m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05473
|
[1] | |||
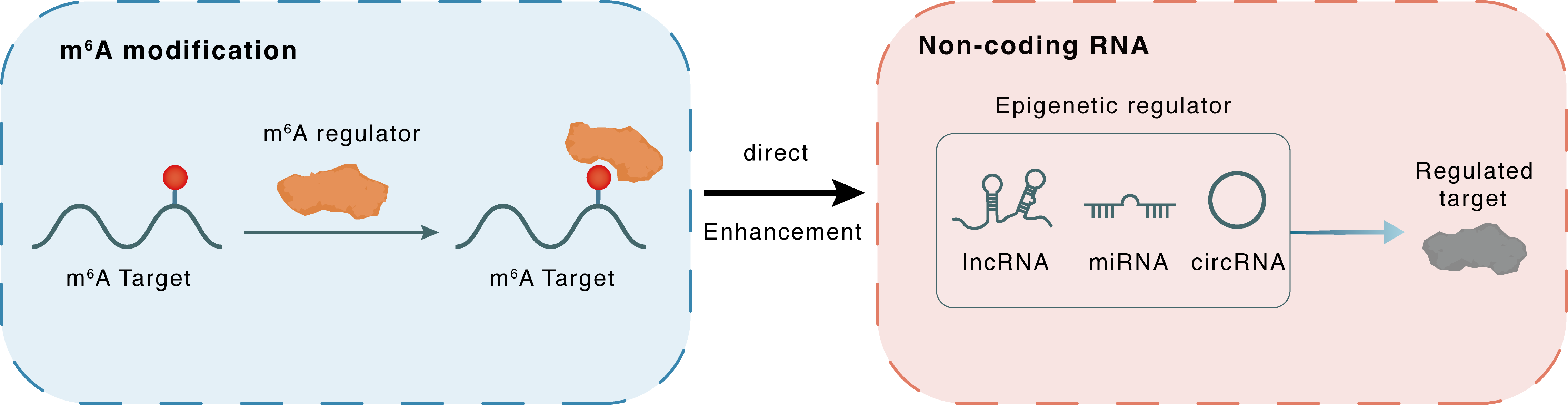 m6A modification
hsa-miR-186-5p
hsa-miR-186-5p
METTL3
Methylation
m6A modification
hsa-miR-186-5p
hsa-miR-186-5p
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR-186-5p
METTL3
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR-186-5p
METTL3
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | hsa-miR-186-5p | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-186-5p | microRNA | View Details | ||
| Regulated Target | Methyltransferase-like protein 3 (METTL3) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | Platinum can increase the overall m6A level of esophageal cancer. SNHG3/hsa-miR-186-5p, induced by platinum, was involved in regulating m6A level by targeting METTL3. miR-186-5p binds to the 3'UTR of METTL3 to inhibit its expression. Our manuscript has provided clues that regulating m6A level was a novel way to enhance the platinum efficacy. | ||||
| Responsed Disease | Melanoma | ICD-11: 2C30 | |||
| Responsed Drug | ML162 | ||||
In-vitro Model |
501-mel | Melanoma | Homo sapiens | CVCL_4633 | |
| SK-MEL-2 | Melanoma | Homo sapiens | CVCL_0069 | ||
| SK-MEL-28 | Cutaneous melanoma | Homo sapiens | CVCL_0526 | ||
| SK-MEL-147 | Melanoma | Homo sapiens | CVCL_3876 | ||
| A-375 | Amelanotic melanoma | Homo sapiens | CVCL_0132 | ||
| SK-MEL-173 | Melanoma | Homo sapiens | CVCL_6090 | ||
| SK-MEL-239 | Melanoma | Homo sapiens | CVCL_6122 | ||
| 451Lu | Cutaneous melanoma | Homo sapiens | CVCL_6357 | ||
| WM278 | Cutaneous melanoma | Homo sapiens | CVCL_6473 | ||
| WM1361A | Cutaneous melanoma | Homo sapiens | CVCL_6788 | ||
| WM793 | Melanoma | Homo sapiens | CVCL_8787 | ||
| WM35 | Melanoma | Homo sapiens | CVCL_0580 | ||
| WM902B | Melanoma | Homo sapiens | CVCL_6807 | ||
| WM115 | Melanoma | Homo sapiens | CVCL_0040 | ||
| WM266-4 | Melanoma | Homo sapiens | CVCL_2765 | ||
| WM1552C | Cutaneous melanoma | Homo sapiens | CVCL_6472 | ||
| In-vivo Model | 4-6 weeks old NOD/Shi-scid/IL-2Rgamma null (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NSG)) mice (female). | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 2C30: Melanoma | 253 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Toripalimab | Approved in China | [2] | ||
| External Link | ||||
| Atezolizumab | Approved | [3] | ||
| External Link | ||||
| Vemurafenib | Approved | [4] | ||
| Synonyms |
PLX4032; RG7204; RO5185426; Zelboraf (TN); Vemurafenib (BRAF inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| Dacarbazine | Approved | [5] | ||
| Synonyms |
Biocarbazin; Biocarbazine; DTIC; DTICDome; DTIE; Dacarbazino; Dacarbazinum; Dacatic; Decarbazine; Deticene; Dimethyltriazenoimidazolecarboxamide; ICDMT; ICDT; Biocarbazine R; DTIC Dome; Dimethyl Imidazole Carboxamide; Dimethyl Triazeno Imidazole Carboxamide; Imidazole carboxamide; HE1150000; Carboxamide (TN); Carboxamide, Dimethyl Imidazole; DIC (TN); DTIC (TN); DTIC-Dome; Dacarbazino [INN-Spanish]; Dacarbazinum [INN-Latin]; Imidazole (TN); Imidazole Carboxamide, Dimethyl; NPFAPI-05; DTIC-Dome (TN); Di-me-triazenoimidazolecarboxamide; Di-methyl-triazenoimidazolecarboxamide; DTIC, DTIC-Dome, Dacarbazine; Dacarbazine (JAN/USP/INN); Dacarbazine [USAN:INN:BAN:JAN]; (5E)-5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; (5Z)-5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; (Dimethyltriazeno)imidazolecarboxamide; 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide; 4(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide; 4-(3,3-Dimethyl-1-triazeno)imidazole-5-carboxamide; 4-(3,3-Dimethyltriazeno)imidazole-5-carboxamide; 4-(5)-(3,3-Dimethyl-1-triazeno)imidazole-5(4)-carboxamide; 4-(Dimethyltriazeno)imidazole-5-c arboxamide; 4-(Dimethyltriazeno)imidazole-5-carboxamide; 4-(or 5)-(3,3-Dimethyl-1-triazeno)imidazole-5(or 4)-carboxamide; 4-[(1E)-3,3-Dimethyltriaz-1-en-1-yl]-1H-imidazole-5-carboxamide; 4-[3,3-dimethyltriaz-1-en-1-yl]-1H-imidazole-5-carboxamide; 5(or 4)-(dimethyltriazeno)imidazol e-4(or 5)-carboxamide; 5(or 4)-(dimethyltriazeno)imidazole-4(or 5)-carboxamide; 5-(3,3-Dimethyl-1-triazeno)imidazole-4-carboxamide; 5-(3,3-Dimethyl-1-triazenyl)-1H-imidazole-4-carboxamide; 5-(3,3-Dimethyl-1-triazenyl)imidazole-4-carboxamide; 5-(3,3-Dimethyltri azeno)imidazole-4-carboxamide; 5-(3,3-Dimethyltriazeno)-imidazole-4-carbamide; 5-(3,3-Dimethyltriazeno)imidazole-4-carboxamide; 5-(3,3-dimethyltriaz-1-en-1-yl)-1H-imidazole-4-carboxamide; 5-(Dimethyltriazeno)-4-imidazolecarboxamide; 5-(Dimethyltriazeno)imidazole-4-carboxamide; 5-(Dimethyltriazeno)imidazole-4-carboximide; 5-(dimethylaminohydrazinylidene)imidazole-4-carboxamide; 5-[3,3-Dimethyl-1-triazenyl]imidazole-4-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Bavencio | Approved | [3] | ||
| External Link | ||||
| Teplizumab | Phase 3 | [6] | ||
| External Link | ||||
| LGX818 | Approved | [7] | ||
| Synonyms |
Encorafenib; 1269440-17-6; LGX-818; Encorafenib (LGX818); UNII-8L7891MRB6; LGX 818; 8L7891MRB6; Encorafenib [USAN:INN]; LGX-818(Encorafenib)
Click to Show/Hide
|
|||
| External Link | ||||
| Tebentafusp | Approved | [8] | ||
| External Link | ||||
| Lenvatinib | Approved | [9] | ||
| Synonyms |
E 7080; E-7080, E7080; 4-[3-chloro-4-(cyclopropylcarbamoylamino)phenoxy]-7-methoxy-quinoline-6-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| Interferon Alfa-2b | Approved | [10] | ||
| Synonyms |
Intron A; Viraferon
Click to Show/Hide
|
|||
| External Link | ||||
| Osimertinib | Approved | [11] | ||
| Synonyms |
Tagrisso
Click to Show/Hide
|
|||
| External Link | ||||
| Tremetinib | Approved | [12] | ||
| External Link | ||||
| Durvalumab | Approved | [13] | ||
| External Link | ||||
| Cemiplimab | Approved | [7] | ||
| Synonyms |
REGN2810; REGN-2810; REGN 2810
Click to Show/Hide
|
|||
| External Link | ||||
| Avelumab | Approved | [13] | ||
| External Link | ||||
| Omaveloxolone | Approved | [3] | ||
| Synonyms |
RJCWBNBKOKFWNY-HGNIWHNWSA-N; N-((4aR,6aR,6bS,12aS,14aR,14bR)-11-cyano-2,2,6a,6b,9,9,12a-heptamethyl-10,14-dioxo-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,14,14a,14b-octadecahydropicen-4a-yl)-2,2-difluoropropanamide; 1474034-05-3; AKOS030526563
Click to Show/Hide
|
|||
| External Link | ||||
| Isoproterenol | Approved | [14] | ||
| Synonyms |
