m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05430
|
[1] | |||
 m6A modification
mmu-miR-7212-5p
mmu-miR-7212-5p
METTL3
Methylation
m6A modification
mmu-miR-7212-5p
mmu-miR-7212-5p
METTL3
Methylation
 : m6A sites
Direct
Enhancement
Non-coding RNA
miR-7212-5p
FGFR3
lncRNA miRNA circRNA : m6A sites
Direct
Enhancement
Non-coding RNA
miR-7212-5p
FGFR3
lncRNA miRNA circRNA
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
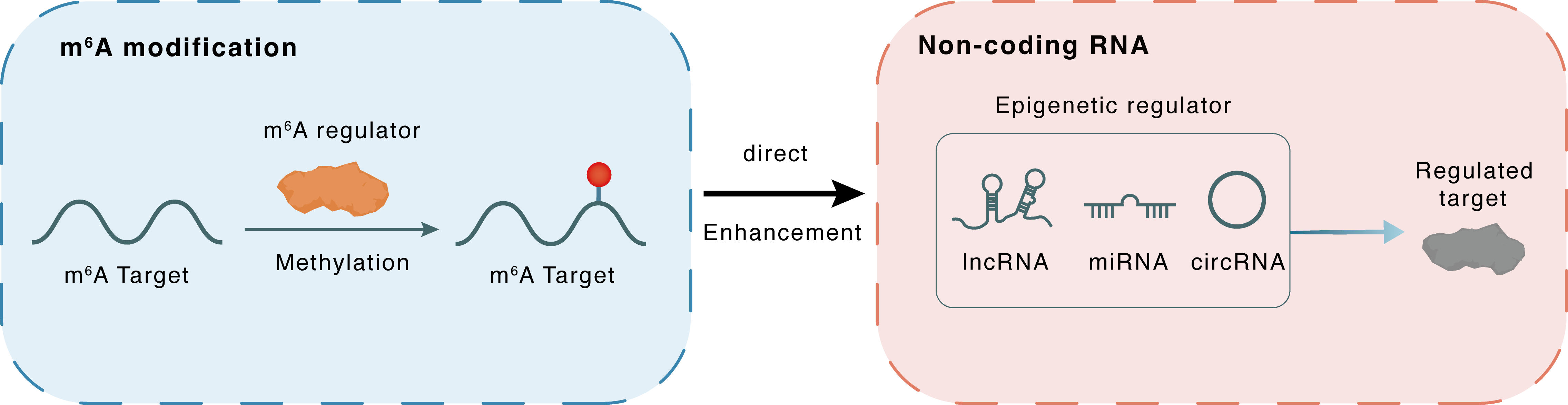
| m6A Regulator | Methyltransferase-like 3 (METTL3) | WRITER | |||
| m6A Target | mmu-miR-7212-5p | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | mmu-miR-7212-5p | microRNA | View Details | ||
| Regulated Target | Fibroblast growth factor receptor 3 (FGFR3) | View Details | |||
| Crosstalk Relationship | m6A → ncRNA | Enhancement | |||
| Crosstalk Mechanism | m6A regulators directly modulate the functionality of ncRNAs through specific targeting ncRNA | ||||
| Crosstalk Summary | Down-regulation of METTL3 promotes osteogenic processes both in vitro and in vivo, and this effect is recapitulated by the suppression of mmu-miR-7212-5p maturation. miR-7212-5p inhibits osteoblast differentiation in MC3T3-E1 cells by targeting Fibroblast growth factor receptor 3 (FGFR3). | ||||
| Responsed Disease | Unspecific body region injury | ICD-11: ND56 | |||
| Cell Process | Cell differentiation | ||||
In-vitro Model |
MC3T3-E1 | Normal | Mus musculus | CVCL_0409 | |
| In-vivo Model | A longitudinal incision was made on the skin and the muscles were separated to expose the femur. A transverse osteotomy was performed in the mid-diaphysis of the femur, and the bones were stabilized by inserting a 23-gauge intramedullary needle. Equal amounts (100 uL) of phosphate-buffered saline (PBS), plasmid METTL3 and agomiR-7212-5p (10 mg/kg body weight) were locally injected into the femoral fracture site. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Fibroblast growth factor receptor 3 (FGFR3) | 19 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Pemigatinib | Approved | [2] | ||
| Synonyms |
Unii-Y6BX7BL23K; Y6BX7BL23K; GTPL9767; SCHEMBL15556271; HCDMJFOHIXMBOV-UHFFFAOYSA-N; example 126 [WO2014007951]; 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(morpholin-4-ylmethyl)-4,7-dihydropyrrolo[4,5]pyrido[1,2-d]pyrimidin-2-one; 3-(2,6-difluoro-3,5-dimethoxyphenyl)-1-ethyl-8-(morpholin-4-ylmethyl)-1,3,4,7-tetrahydro-2H-pyrrolo[3',2':5,6]pyrido[4,3-d]pyrimidin-2-one; INCB54828
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Trapidil | Phase 4 | [3] | ||
| Synonyms |
