-mediated IFN-alpha (gene therapy, cancer), MD Anderson Cancer Center/NCI
Click to Show/Hide
|
|||
| External Link | ||||
| LNK-754 | Phase 1 | [55] | ||
| Synonyms |
LNK 754; 439153-64-7; OSI 754; CP 609754; CP-609,754; DTXSID60195986; 2(1H)-Quinolinone, 6-((4-chlorophenyl)hydroxy(1-methyl-1H-imidazol-5-yl)methyl)-4-(3-ethynylphenyl)-1-methyl-
Click to Show/Hide
|
|||
| External Link | ||||
| example 7 [US8664233] | Clinical trial | [56] | ||
| Synonyms |
SCHEMBL977927; GTPL8283; HFGHRUCCKVYFKL-UHFFFAOYSA-N; SB19793; 4-ethoxy-2-(piperazin-1-yl)-7-(pyridin-4-yl)-5H-pyrimido[5,4-b]indole; 4-ethoxy-2-piperazin-1-yl-7-pyridin-4-yl-5H-pyrimido[5,4-b]indole
Click to Show/Hide
|
|||
| External Link | ||||
| (S)-DRF-1042 | Clinical trial | [57] | ||
| Synonyms |
5(S)-(2'-hydroxyethoxy)-20(S)-camptothecin; 5(S)-(2'-hydroxyethoxy)-20(S)-CPT
Click to Show/Hide
|
|||
| External Link | ||||
| Larotaxel | Discontinued in Phase 3 | [58] | ||
| Synonyms |
Benzenepropanoic acid; PNU 100940; XRP 9881; XRP9881
Click to Show/Hide
|
|||
| External Link | ||||
| Keyhole limpet hemocyanin | Discontinued in Phase 3 | [59] | ||
| External Link | ||||
| IDM-2 | Discontinued in Phase 2/3 | [60] | ||
| Synonyms |
Bexidem; MAK anticancer agents (2), Immuno-Designed Molecules/IDM Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| NKS-01 | Discontinued in Phase 2 | [61] | ||
| Synonyms |
14alpha-Hydroxy-4-androstene-3,6,17-trione
Click to Show/Hide
|
|||
| External Link | ||||
| S-288310 | Discontinued in Phase 1/2 | [62] | ||
| Synonyms |
Peptide vaccine (bladder cancer), OncoTherapy Science; Peptide vaccine (bladder cancer), Shionogi
Click to Show/Hide
|
|||
| External Link | ||||
| Capzola | Application submitted | [8] | ||
| External Link | ||||
| MINAMESTANE | Terminated | [63] | ||
| Synonyms |
FCE-24928; 4-Aminoandrosta-1,4,6-triene-3,17-dione
Click to Show/Hide
|
|||
| External Link | ||||
| CG-8840 | Terminated | [64] | ||
| Synonyms |
CV-884
Click to Show/Hide
|
|||
| External Link | ||||
| ET-009 | Investigative | [65] | ||
| External Link | ||||
| TD-6989 | Investigative | [65] | ||
| External Link | ||||
| SL-601 | Investigative | [65] | ||
| External Link | ||||
| ASC-JMZ1 | Investigative | [65] | ||
| External Link | ||||
| TD-3633 | Investigative | [65] | ||
| External Link | ||||
| CEL-011 | Investigative | [65] | ||
| External Link | ||||
| Debio-1141 | Investigative | [65] | ||
| Synonyms |
PLK-1 targeting UsiRNA (liposomal, cancer), Marina; PLK1-targeting UsiRNA (liposomal, cancer), MDRNA; PLK-1 targeting UsiRNA (liposomal, bladder cancer), Marina/Debiopharm
Click to Show/Hide
|
|||
| External Link | ||||
| TD-1770 | Investigative | [65] | ||
| External Link | ||||
| Chitosan/IL-12 | Investigative | [65] | ||
| Synonyms |
Chitosan/IL-12 immunotherapy (intravesical, cancer), National Cancer Institute
Click to Show/Hide
|
|||
| External Link | ||||
| BAMLET | Investigative | [65] | ||
| Synonyms |
BAMLET (liquid formulation, bladder cancer); BAMLET instillation (bladder cancer), NatImmune; BAMLET (liquid formulation, bladder cancer), NatImmune; Bovine alpha-lactalbumin-lipid complex (liquid formulation, bladder cancer), NatImmune
Click to Show/Hide
|
|||
| External Link | ||||
| TMX-202 | Investigative | [66] | ||
| Synonyms |
TMX-20X
Click to Show/Hide
|
|||
| External Link | ||||
| SX-MTR1 | Investigative | [67] | ||
| Synonyms |
MTOR modulators (small peptide mimetics, bladder cancer), Serometrix
Click to Show/Hide
|
|||
| External Link | ||||
| AP-300 | Investigative | [65] | ||
| External Link | ||||
| BC-821 | Investigative | [65] | ||
| Synonyms |
IGF2-DTA; Diphtheria toxin A conjugated insulin-like growth factor 2 (cancer), BioCancell
Click to Show/Hide
|
|||
| External Link | ||||
| OGX-427 + Paclitaxel | Investigative | [68] | ||
| External Link | ||||
References