Isoprenaline; Isoprenalin; Norisodrine; Novodrin; Isopropydrin; Isopropylarterenol; Respifral; Assiprenol; Asiprenol; Bellasthman; Asmalar; Aludrine; Aludrin; N-Isopropylnoradrenaline; Bronkephrine; Neodrenal; Lomupren; Isonorene; Isopropyladrenaline; 7683-59-2; N-Isopropylnorepinephrine; Isopropylnorepinephrine; neo-Epinine; Isadrine; Saventrine; Isorenin; Isonorin; Proternol; Isopropylnoradrenaline; Isopropyl noradrenaline; Racemic isoprenaline; dl-Isadrine; Racemic isoproterenol; (+-)-Isoproterenol; Vapo-N-iso; Aerolone; Aleudrine; Dihydroxyphenylethanolisopropylamine; Euspiran; ISOPROP; Isadrin; Isoproterenolum; Isuprel; Isupren; Izadrin; Epinephrine isopropyl homolog; Isoprenaline hydrochloride; Isoproterenol Chloride; Isoproterenol [JAN]; Isuprel Mistometer; WIN 5162; D-Isoprenaline; D-Isopropylarterenol; D-Isoproterenol; DL-Isopropylnorepinephrine; Dl-Ipr; Dl-Isopropylnoradrenaline; Isoprenalina [INN-Spanish]; Isoprenaline (INN); Isoprenalinum [INN-Latin]; Isuprel (TN); L-Isopropylnoradrenaline; L-Isoproterenol; Medihaler-ISO; Vapo-Iso; Alpha-(Isopropylaminomethyl)protocatechuyl alcohol; Alpha-(Isopropylaminomoethyl)protocatechuyl alcohol; D-N-Isopropylnorepinephrine; Dl-N-Isopropylnoradrenaline; Isopropylaminomethyl(3,4-dihydroxyphenyl)carbinol; Isopropylaminomethyl-3,4-dihydroxyphenyl carbinol; DL(+-)-Isoproterenol; N-Isopropyl-beta-dihydroxyphenyl-beta-hydroxyethylamine; (+)-Isoprenaline; (+)-Isoproterenol; (+-)-Isoprenaline; (-)-Isoproterenol hydrochloride; (S)-(+)-Isoproterenol; (S)-Isoprenaline; (S)-Isoproterenol; 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-(9CI); 1,2-Benzenediol, 4-(1-hydroxy-2-((1-methylethyl)amino)ethyl)-, (S)-(9CI); 1-(3,4-Dihydroxyphenyl)-2-(isopropylamino)ethanol; 1-(3,4-Dihydroxyphenyl)-2-isopropylaminoethanol; 3,4-Dihydroxy-alpha-(isopropylaminomethyl)-benzyl alcohol; 3,4-Dihydroxy-alpha-[(isopropylamino)methyl]benzyl alcohol; 3,4-Dihydroxy-alpha-((isopropylamino)methyl)benzyl alcohol; 4-(1-Hydroxy-2(isopropylamino)ethyl)-benzene 1,2-diol; 4-(1-Hydroxy-2-((1-methylethyl)amino)ethyl)-1,2-benzenediol; 4-{1-hydroxy-2-[(1-methylethyl)amino]ethyl}benzene-1,2-diol; AS1409
Click to Show/Hide
|
|||
| External Link | ||||
| Thymalfasin | Phase 2 | [15] | ||
| Synonyms |
Zadaxin; 62304-98-7; Thymosin alpha1; Thymosin alpha 1; Thymosin alpha1 (ox); Thymosin alpha1 (human); Thymalfasin [USAN:INN]; UNII-W0B22ISQ1C; Thymosin-alpha-1; 69440-99-9; Thymosin alpha1 (cattle); C129H215N33O55; W0B22ISQ1C; Zadaxin (TN); Thymalfasin alfa 1
Click to Show/Hide
|
|||
| External Link | ||||
| Melacine | Approved | [10] | ||
| Synonyms |
Melanoma vaccine (Detox-B), Corixa; Melanoma theraccine (Detox-B), Biomira/Ribi; Melanoma vaccine (Detox-B), Biomira/Ribi; Melanomavaccine (Detox-B), Corixa/Schering-Plough
Click to Show/Hide
|
|||
| External Link | ||||
| Ingenol mebutate | Phase 3 | [16] | ||
| Synonyms |
Picato; Ingenol 3-angelate; 75567-37-2; PEP-005; PEP005; 3-Angeloylingenol; UNII-7686S50JAH; PEP 005; 3-Ingenyl angelate; Ingenol-3-angelate; 7686S50JAH; Picato (TN); Euphorbia factor H1; Euphorbia factor An1; Ingenol mebutate [USAN:INN]; [dihydroxy-(hydroxymethyl)-tetramethyl-oxo-[ ]yl] (Z)-2-methylbut-2-enoate; I3A; Ingenol-3- angelate; Ingenol mebutate (USAN); (Z)-2-methylbut-2-enoate; SCHEMBL2526605; GTPL7443; CHEMBL1863513; HSDB 8308; VDJHFHXMUKFKET-WDUFCVPESA-N; MolPort-003-941-761; AN-262; POL-103A
Click to Show/Hide
|
|||
| External Link | ||||
| ARRY-162 | Approved | [7] | ||
| Synonyms |
606143-89-9; Binimetinib; MEK162; MEK-162; ARRY-438162; 5-[(4-Bromo-2-fluorophenyl)amino]-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzimidazole-6-carboxamide; ARRY 162; UNII-181R97MR71; Binimetinib (MEK162, ARRY-162, ARRY-438162); 181R97MR71; MEK162 (ARRY-162, ARRY-438162); 6-(4-bromo-2-fluorophenylamino)-7-fluoro-N-(2-hydroxyethoxy)-3-methyl-3H-benzo[d]imidazole-5-carboxamide; Binimetinib [USAN:INN]; MEK 162; ARRY 438162; MEK162(Binimetinib); Binimetinib (JAN/USAN); NVP-ME
Click to Show/Hide
|
|||
| External Link | ||||
| Relatlimab | Approved | [17] | ||
| External Link | ||||
| Tc-99m tilmanocept | Approved | [18] | ||
| Synonyms |
Lymphoseek (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Palladium Pd 103 | Approved | [10] | ||
| Synonyms |
Palladium-103
Click to Show/Hide
|
|||
| External Link | ||||
| Pembrolizumab | Approved | [3] | ||
| External Link | ||||
| HybriCell | Approved | [10] | ||
| Synonyms |
Dendritic cell-tumor cell hybrid vaccine (melanoma, RCC), Genoa
Click to Show/Hide
|
|||
| External Link | ||||
| MK-3475 | Approved | [19] | ||
| External Link | ||||
| Ipilimumab | Approved | [20] | ||
| Synonyms |
BMS-734016; MDX-010; MDX-101; Yervoy (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Trametinib + dabrafenib | Approved | [21] | ||
| External Link | ||||
| Dabrafenib | Approved | [12] | ||
| Synonyms |
1195765-45-7; Dabrafenib (GSK2118436); Tafinlar; GSK2118436A; UNII-QGP4HA4G1B; GSK 2118436; QGP4HA4G1B; N-(3-(5-(2-aminopyrimidin-4-yl)-2-tert-butylthiazol-4-yl)-2-fluorophenyl)-2,6-difluorobenzenesulfonamide; CHEBI:75045; N-[3-[5-(2-Amino-4-pyrimidinyl)-2-(tert-butyl)-4-thiazolyl]-2-fluorophenyl]-2,6-difluorobenzenesulfonamide; N-{3-[5-(2-aminopyrimidin-4-yl)-2-tert-butyl-1,3-thiazol-4-yl]-2-fluorophenyl}-2,6-difluorobenzenesulfonamide; GSK-2118436A; Dabrafenib [USAN:INN]; GSK2118436
Click to Show/Hide
|
|||
| External Link | ||||
| 99mTc-depreotide | Approved | [10] | ||
| Synonyms |
NeoSpect; NeoTect; P829 Techtide; Technetium-99m P829; Technetium-99m depreotide; 99mTc-P829
Click to Show/Hide
|
|||
| External Link | ||||
| Talimogene Laherparepvec | Approved | [10] | ||
| Synonyms |
IMLYGIC
Click to Show/Hide
|
|||
| External Link | ||||
| Treosulfan | Approved | [22] | ||
| Synonyms |
Tresulfan; Dimesyl-meso-erythritol; Erythritol, 1,4-dimethanesulfonate; Erythritol, dimesyl-, meso-; Dihydroxymyleran; Dihydroxybusulfan; 1,4-Di(methanesulfonate)erythritol; NSC 94160; L-Threityl dimesylate; Erythritol, 1,4-bis(methanesulfonate); Threitol-1,4-bismethanesulfonate, meso-; CB 40069; 1,4-Bis(methanesulfonyloxy)-2,3-butanediol; 1,4-Bis(methylsulfonyloxy)threitol; Threitol 1,4-bis(methanesulfonate); ERYTHRITOL, 1,4-DIMETHANESULFONATE, (meso)-; NCGC00181153-01; L-Threitol 1,4-dimethanesulfonate
Click to Show/Hide
|
|||
| External Link | ||||
| Trametinib | Approved | [23] | ||
| Synonyms |
GSK 1120212; GSK-1120212; GSK1120212; JTP 74057; JTP-74057; Mekinist; Trametinib (GSK1120212); Trametinib (GSK1120212JTP 74057); Trametinib (MEK inhibitor); 33E86K87QN; A1-01871; AK174783; CHEBI:75998; UNII-33E86K87QN
Click to Show/Hide
|
|||
| External Link | ||||
| Nivolumab | Approved | [24] | ||
| External Link | ||||
| HBI-8000 | Phase 1/2 | [13] | ||
| Synonyms |
CS055; SCHEMBL5500152; GTPL8305; AKOS026750315; N-(2-amino-5-fluorophenyl)-4-{[3-(pyridin-3-yl)prop-2-enamido]methyl}benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| OncoVAX | Preregistration | [25] | ||
| Synonyms |
Cancer vaccine, Vaccinogen; OncoVAX, Vaccinogen; Autologous vaccine (colon cancer), Vaccinogen; OncoVAX-CL, Intracel; Vaccine(colon cancer), Intracel; Vaccine (colon cancer), Vaccinogen
Click to Show/Hide
|
|||
| External Link | ||||
| MPL-containing Pollinex allergy desensitization vaccine | Preregistration | [26] | ||
| Synonyms |
HDM SC vaccine, Allergy Therapeutics; House dust mite SC vaccine, Allergy Therapeutics; Pollinex Quattro (house dust mite allergy vaccine); Quattro MPL (house dust mite allergy vaccine); MPL-containing Pollinex allergy desensitization vaccine (house dust mites); MPL-containing Pollinex allergy desensitization vaccine (house dust mites), Allergy Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Tilsotolimod | Phase 3 | [27] | ||
| Synonyms |
IMO-2125
Click to Show/Hide
|
|||
| External Link | ||||
| NKTR-214 | Phase 3 | [28] | ||
| Synonyms |
bempegaldesleukin
Click to Show/Hide
|
|||
| External Link | ||||
| Nemvaleukin alfa | Phase 3 | [29] | ||
| External Link | ||||
| Melanoma vaccine, University of Virginia | Phase 3 | [16] | ||
| Synonyms |
Vaccine (melanoma); Melanoma-specific antigens, Argonex; Peptide vaccine/autologous dendritic cells, Argonex; Vaccine (melanoma), Argonex; Vaccine (melanoma), University of Virginia
Click to Show/Hide
|
|||
| External Link | ||||
| Velimogene aliplasmid | Phase 3 | [30] | ||
| Synonyms |
Allovectin (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| MPDL-3280A | Phase 3 | [31] | ||
| External Link | ||||
| Seviprotimut-L | Phase 3 | [32] | ||
| External Link | ||||
| Melanoma vaccine | Phase 3 | [33] | ||
| Synonyms |
NA17.A2 peptides, Institut Curie; Vaccine (melanoma), Institut Curie; Melanoma vaccine (NA17.A2/tyrosinase/MART-1, gp100); Melanoma vaccine (NA17.A2/tyrosinase/MART-1, gp100), Institut Curie
Click to Show/Hide
|
|||
| External Link | ||||
| Selumetinib | Phase 3 | [34] | ||
| Synonyms |
AZD-6244; ARRY142886; AZD6244; AZD 6244; 6UH91I579U; ARRY 142886; ARRY-142886; AZD6244 (Selumetinib); AZD6244(Selumetinib); CHEBI:90227; CHEMBL1614701; MEK inhibitors; Selumetinib (AZD6244); UNII-6UH91I579U
Click to Show/Hide
|
|||
| External Link | ||||
| ABT-888 | Phase 3 | [9] | ||
| Synonyms |
Veliparib; 912444-00-9; ABT 888; ABT-888 (Veliparib); Veliparib (ABT-888); ABT888; UNII-01O4K0631N; 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide; CHEBI:62880; 2-[(R)-2-methylpyrrolidin-2-yl]-1H-benzimidazole-4-carboxamide; (R)-2-(2-methylpyrrolidin-2-yl)-1H-benzo[d]imidazole-4-carboxamide; 01O4K0631N; 2-[(2R)-2-Methylpyrrolidin-2-yl]-1H-benimidazole-4-; (2r)-2-(7-Carbamoyl-1h-Benzimidazol-2-Yl)-2-Methylpyrrolidinium; Veliparib dihydrochloride
Click to Show/Hide
|
|||
| External Link | ||||
| Multi-epitope peptide melanoma vaccine | Phase 3 | [33] | ||
| Synonyms |
Multi-epitope peptide melanoma vaccine (MART-1, gp100, tyrosinase); Multi-epitope peptide melanoma vaccine (MART-1, gp100, tyrosinase), University of Pittsburgh; MART-1 (51-73) + MART-1(27-35); MART-1(NSC-672643), gp100(NSC-683472), tyrosinase (NSC-699048); MGT (MART-1 (27-35), gp100(209-217, 210M), tyrosinase (368-376, 370D)); (MART-1, gp100, tyrosinase) + (IFNalfa2b + GM-CSF); (MART-1, gp100, tyrosinase) + GM-CSF; (MART-1, gp100, tyrosinase) + IFNalfa2b
Click to Show/Hide
|
|||
| External Link | ||||
| AMD-070 | Phase 3 | [13] | ||
| Synonyms |
AMD 070; AMD070; AMD11070; S14-0353; N-(1H-benzoimidazol-2-ylmethyl)-N-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine; N'-(1H-Benzo[d]imidazol-2-ylmethyl)-N'-(5,6,7,8-tetrahydroquinolin-8-yl)butane-1,4-diamine Trihydrobromide Dihydrate
Click to Show/Hide
|
|||
| External Link | ||||
| SNDX-275 | Phase 3 | [13] | ||
| Synonyms |
Entinostat; Histone Deacetylase Inhibitor I; IN1470; MS 275; SNDX 275; MS 27-275; Ms-275; Entinostat (USAN/INN); MS-27-275; Pyridin-3-ylmethyl 4-(2-aminophenylcarbamoyl)benzylcarbamate; Pyridin-3-ylmethyl N-[[4-[(2-aminophenyl)carbamoyl]phenyl]methyl]carbamate; Pyridin-3-ylmethyl {4-[(2-aminophenyl)carbamoyl]benzyl}carbamate; Pyridin-3-ylmethyl{4-[(2-aminophenyl)carbamoyl]benzyl}carbamate; Carbamic acid, [[4-[[(2-aminophenyl)amino]carbonyl]phenyl] methyl]-, 3-pyridinylmethyl ester; Carbamic acid, [[4-[[(2-aminophenyl)carbaonyl]phenyl]methyl]-, 3-pyridinylmethyl ester; Entinostat, SNDX-275, MS-27-275, MS-275; N-(2-Aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)aminomethyl]benzamide; N-(2-aminophenyl)-4-(N-(pyridin-3-ylmethoxycarbonyl)aminomethyl)benzamide; Carbamic acid, ((4-(((2-aminophenyl)amino)carbonyl)phenyl)methyl)-, 3-pyridinylmethyl ester; 3-Pyridinylmethyl ((4-(((2-aminophenyl)amino)carbonyl)phenyl)methyl)carbamate
Click to Show/Hide
|
|||
| External Link | ||||
| Canvaxin | Phase 3 | [35] | ||
| Synonyms |
Cancer vaccine, CancerVax; Melanoma vaccine, John Wayne Cancer Institute; Polyvalent melanoma cancer vaccine (PMCV)
Click to Show/Hide
|
|||
| External Link | ||||
| PV-10 | Phase 3 | [36] | ||
| Synonyms |
632-69-9; Rose bengal sodium; Rose bengal disodium salt; R105 sodium; Rose-bengal (131 I) natrium; Food Red No 105, sodium salt; EINECS 211-183-3; Food Red Color No 105, sodium salt; Sel disodique de rose bengale iodee (131 I); Rose bengale (131 I) sodique [INN-French]; Rosa bengala sodica (131 I) [INN-Spanish]; Roseum bengalense (131 I) natricum [INN-Latin]; 2,4,5,7-Tetraido(m,p,o',m')tetrachlorofluorescein, disodium salt; Fluorescein, 4,5,6,7-tetrachloro-2',4',5',7'-tetraiodo-, disodium salt; Disodium
Click to Show/Hide
|
|||
| External Link | ||||
| Pegargiminase | Phase 2 | [9] | ||
| Synonyms |
ADI-PEG-20
Click to Show/Hide
|
|||
| External Link | ||||
| RG7421+RG7204 | Phase 3 | [37] | ||
| Synonyms |
Cobimetinib+vemurafenib; Vemurafenib + Cobimetinib (BRAF inhibitor + MEK inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| INCB24360 | Phase 2 | [13] | ||
| Synonyms |
Epacadostat
Click to Show/Hide
|
|||
| External Link | ||||
| IMO-2125 | Phase 3 | [13] | ||
| External Link | ||||
| Cotellic | Phase 3 | [3] | ||
| Synonyms |
Cobimetinib fumarate; Cobimetinib hemifumarate; Xl-518 hemifumarate; GDC-0973 hemifumarate; UNII-6EXI96H8SV; 6EXI96H8SV; Cobimetinib fumarate [USAN]; 1369665-02-0; Cobimetinib fumarate (USAN); Cotellic (TN); CHEMBL2364607; CHEBI:90853; Methanone, (3,4-difluoro-2-((2-fluoro-4-iodophenyl)amino)phenyl)(3-hydroxy-3-(2S)-2-piperidinyl-1-azetidinyl)-, (2E)-2-butenedioate (2:1); D10615; bis[(2S)-2-{1-[3,4-difluoro-2-(2-fluoro-4-iodoanilino)benzoyl]-3-hydroxyazetidin-3-yl}piperidin-1-ium] (2E)-but-2-enedioate
Click to Show/Hide
|
|||
| External Link | ||||
| Tesetaxel | Phase 2 | [38] | ||
| Synonyms |
DJ-927; 333754-36-2; UNII-UG97LO5M8Y; UG97LO5M8Y; Tesetaxel [INN]; DJ927; DJ 927; CHEMBL2107787; SCHEMBL12060837; DB12019; Z-3104; (2AS,2BR,3S,4S,6S,8AR,10R,11AS,11BR,13AR)-2A-ACETOXY-6-(((2R,3S)-3-((TERT-BUTOXYCARBONYL)AMINO)-3-(3-FLUOROPYRIDIN-2-YL)-2-HYDROXYPROPANOYL)OXY)-10-((DIMETHYLAMINO)METHYL)-4-HYDROXY-7,11B,14,14-TETRAMETHYL-2A,2B,3,4,5,6,8A,11A,11B,12,13,13A-DODECAHYDRO-2H-4,8-METHANOOXETO[3'',2'':3',4']BENZO[1',2':3,4]CYCLODECA[1,2-D][1,3]DIOXOL-3-YL BENZOATE
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986205 | Phase 3 | [13] | ||
| Synonyms |
KRTIYQIPSAGSBP-KLAILNCOSA-N; 1923833-60-6; BMS986205; UNII-0A7729F42K; 0A7729F42K; GTPL9707; SCHEMBL18826792; SCHEMBL17740982; SCHEMBL19105151; EX-A2606; AKOS032954040; HY-101560; CS-0021719; Q29213697; (R)-N-(4-chlorophenyl)-2-((1s,4S)-4-(6-fluoroquinolin-4-yl)cyclohexyl)propanamide; (2R)-N-(4-chlorophenyl)-2-[4-(6-fluoroquinolin-4-yl)cyclohexyl]propanamide; (2R)-N-(4-Chlorophenyl)-2-(4-(6-fluoro-4-quinolyl)cyclohexyl)propanamide, cis; Cyclohexaneacetamide, N-(4-chlorophenyl)-4-(6-fluoro-4-quinolinyl)-alpha-methyl-, cis-(alphaR)-
Click to Show/Hide
|
|||
| External Link | ||||
| Daromun | Phase 3 | [13] | ||
| Synonyms |
darleukin + fibromun
Click to Show/Hide
|
|||
| External Link | ||||
| SD-101 | Phase 1/2 | [13] | ||
| Synonyms |
HCV-ISS; HCV vaccine (ISS), Dynavax/Symphony Dynamo
Click to Show/Hide
|
|||
| External Link | ||||
| Javelin | Phase 3 | [39] | ||
| Synonyms |
MJV-101
Click to Show/Hide
|
|||
| External Link | ||||
| Spartalizumab | Phase 3 | [40] | ||
| External Link | ||||
| Melapuldencel-T | Phase 3 | [41] | ||
| External Link | ||||
| Oblimersen | Phase 3 | [42] | ||
| External Link | ||||
| PDR001 | Phase 3 | [43] | ||
| External Link | ||||
| GMK | Phase 3 | [44] | ||
| Synonyms |
(3-Deoxy-3-(3-Methoxy-Benzamido)-B-D-Galactopyranosyl)-(3-Deoxy-3-(3-Methoxy-Benzamido)-2-O-Sulfo-B-D-Galactopyranosyl)-Sulfide; 3-deoxy-3-[(3-methoxybenzoyl)amino]-beta-D-galactopyranosyl 3-deoxy-3-[(3-methoxybenzoyl)amino]-2-O-sulfo-1-thio-beta-D-galactopyranoside
Click to Show/Hide
|
|||
| External Link | ||||
| Mitumomab | Phase 3 | [45] | ||
| Synonyms |
LuVax; MelVax; BEC-2; EMD-60205; IMC-BEC2; Antimelanoma antibody, ImClone/Merck KGaA; Antimetastatic agents, ImClone/Merck KGaA; Melanoma vaccine, ImClone/Merck KGaA; GD3 ganglioside (vaccine 1), Memorial Sloan-Kettering
Click to Show/Hide
|
|||
| External Link | ||||
| QS-21 | Phase 3 | [46] | ||
| Synonyms |
AC1L1U2U; C92H148O46; LS-173109; 124716-EP2295426A1
Click to Show/Hide
|
|||
| External Link | ||||
| RemuneX | Discontinued in Phase 3 | [47] | ||
| External Link | ||||
| 177Lu-DOTA-octreotate | Phase 3 | [48] | ||
| Synonyms |
Lutate; Octreotate [177Lu]; Lutetium-177 octreotate; 177-Lu octreotate; 177-Lu-radiolabeled somatostatin receptor-targeted peptide (neuroendocrine tumor), Biosynthema; 177-lutetium-radiolabeled somatostatin receptor-targeted peptide (neuroendocrinetumor), Biosynthema; 177Lutetium DOTA0-Tyr3-Octreotate
Click to Show/Hide
|
|||
| External Link | ||||
| NKTR 214 | Phase 3 | [49] | ||
| External Link | ||||
| MAGE-A3 immunotherapeutic | Phase 3 | [50] | ||
| External Link | ||||
| OT-101 | Phase 2/3 | [3] | ||
| External Link | ||||
| Imprime PGG | Phase 1/2 | [13] | ||
| Synonyms |
Imprime PGG (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| NLG8189 | Phase 2/3 | [13] | ||
| Synonyms |
1-Methyl-D-tryptophan; Indoximod; 110117-83-4; D-Tryptophan, 1-methyl-; D-1MT; Indoximod (NLG-8189); D-1-methyltryptophan; UNII-TX5CYN1KMZ; D-(+)-1-Methyltryptophan; TX5CYN1KMZ; (R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methylindol-3-yl)propanoic acid; NSC-721782; (2R)-2-amino-3-(1-methyl-3-indolyl)propanoic acid; 1-MT; (2R)-2-azanyl-3-(1-methylindol-3-yl)propanoic acid; (2R)-2-amino-3-(1-methyl-1H-indol-3-yl)propanoic acid; D-l-Methyltryptophan; Indoximod [USAN:INN]; NLG-8189; NLG 8189
Click to Show/Hide
|
|||
| External Link | ||||
| FX005 | Phase 2/3 | [51] | ||
| Synonyms |
TAFAMIDIS; 594839-88-0; FX-1006; 2-(3,5-Dichlorophenyl)-1,3-Benzoxazole-6-Carboxylic Acid; UNII-8FG9H9D31J; CHEBI:78538; 2-(3,5-Dichlorophenyl)-6-benzoxazole carboxylic acid; 8FG9H9D31J; 2-(3,5-Dichlorophenyl)-6-benzoxazolecarboxylic Acid; C14H7Cl2NO3; 2-(3,5-dichlorophenyl)benzoxazole-6-carboxylic acid; Tafamidis [USAN:INN]; tafamidisum; 4his; Fx 1006; 3MI; Tafamidis (USAN/INN); SCHEMBL442508; GTPL8378; CHEMBL2103837; KS-00000TIG; DTXSID00208185; MolPort-027-352-897; BCP29089; 3096AH; ZINC43206271; BDBM50197883; AKOS017550076
Click to Show/Hide
|
|||
| External Link | ||||
| HF10 | Phase 2 | [13] | ||
| Synonyms |
Canerpaturev
Click to Show/Hide
|
|||
| External Link | ||||
| EDP-1503 | Phase 2 | [52] | ||
| External Link | ||||
| mRNA-4157 | Phase 2 | [53] | ||
| External Link | ||||
| Tavokinogene telseplasmid | Phase 2 | [13] | ||
| Synonyms |
TAVO
Click to Show/Hide
|
|||
| External Link | ||||
| V937 | Phase 2 | [54] | ||
| External Link | ||||
| Lifileucel | Phase 2 | [55] | ||
| Synonyms |
LN-144
Click to Show/Hide
|
|||
| External Link | ||||
| PVSRIPO | Phase 2 | [56] | ||
| External Link | ||||
| Ad-RTS-hIL-12 | Phase 2 | [57] | ||
| External Link | ||||
| BNT111 | Phase 2 | [58] | ||
| External Link | ||||
| Voyager-V1 | Phase 2 | [59] | ||
| Synonyms |
VSV-IFNBeta-NIS
Click to Show/Hide
|
|||
| External Link | ||||
| GRN 1201 | Phase 1 | [60] | ||
| External Link | ||||
| Dorgenmeltucel-L | Phase 2 | [61] | ||
| External Link | ||||
| Cancer vaccine | Phase 2 | [62] | ||
| Synonyms |
Cancer vaccine, AVAX/Thomas Jefferson University; Vaccine (melanoma), Thomas Jefferson; Vaccine (DNP-modified), Thomas Jefferson
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-2302025A | Phase 2 | [63] | ||
| Synonyms |
PRAME ASCI (melanoma), GSK; Recombinant preferentially expressed antigen of melanoma immunotherapeutic vaccine (melanoma), GlaxoSmithKline
Click to Show/Hide
|
|||
| External Link | ||||
| Interleukin-12 gene therapy | Phase 2 | [64] | ||
| External Link | ||||
| PF-4878691 | Phase 2 | [65] | ||
| Synonyms |
PF-04878691; PF-4171455
Click to Show/Hide
|
|||
| External Link | ||||
| SentoClone | Phase 2 | [66] | ||
| Synonyms |
Autologous T lymphocyte therapy (malignant melanoma), SentoClone; Autologous Th1 cell therapy (malignant melanoma), SentoClone
Click to Show/Hide
|
|||
| External Link | ||||
| FANG vaccine | Phase 3 | [67] | ||
| Synonyms |
FANG (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| MAGE-3-transduced autologous T-cell vaccine | Phase 2 | [68] | ||
| Synonyms |
M3TK anticancer vaccine, MolMed/Takara; MAGE-3-transduced autologous T-cell vaccine (anticancer);MAGE-3-transduced autologous T-cell vaccine (anticancer), MolMed SpA/Takara Bio
Click to Show/Hide
|
|||
| External Link | ||||
| CB-10-01 | Phase 2 | [69] | ||
| External Link | ||||
| APN-301 | Phase 2 | [70] | ||
| Synonyms |
EMD-273063; Ch14.18-IL-2; Hu14.18-IL-2
Click to Show/Hide
|
|||
| External Link | ||||
| Multi-epitope tyrosinase/gp100 vaccine | Phase 2 | [71] | ||
| Synonyms |
Melanoma vaccine, Memorial Sloan-Kettering; Multi-epitope tyrosinase/gp100 vaccine (melanoma); Multi-epitope tyrosinase/gp100 vaccine (melanoma), Memorial Sloan-Kettering
Click to Show/Hide
|
|||
| External Link | ||||
| Antigen-pulsed dendritic cell vaccine | Phase 2 | [72] | ||
| Synonyms |
Antigen-pulsed dendritic cell vaccine (melanoma)
Click to Show/Hide
|
|||
| External Link | ||||
| Stage IV melanoma vaccine | Phase 2 | [73] | ||
| Synonyms |
Stage IV melanoma vaccine, ARI; Stage IV melanoma vaccine, Adelaide Research & Innovation
Click to Show/Hide
|
|||
| External Link | ||||
| MKC-1106-MT | Phase 2 | [74] | ||
| Synonyms |
Melanoma vaccine (MART-1/tyrosinase), Mannkind
Click to Show/Hide
|
|||
| External Link | ||||
| Telomelysin | Phase 2 | [49] | ||
| External Link | ||||
| Autologous anti-gp100 T-cell receptor gene-engineered peripheral blood lymphocytes | Phase 2 | [75] | ||
| Synonyms |
Autologous anti-gp100 T-cell receptor gene-engineered peripheral blood lymphocytes (melanoma); Autologous anti-gp100 TCR gene-engineered PBLs (melanoma), NCI; Autologous anti-gp100 T-cell receptor gene-engineered peripheral blood lymphocytes (melanoma), National Cancer Institute
Click to Show/Hide
|
|||
| External Link | ||||
| IPI-549 | Phase 2 | [13] | ||
| Synonyms |
XUMALORDVCFWKV-IBGZPJMESA-N; IPI549; 1693758-51-8; CHEMBL3984425; GTPL9563; SCHEMBL16629991; IPI 549; MolPort-044-756-207; EX-A1057; s8330; BDBM50192880; ZINC584906867; AKOS030627132; CS-6106; compound 26 [PMID: 27660692]; AC-29898; HY-100716; Pyrazolo[1,5-a]pyrimidine-3-carboxamide, 2-amino-N-[(1S)-1-[1,2-dihydro-8-[2-(1-methyl-1H-pyrazol-4-yl)ethynyl]-1-oxo-2-phenyl-3-isoquinolinyl]ethyl]-; 2-amino-N-[(1S)-1-[8-[2-(1-methylpyrazol-4-yl)ethynyl]-1-oxo-2-phenylisoquinolin-3-yl]ethyl]pyrazolo[1,5-a]pyrimidine-3-carboxamide; (S)-2-amino-N-(1-(8-((
Click to Show/Hide
|
|||
| External Link | ||||
| Tumor infiltrating lymphocyte | Phase 2 | [76] | ||
| External Link | ||||
| TH-302 | Phase 2 | [13] | ||
| Synonyms |
evofosfamide; 918633-87-1; TH 302; TH302; UNII-8A9RZ3HN8W; Evofosfamide(TH 302); n,n'-bis(2-bromoethyl)phosphorodiamidic acid (1-methyl-2-nitro-1h-imidazol-5-yl)methyl ester; 8A9RZ3HN8W; compound 3b; Evofosfamide;HAP-302; Phosphorodiamidic acid, N,N'-bis(2-bromoethyl)-, (1-methyl-2-nitro-1H-imidazol-5-yl)methyl ester; 2-bromo-N-[(2-bromoethylamino)-[(3-methyl-2-nitroimidazol-4-yl)methoxy]phosphoryl]ethanamine; Evofosfamide [USAN:INN]; Evofosfamide(TH-302); C9H16Br2N5O4P; CHEMBL260046; SCHEMBL2357174
Click to Show/Hide
|
|||
| External Link | ||||
| Ontuxizumab | Phase 2 | [49] | ||
| Synonyms |
Amatuximab
Click to Show/Hide
|
|||
| External Link | ||||
| Anti-CD3 activated vaccine-primed lymphocytes | Phase 2 | [77] | ||
| Synonyms |
VPLN tumor vaccine, University of Michigan; Anti-CD3 activated vaccine-primed lymphocytes (cancer); Monoclonal antiobody activated vaccine-primed lymphocytes (cancer), University of Michigan; Vaccine-primed lymph node (VPLN) cells, University of Michigan; Anti-CD3 activated vaccine-primed lymphocytes (cancer), University of Michigan; Anti-CD3/anti-CD28 activated vaccine-primed lymphocytes (cancer), University of Michigan
Click to Show/Hide
|
|||
| External Link | ||||
| TLPLDC | Phase 2 | [78] | ||
| External Link | ||||
| Coxsackievirus A21 | Phase 1/2 | [49] | ||
| Synonyms |
Cavatak (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Resimmune | Phase 1/2 | [79] | ||
| Synonyms |
A-dmDT390-bisFv immunotoxin
Click to Show/Hide
|
|||
| External Link | ||||
| ABX-IL8 | Phase 2 | [80] | ||
| External Link | ||||
| ITIL-168 | Phase 2 | [81] | ||
| External Link | ||||
| Hi-8 PrimeBoost therapeutic melanoma vaccine | Phase 2 | [82] | ||
| Synonyms |
Hi-8 MEL; Melanoma therapeutic vaccine, Oxxon; PSG2.Mel3; Hi-8 PrimeBoost therapeutic melanoma vaccine, Oxford BioMedica; Hi-8 PrimeBoost therapeutic melanoma vaccine, Oxxon; Lead Hi-8 PrimeBoost therapeutic melanoma vaccine, Oxxon; Pharmaccine therapy (melanoma), Oxxon; DNA/MVA immunotherapy (melanoma), Oxxon; MEL-1 immunotherapy (melanoma), Oxxon; Melanoma immunotherapy (Prime-Boost), Oxxon
Click to Show/Hide
|
|||
| External Link | ||||
| GRN-1201 | Phase 1 | [49] | ||
| External Link | ||||
| CYT-004-MelQbG10 | Phase 2 | [83] | ||
| Synonyms |
Immunodrug vaccine (melanoma), Cytos; QbG10 Immunodrug carrier (cancer), Cytos
Click to Show/Hide
|
|||
| External Link | ||||
| GSK3377794 | Phase 2 | [13] | ||
| External Link | ||||
| Gp100:209-217(210M) peptide vaccine | Phase 2 | [84] | ||
| Synonyms |
Gp100 melanoma-associated antigen vaccine, NCI; Gp100:209-217(210M) peptide vaccine (melanoma); Gp100:209-217(210M) peptide vaccine (melanoma), NCI
Click to Show/Hide
|
|||
| External Link | ||||
| LN-144 | Phase 2 | [49] | ||
| External Link | ||||
| MLN2480 | Phase 2 | [85] | ||
| Synonyms |
VWMJHAFYPMOMGF-ZCFIWIBFSA-N; 1096708-71-2; MLN 2480; BIIB-024; Tak-580; MLN-2480; BIIB024; UNII-ZN90E4027M; BIIB 024; 4-Pyrimidinecarboxamide, 6-amino-5-chloro-N-[(1R)-1-[5-[[[5-chloro-4-(trifluoromethyl)-2-pyridinyl]amino]carbonyl]-2-thiazolyl]ethyl]-; ZN90E4027M; (r)-2-(1-(6-amino-5-chloropyrimidine-4-carboxamido)ethyl)-n-(5-chloro-4-(trifluoromethyl)pyridin-2-yl)thiazole-5-carboxamide; 4-Pyrimidinecarboxamide, 6-amino-5-chloro-N-((1R)-1-(5-(((5-chloro-4-(trifluoromethyl)-2-pyridinyl)amino)carbonyl)-2-thiazolyl)ethyl]-; AMG 2112819
Click to Show/Hide
|
|||
| External Link | ||||
| ARC-100 | Phase 2 | [86] | ||
| Synonyms |
SCHEMBL13011610
Click to Show/Hide
|
|||
| External Link | ||||
| AG140699 | Phase 2 | [87] | ||
| External Link | ||||
| E7016 | Phase 2 | [88] | ||
| Synonyms |
UNII-M8926C7ILX; E-7016; M8926C7ILX; 902128-92-1; SCHEMBL1319757; CHEMBL3527000; BDBM97563; GPI 21016; GPI-21016; SB16889; Benzopyrano(4,3,2-de)phthalazin-3(2H)-one, 10-((4-hydroxy-1-piperidinyl)methyl)-; US8470825, 4i; E 7016
Click to Show/Hide
|
|||
| External Link | ||||
| GR-MD-02 | Phase 2 | [89] | ||
| Synonyms |
Belapectin
Click to Show/Hide
|
|||
| External Link | ||||
| PLX8394 | Phase 2 | [90] | ||
| External Link | ||||
| Hi8 melanoma vaccine | Phase 2 | [91] | ||
| External Link | ||||
| GCAN 101 | Phase 2 | [92] | ||
| External Link | ||||
| MSB0010445 | Phase 2 | [93] | ||
| External Link | ||||
| RAF265 | Phase 2 | [94] | ||
| Synonyms |
927880-90-8; CHIR-265; RAF 265; RAF-265; CHIR 265; RAF265 (CHIR-265); 1-Methyl-5-[[2-[5-(trifluoromethyl)-1h-imidazol-2-yl]-4-pyridinyl]oxy]-n-[4-(trifluoromethyl)phenyl]-1h-benzimidazol-2-amine; CHIR265; RAF265(CHIR-265); UNII-8O434L3768; 8O434L3768; 1-methyl-5-[2-[5-(trifluoromethyl)-1H-imidazol-2-yl]pyridin-4-yl]oxy-N-[4-(trifluoromethyl)phenyl]benzimidazol-2-amine; 1-Methyl-5-({2-[5-(Trifluoromethyl)-1h-Imidazol-2-Yl]pyridin-4-Yl}oxy)-N-[4-(Trifluoromethyl)phenyl]-1h-Benzimidazol-2-Amine; PubChem22594
Click to Show/Hide
|
|||
| External Link | ||||
| Urelumab | Phase 2 | [95] | ||
| External Link | ||||
| MORAb-004 | Phase 2 | [96] | ||
| External Link | ||||
| APX005M | Phase 2 | [97] | ||
| External Link | ||||
| Leuvectin | Phase 2 | [98] | ||
| Synonyms |
IL-2 gene therapy, Vical; Cytofectin (IL-2), Vical; Gene therapy (interleukin-2), Vical
Click to Show/Hide
|
|||
| External Link | ||||
| BVD-523 | Phase 2 | [99] | ||
| External Link | ||||
| MGCD-0103 | Phase 2 | [3] | ||
| Synonyms |
Mocetinostat; MG 0103; MG 4230; MG 4915; MG 5026; MG0103; MG4230; MG4915; MG5206; MGCD 0103; MGCD0103; MG-0103; MG-4230; MG-4915; MG-5026; Mocetinostat, MGCD0103; N-(2-aminophenyl)-4-[[(4-pyridin-3-ylpyrimidin-2-yl)amino]methyl]benzamide; N-(2-Aminophenyl)-4-((4-pyridin-3-ylpyrimidin-2-ylamino)methyl)benzamide
Click to Show/Hide
|
|||
| External Link | ||||
| Polynoma-1 | Phase 2 | [100] | ||
| Synonyms |
Polyvalent melanoma vaccine, Vaccinoma
Click to Show/Hide
|
|||
| External Link | ||||
| ENB003 | Phase 1/2 | [101] | ||
| Synonyms |
2364572-10-9; AAY8R26VDX; BQ 788-d2; BQ-788, di(methyl-d)-; CHEMBL5095234; D-Norleucine, N-((cis-2,6-di(methyl-d)-1-piperidinyl)carbonyl)-4-methyl-L-leucyl-1-(methoxycarbonyl)-D-tryptophyl-, sodium salt; Enb 003; ENB 003 [WHO-DD]; ENB003; ENB-003; UNII-AAY8R26VDX; Vodudeutentan sodium
Click to Show/Hide
|
|||
| External Link | ||||
| HL-085 | Phase 1/2 | [102] | ||
| External Link | ||||
| NKTR-262 | Phase 1/2 | [3] | ||
| External Link | ||||
| Delolimogene mupadenorepvec | Phase 1/2 | [103] | ||
| Synonyms |
LOAd703
Click to Show/Hide
|
|||
| External Link | ||||
| Antigen-specific melanoma vaccine | Phase 1/2 | [104] | ||
| External Link | ||||
| ImmuFact IMP321 | Phase 1/2 | [105] | ||
| External Link | ||||
| NY-ESO-TCR | Phase 1/2 | [49] | ||
| External Link | ||||
| LXS196 | Phase 1/2 | [13] | ||
| Synonyms |
XXJXHXJWQSCNPX-UHFFFAOYSA-N; 1874276-76-2; LXS-196; NVP-LXS196; SCHEMBL17506262; CHEMBL3982723; LXS 196; BDBM251460; EX-A2690; BCP20781; ZINC584641445; CS-7529; HY-101569; US9452998, 9; 3-amino-N-(3-(4-amino-4-methylpiperidin-1-yl)pyridin-2-yl)-6-(3-(trifluoromethyl)pyridin-2-yl)pyrazine-2-carboxamide
Click to Show/Hide
|
|||
| External Link | ||||
| AU-011 | Phase 1/2 | [13] | ||
| External Link | ||||
| MAGE-A10 TCR | Phase 1/2 | [3] | ||
| External Link | ||||
| TRX-518 | Phase 1/2 | [106] | ||
| Synonyms |
GITR monoclonal antibody (cancer/viral infection), TolerRx; Glucocorticoid-induced TNF receptor monoclonal antibody (viral infection/cancer), TolerRx; GITR mAb (IgG/iv, cancer/viral infection), TolerRx
Click to Show/Hide
|
|||
| External Link | ||||
| IMCnyeso | Phase 1/2 | [3] | ||
| External Link | ||||
| LAG525 | Phase 1/2 | [13] | ||
| External Link | ||||
| NCI-4650 | Phase 1/2 | [3] | ||
| External Link | ||||
| Ad-IL-12 DNA therapeutic | Phase 1/2 | [107] | ||
| Synonyms |
INXN-2001
Click to Show/Hide
|
|||
| External Link | ||||
| SCIB-1 | Phase 1/2 | [108] | ||
| Synonyms |
SCIB-5; SCIB-1 DNA vaccine (melanoma, TriGrid), Scancell; Anti-TRP-2/ anti-gp100 monoclonal antibody vaccine (melanoma), Scancell
Click to Show/Hide
|
|||
| External Link | ||||
| ImmunoVEX-timelan | Phase 1/2 | [109] | ||
| External Link | ||||
| CLL442 | Phase 1/2 | [13] | ||
| External Link | ||||
| ONCOS-102 | Phase 1/2 | [49] | ||
| Synonyms |
CGTG-102
Click to Show/Hide
|
|||
| External Link | ||||
| AV-MEL-1 | Phase 1 | [110] | ||
| External Link | ||||
| CV8102 | Phase 1 | [111] | ||
| External Link | ||||
| GEN-011 | Phase 1 | [112] | ||
| External Link | ||||
| INXN 2001 | Phase 1 | [113] | ||
| External Link | ||||
| ADP-A2M10 | Phase 1 | [114] | ||
| External Link | ||||
| CDX-1140 | Phase 1 | [115] | ||
| External Link | ||||
| PF-07284890 | Phase 1 | [116] | ||
| Synonyms |
1-Propanesulfonamide, N-(2-chloro-3-((3,4-dihydro-3,5-dimethyl-4-oxo-6-quinazolinyl)amino)-4-fluorophenyl)-3-fluoro-; 2573781-75-4; AR00504461; ARRY461; ARRY-461; ARRY-461; BDBM565133; CHEMBL5095256; CS-0564141; EX-A7593; GTPL12096; HY-147405; JNJ5N2RH5W; N-(2-chloro-3-((3,5-dimethyl-4-oxo-3,4-dihydroquinazolin-6-yl)amino)-4-fluorophenyl)-3-fluoropropane-1-sulfonamide; PF07284890; PF-07284890; PF-07284890; SCHEMBL22831935; Tinlorafenib; tinlorafenib [INN]; Tinlorafenib [USAN]; UNII-JNJ5N2RH5W; US11414404, Ex. # 10; WHO 12353
Click to Show/Hide
|
|||
| External Link | ||||
| XmAb20717 | Phase 1 | [117] | ||
| Synonyms |
XmAb717
Click to Show/Hide
|
|||
| External Link | ||||
| SX-682 | Phase 1 | [118] | ||
| Synonyms |
1648843-04-2; UNII-H5212R2DPM; H5212R2DPM; SX682; (2-(((5-((4-Fluorophenyl)carbamoyl)pyrimidin-2-yl)thio)methyl)-4-(trifluoromethoxy)phenyl)boronic acid; CHEMBL4297480; GTPL10165; BCP32154; EX-A4295; MFCD28502254; s8947; SX 682; SB17394; HY-119339; CS-0067128; [2-[[5-[(4-fluorophenyl)carbamoyl]pyrimidin-2-yl]sulfanylmethyl]-4-(trifluoromethoxy)phenyl]boronic acid; 2-(2-Dihydroxyboryl-5-trifluoromethoxybenzylsulfanyl)pyrimidine-5-carboxylic acid (4-fluorophenyl)amide; B-(2-(((5-(((4-Fluorophenyl)amino)carbonyl)-2-pyrimidinyl)thio)methyl)-4-(trifluoromethoxy)phenyl)boronic acid; Boronic acid, B-(2-(((5-(((4-fluorophenyl)amino)carbonyl)-2-pyrimidinyl)thio)methyl)-4-(trifluoromethoxy)phenyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| SB 11285 | Phase 1 | [119] | ||
| External Link | ||||
| CMP-001 | Phase 1 | [3] | ||
| Synonyms |
vidutolimod
Click to Show/Hide
|
|||
| External Link | ||||
| PRT1419 | Phase 1 | [120] | ||
| External Link | ||||
| INCAGN2385 | Phase 1 | [121] | ||
| External Link | ||||
| TBX-3400 | Phase 1 | [122] | ||
| External Link | ||||
| Ifx-Hu2.0 | Phase 1 | [123] | ||
| External Link | ||||
| VCL-1M01 | Phase 1 | [124] | ||
| Synonyms |
VCL-IM01; EPT (plasmid DNA), Vical; IL-2/EP; MedPulser, cancer (plasmid DNA), Vical; Plasmid DNA (transdermal, EPT), Vical; IL-2 gene therapy (plasmid, transdermal, melanoma); IL-2 gene therapy (pVCL-1102 plasmid DNA vector, melanoma), Vical; IL-2 gene therapy (plasmid, transdermal, melanoma), Vical
Click to Show/Hide
|
|||
| External Link | ||||
| GPA-TriMAR-T cells | Phase 1 | [125] | ||
| External Link | ||||
| PTI-188 | Phase 1 | [126] | ||
| External Link | ||||
| Dendritic cancer cell vaccine | Phase 1 | [127] | ||
| External Link | ||||
| BETULINIC ACID | Phase 1 | [128] | ||
| Synonyms |
472-15-1; Mairin; Betulic acid; NSC 113090; CCRIS 6748; 3-Hydroxylup-20(29)-en-28-oic acid; UNII-4G6A18707N; 3beta-Hydroxy-20(29)-lupaene-28-oic acid; EINECS 207-448-8; NSC677578; als-357; NSC 677578; CHEMBL269277; CHEBI:3087; 3beta-Hydroxy-lup-20(29)-en-28-oic acid; 4G6A18707N; Lup-20(29)-en-28-oic acid, 3-hydroxy-, (3beta)-; AK-72848; Lupatic Acid; SMR000445624
Click to Show/Hide
|
|||
| External Link | ||||
| Multipeptide vaccine combination | Phase 1 | [129] | ||
| Synonyms |
Multipeptide vaccine combination (melanoma); Multipeptide vaccine combination (melanoma), Ludwig; MAGE-10.A2+ Melan-A ELA + NY-ESO-1b + tyrosinase + GM-CSF multipeptide vaccine (melanoma), Ludwig; MAGE-10.A2 + Melan-A ELA + NY-ESO-1b + tyrosinase + GM-CSF multipeptide vaccine (melanoma), Ludwig/Columbia
Click to Show/Hide
|
|||
| External Link | ||||
| NEO-PV-01 | Phase 1 | [3] | ||
| External Link | ||||
| Adoptive T-cell therapy | Phase 1 | [130] | ||
| Synonyms |
Adoptive T-cell therapy (cancer)
Click to Show/Hide
|
|||
| External Link | ||||
| Vaccimel | Phase 1 | [131] | ||
| Synonyms |
Melanoma cell line vaccine (cancer), Centro de Investigaciones Oncologicas; Autologous dendritic cells + allogeneic melanoma cell lines vaccine, Centro de Investigaciones Oncologicas/ Universidad de Buenos Aires; Dendritic cells + apoptotic/necrotic allogeneic melanoma cell lines vaccine, Centro de Investigaciones Oncologicas/ Universidad de Buenos Aires; DC/Apo-Nec vaccine (melanoma), Centro de Investigaciones Oncologicas/ Universidad de Buenos Aires
Click to Show/Hide
|
|||
| External Link | ||||
| Lipovaxin-MM | Phase 1 | [132] | ||
| Synonyms |
Dendritic cell-specific antigen targeting vaccine (liposome/nanoparticle/intravenous, melanoma), Lipotek
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-2241658A | Phase 1 | [133] | ||
| Synonyms |
NY-ESO-1 ASCI (melanoma), GSK; Recombinant NY-ESO-1 antigen immunotherapeutic vaccine (melanoma), GlaxoSmithKline; 2241658A
Click to Show/Hide
|
|||
| External Link | ||||
| ICON-1 | Phase 1 | [49] | ||
| External Link | ||||
| PLX-4720 | Phase 1 | [134] | ||
| Synonyms |
PLX 4720; PLX4720 (BRAF inhibitor)
Click to Show/Hide
|
|||
| External Link | ||||
| AE-08 | Phase 1 | [135] | ||
| External Link | ||||
| TAK-202 | Phase 1 | [49] | ||
| External Link | ||||
| Autologous T Cells Expressing MET scFv CAR | Phase 1 | [136] | ||
| External Link | ||||
| AEB701 | Phase 1 | [137] | ||
| External Link | ||||
| UV1 + GM-CSF vaccine | Phase 1 | [3] | ||
| External Link | ||||
| AMEP | Phase 1 | [138] | ||
| Synonyms |
(Aminomethyl)phosphonic acid; 1066-51-9; AMINOMETHYLPHOSPHONIC ACID; Aminomethanephosphonic acid; 1-Aminomethylphosphonic acid; 1-Aminomethylphosphonate; Amino methane phosphoric acid; Caswell No. 037C; Phosphaglycine; (1-Aminomethyl)phosphonic acid; Phosphonic acid, aminomethyl-; UNII-90825O5C1U; Phosphonic acid, (aminomethyl)-; Aminomethylphosphonic acid (AMPA); NSC 30076; EPA Pesticide Chemical Code 207800; CHEBI:28812; MGRVRXRGTBOSHW-UHFFFAOYSA-N; (Aminomethyl)phosphonic acid, 99%; MFCD00008105; GEO-00169; 90825O5C1U
Click to Show/Hide
|
|||
| External Link | ||||
| Melanoma DNA vaccine | Phase 1 | [139] | ||
| External Link | ||||
| DEDN-6526A | Phase 1 | [140] | ||
| External Link | ||||
| IL-2 pDNA | Phase 1 | [141] | ||
| External Link | ||||
| AT13387 | Phase 1 | [142] | ||
| Synonyms |
AT-13387
Click to Show/Hide
|
|||
| External Link | ||||
| Ioflubenzamide (131I) | Phase 1 | [143] | ||
| Synonyms |
Solazed; Ioflubenzamide I-131; MIP-1104; MIP-1143; MIP-1144; MIP-1145; ZK-BA; Iodine-123-Solazed; Iodine-131-Solazed; MIP-1145-[123I]; MIP-1145-[131I]; Radiolabeled benzamides (melanoma), Bayer; Radiolabeled benzamides (melanoma), Molecular Insight; Iodine-123-ZK-BA; Iodine-131-ZK-BA; Melanin-targeting radiodiagnostic (melanoma), Bayer/Molecular Insight; Melanin-targeting radiotherapeutic (melanoma), Bayer/Molecular Insight; 123I-MIP-1145; 123I-Solazed; 123I-ZK-BA
Click to Show/Hide
|
|||
| External Link | ||||
| SAM-6 | Phase 1 | [144] | ||
| External Link | ||||
| Flanvotumab | Phase 1 | [145] | ||
| Synonyms |
IMC-20D7S; Gp75 monoclonal antibodies (melanoma), ImClone; Gp75 mAb (melanoma), ImClone/Eli Lilly; GP75/TRP1 mAbs (melanoma), ImClone/Eli Lilly
Click to Show/Hide
|
|||
| External Link | ||||
| CAR-T cells recognizing EpCAM | Phase 1 | [146] | ||
| External Link | ||||
| DC-IL-12 DNA therapeutic | Phase 1 | [147] | ||
| Synonyms |
INXN-3001
Click to Show/Hide
|
|||
| External Link | ||||
| LV305 | Phase 1 | [148] | ||
| Synonyms |
NY-ESO-1 Zvex
Click to Show/Hide
|
|||
| External Link | ||||
| TTI-621 | Phase 1 | [3] | ||
| External Link | ||||
| PRAME antigen-specific cancer immunotherapeutic | Phase 1 | [149] | ||
| External Link | ||||
| Nuleusin | Discontinued in Phase 3 | [150] | ||
| Synonyms |
IL-2 (subcutaneous formulation, RCC/MM), 3SBio
Click to Show/Hide
|
|||
| External Link | ||||
| IDD-3 | Discontinued in Phase 2 | [151] | ||
| Synonyms |
Uvidem; Dendritophage + melanoma tumor cell lysate; Melanoma vaccine, Immuno-Designed Molecules/ IDM Pharma; Vaccine (melanoma), Immuno-Designed Molecules /IDM Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| AN-9 | Discontinued in Phase 2 | [152] | ||
| Synonyms |
Pivanex; Pivalyloxymethyl butyrate; AN 9; AN9; AN 9 (ion exchanger); Butanoyloxymethyl 2,2-dimethylpropanoate; Butanoicacid, (2,2-dimethyl-1-oxopropoxy)methyl ester; N-(5-amino-9,10-dioxoanthracen-1-yl)acetamide; N-(5-amino-9,10-dioxo-9,10-dihydroanthracen-1-yl)acetamide; ((2,2-Dimethylpropanoyl)oxy)methyl butanoate;1,5-BIS[3-(DIETHYLAMINO)PROPIONAMIDO]ANTHRACENE-9,10-DIONE
Click to Show/Hide
|
|||
| External Link | ||||
| BIWB-1 | Discontinued in Phase 2 | [153] | ||
| Synonyms |
Melanoma vaccine (autologous), Boehringer Ingelheim/Bender; IL-2 gene therapy (autologous), Boehringer Ingelheim/Bender
Click to Show/Hide
|
|||
| External Link | ||||
| Melanoma vaccine (ALVAC) | Discontinued in Phase 2 | [154] | ||
| Synonyms |
Melanoma vaccine (ALVAC), sanofi pasteur; Melanoma vaccine (gp100/MAGE-1/MAGE-3/MART-1/NY-ESO-1, ALVAC), sanofi pasteur
Click to Show/Hide
|
|||
| External Link | ||||
| TriGem | Discontinued in Phase 2 | [155] | ||
| Synonyms |
Monoclonal antibody 1A7, Titan Pharmaceuticals; Anti-I17 antibody, Trilex
Click to Show/Hide
|
|||
| External Link | ||||
| Melaxin | Discontinued in Phase 2 | [156] | ||
| Synonyms |
Autologous dendritoma vaccine (sc, melanoma), Oncolix
Click to Show/Hide
|
|||
| External Link | ||||
| Ecromeximab | Discontinued in Phase 2 | [157] | ||
| External Link | ||||
| XomaZyme-Mel | Discontinued in Phase 2 | [158] | ||
| Synonyms |
XMMME-0001-RTA
Click to Show/Hide
|
|||
| External Link | ||||
| Zeniplatin | Discontinued in Phase 2 | [159] | ||
| Synonyms |
CL-286558
Click to Show/Hide
|
|||
| External Link | ||||
| ODC-0501 | Discontinued in Phase 1/2 | [160] | ||
| Synonyms |
Dendritic cell vaccine (melanoma), ODC; Vaccine (melanoma), ODC
Click to Show/Hide
|
|||
| External Link | ||||
| RG-7256 | Discontinued in Phase 1 | [161] | ||
| Synonyms |
B-raf inhibitor (melanoma), Roche/Plexxikon
Click to Show/Hide
|
|||
| External Link | ||||
| RG7636 | Discontinued in Phase 1 | [162] | ||
| External Link | ||||
| ABX-MA1 | Discontinued in Phase 1 | [163] | ||
| Synonyms |
Anti-MUC18 MAb, Abgenix
Click to Show/Hide
|
|||
| External Link | ||||
| F-50040 | Discontinued in Phase 1 | [164] | ||
| Synonyms |
Metastatic melanoma vaccine, Pierre Fabre; P40-ELA; Metastatic melanoma vaccine, BioMerieux-Pierre Fabre; Vaccine (melanoma), Pierre Fabre; Vaccine (melanoma), BioMerieux-Pierre Fabre
Click to Show/Hide
|
|||
| External Link | ||||
| ANAVEX 1007 | Preclinical | [165] | ||
| External Link | ||||
| DISC GM-CSF | Terminated | [166] | ||
| External Link | ||||
| Withaferin A | Terminated | [167] | ||
| Synonyms |
Heat shock factor 1 inhibitor (melanoma), University of Kansas; HSF-1 inhibitor (melanoma), University of Kansas
Click to Show/Hide
|
|||
| External Link | ||||
| SBP-002 | Terminated | [168] | ||
| Synonyms |
Coramsine; Solasonine + solamargine; Solasodine glycosides (cancer, injectable formulation), Solbec; Solasodine glycosides (psoriasis, cream formulation), Solbec; Solasonine + solamargine (cancer), Solbec; Solasonine + solamargine (psoriasis), Solbec; SBP-002 (intravenous, cancer), Solbec; SBP-002 (psoriasis, cream formulation), Solbec
Click to Show/Hide
|
|||
| External Link | ||||
| RAAV vaccine | Terminated | [169] | ||
| External Link | ||||
| Novovac-M1 | Terminated | [170] | ||
| External Link | ||||
| VLD-631 | Investigative | [171] | ||
| External Link | ||||
| SMT-C2100 | Investigative | [171] | ||
| External Link | ||||
| TOCA-621 | Investigative | [171] | ||
| Synonyms |
Viral vector-delivered interferon gamma gene therapy (malignant melanoma), Tocagen
Click to Show/Hide
|
|||
| External Link | ||||
| NVX-412 | Investigative | [171] | ||
| External Link | ||||
| G3139 + Dacarbazine | Investigative | [172] | ||
| External Link | ||||
| VitiGam | Investigative | [171] | ||
| External Link | ||||
| CGEN-791 | Investigative | [171] | ||
| External Link | ||||
| IMX-MEL1 | Investigative | [171] | ||
| Synonyms |
Melanoma therapeutic vaccine, Imaxio
Click to Show/Hide
|
|||
| External Link | ||||
| Lm Melanoma | Investigative | [171] | ||
| Synonyms |
Recombinant bacterial vector vaccine (melanoma), Aduro BioTech/New York University
Click to Show/Hide
|
|||
| External Link | ||||
| ADS-NPTMZ-1 | Investigative | [171] | ||
| External Link | ||||
| Narciclasine | Investigative | [173] | ||
| Synonyms |
Lycoricidinol; 29477-83-6; Lycoricidin-A; Narciclasina; NSC 266535; BRN 1087400; NSC266535; Lycorcidinol; Nacriclasine; C14H13NO7; CHEMBL98745; 3,4,4a,5-Tetrahydro-2,3,4,7-tetrahydroxy-(1,3)dioxolo(4,5-j)phenanthridin-6(2H)-one; CHEBI:70169; (2s,3r,4s,4ar)-2,3,4,7-tetrahydroxy-3,4,4a,5-tetrahydro[1,3]dioxolo[4,5-j]phenanthridin-6(2h)-one; (2S,3R,4S,4aR)-2,3,4,7-Tetrahydroxy-3,4,4a,5-tetrahydro-2H-[1,3]dioxolo[4,5-j]phenanthridin-6-one
Click to Show/Hide
|
|||
| External Link | ||||
| 111In-labeled lactam bridge-cyclized alpha-melanocyte-stimulating hormone peptide (melanoma), NuView/University of New Mexico | Investigative | [171] | ||
| Synonyms |
Imaging agent (melanoma), NuView; 111In-DOTA-GlyGlu-CycMSH (melanoma), NuView/University of New Mexico
Click to Show/Hide
|
|||
| External Link | ||||
| Ektomun | Investigative | [171] | ||
| Synonyms |
Antibody (melanoma), Trion
Click to Show/Hide
|
|||
| External Link | ||||
| PDS-0102 | Investigative | [171] | ||
| Synonyms |
Versamune-Melanoma; Vaccine (cationic lipid nanoparticle formulation, melanoma), PDS Biotechnology
Click to Show/Hide
|
|||
| External Link | ||||
| MTI-SAM3 | Investigative | [171] | ||
| External Link | ||||
| Box-5 | Investigative | [171] | ||
| Synonyms |
Wnt-5a antagonist (peptide, melanoma/metastasis), WntResearch
Click to Show/Hide
|
|||
| External Link | ||||
| 20D75 | Investigative | [171] | ||
| External Link | ||||
| BCL-005 | Investigative | [171] | ||
| External Link | ||||
| 3-benzofuran-2-yl-2-benzothiazol-2-yl-3-oxo-propanenitrile | Investigative | [174] | ||
| External Link | ||||
| MT-062 | Investigative | [175] | ||
| Synonyms |
EGFR inhibitor (melanoma), Medisyn Technologies
Click to Show/Hide
|
|||
| External Link | ||||
| 1,3-bis(1,3-benzothiazol-2-ylthio)acetone | Investigative | [174] | ||
| Synonyms |
1,3-bis(1,3-benzothiazol-2-ylsulfanyl)propan-2-one; MLS000533100; AC1LOHTK; CBMicro_026328; CHEMBL1526448; MolPort-002-158-967; HMS2496A11; ZINC1029672; 1,3-dibenzothiazol-2-ylthioacetone; STK733322; AKOS001750462; MCULE-2850305009; ST006263; SMR000140538; BIM-0026345.P001; 1,3-bis(benzo[d]thiazol-2-ylthio)propan-2-one; SR-01000535355; SR-01000535355-1; 1,3-bis(1,3-benzothiazol-2-ylsulfanyl)propane-2-one; F1563-0006; A2660/0113409
Click to Show/Hide
|
|||
| External Link | ||||
| PRAME-SLP | Investigative | [171] | ||
| Synonyms |
Peptide vaccine(cancer), ISA Pharmaceuticals; Preferentially expressed antigen based vaccine (SLP technology/peptide, melanoma), ISA
Click to Show/Hide
|
|||
| External Link | ||||
| AEZS-120 | Investigative | [171] | ||
| Synonyms |
Cancer vaccine (bacterial vector), University of Wurzburg/AEterna Zentaris
Click to Show/Hide
|
|||
| External Link | ||||
| 5-fluoro-1H-indole-2-carboxylic acid | Investigative | [174] | ||
| Synonyms |
5-Fluoroindole-2-carboxylic acid; 399-76-8; 2-Carboxy-5-fluoroindole; 1H-Indole-2-carboxylic acid, 5-fluoro-; CHEMBL23507; MLS000080089; WTXBRZCVLDTWLP-UHFFFAOYSA-N; MFCD00005612; SMR000037735; 5-Fluoroindole-2-carboxylic acid, 98%; Spectrum_001495; EINECS 206-919-5; PubChem1683; SpecPlus_000678; Spectrum5_001733; Opera_ID_1340; Spectrum4_001182; Spectrum3_001043; Spectrum2_001469; ACMC-209j9k; AC1Q4NE3; Lopac-265128; cid_1820; AC1Q73TI; AC1L1CB9; AC1Q73TJ; Lopac0_000071; Oprea1_012690
Click to Show/Hide
|
|||
| External Link | ||||
| TriMixDC | Investigative | [176] | ||
| Synonyms |
Dendritic cell vaccine (melanoma), Vrije Universiteit Brussel; TriMixDC (melanoma, intrademal), Universite Libre de Bruxelles
Click to Show/Hide
|
|||
| External Link | ||||
| OP-03 | Investigative | [171] | ||
| Synonyms |
OP-03 program (prodrug, melanoma); OP-03 program (prodrug, melanoma), Onco-Pharmakon
Click to Show/Hide
|
|||
| External Link | ||||
| LG-723 | Investigative | [171] | ||
| External Link | ||||
| LY-1-100 | Investigative | [171] | ||
| Synonyms |
SMART derivatives (melanoma); LY-2-45; SMART derivatives (melanoma), University of Tennessee
Click to Show/Hide
|
|||
| External Link | ||||
| Lovaxin M | Investigative | [171] | ||
| Synonyms |
HMW-MAA cancer vaccine (Listeria vector), Advaxis
Click to Show/Hide
|
|||
| External Link | ||||
References