Trapymin; Rocornal; 15421-84-8; Avantrin; Trapymine; N,N-diethyl-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidin-7-amine; AR 12008; Trapidilum [INN-Latin]; UNII-EYG5Y6355E; EINECS 239-434-2; BRN 0186842; 7-Diethylamino-5-methyl-s-triazolo(1,5-a)pyrimidine; MLS000567667; EYG5Y6355E; N,N-Diethyl-5-methyl-(1,2,4)triazolo(1,5-a)pyrimidine-7-amine; (1,2,4)Triazolo(1,5-a)pyrimidin-7-amine, N,N-diethyl-5-methyl-; 5-Methyl-7-diethylamino-s-triazolo-(1,5-a)-pyrimidine; NCGC00016715-01; AR-12008; SMR000154170; SU10991
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BMS-582664 | Phase 3 | [4] | ||
| Synonyms |
Brivanib alaninate; BMS 582664; BMS582664; BMS-582664, Brivanib alaninate; Brivanib alaninate (INN/USAN); L-Alanine, (1R)-2-((4-((4-fluoro-2-methyl-1H-indol-5-yl)oxy)-5-methylpyrrolo(2,1-f)(1,2,4)triazin-6-yl)oxy)-1-methylethyl ester
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| TKI258 | Phase 3 | [4] | ||
| Synonyms |
Dovitinib; 405169-16-6; CHIR-258; TKI-258; Chir 258; 4-Amino-5-fluoro-3-[5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl]quinolin-2(1H)-one; CHIR258; Dovitinib (TKI-258, CHIR-258); UNII-I35H55G906; CHEMBL522892; 804551-71-1; I35H55G906; TKI 258; 1027263-12-2; (3Z)-4-Amino-5-fluoro-3-[5-(4-methyl-1-piperazinyl)-1,3-dihydro-2H-benzimidazol-2-ylidene]-2(3H)-quinolinone; C21H21FN6O; 4-Amino-5-fluoro-3-(5-(4-methylpiperazin-1-yl)-1H-benzimidazol-2-yl)quinolin-2(1H)-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| E-3810 | Phase 3 | [5] | ||
| Synonyms |
Lucitanib; AL-3810; E-3810, EOS
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 237.5 nM | |||
| External Link | ||||
| B-701 | Phase 2 | [6] | ||
| Synonyms |
VKRFJPYJBOIVPD-UHFFFAOYSA-N; B 701; NSC 46406; 78218-88-9; Phosphorodiamidic acid, N,N-bis(2-chloroethyl)-N'-(3-hydroxypropyl)-, (3-chloropropyl) ester; AC1L3VIX; AC1Q6T2K; NSC46406; NSC-46406; 3-chloropropyl n,n-bis(2-chloroethyl)-n'-(3-hydroxypropyl)phosphorodiamidate; LS-107974; 3-[[bis(2-chloroethyl)amino-(3-chloropropoxy)phosphoryl]amin; 3-[[bis(2-chloroethyl)amino-(3-chloropropoxy)phosphoryl]amino]propan-1-ol; Phosphorodiamidic acid,N-bis(2-chloroethyl)-N'-(3-hydroxypropyl)-, 3-chloropropyl ester
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Recifercept | Phase 2 | [7] | ||
| Synonyms |
TA-46
Click to Show/Hide
|
|||
| External Link | ||||
| Debio 1347 | Phase 2 | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 22 nM | |||
| External Link | ||||
| AEE-788 | Phase 1/2 | [4] | ||
| Synonyms |
AEE; AEE 788; AEE788; GNF-Pf-5343; AEE-788, NVP-AEE 788, AEE788
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| MK-2461 | Phase 1/2 | [9] | ||
| Synonyms |
MK 2461
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 50 nM | |||
| External Link | ||||
| Anti-FGFR3 | Phase 1 | [10] | ||
| External Link | ||||
| SAR442501 | Phase 1 | [11] | ||
| External Link | ||||
| PD-0183812 | Terminated | [12] | ||
| Synonyms |
PETCVZZPKYJZAU-UHFFFAOYSA-N; PD183812; AC1NS8PJ; CHEMBL139653; SCHEMBL5268115; BDBM6280; PD 0183812; N8 Pyrido[2,3-d]pyrimidin-7-one deriv 72; 8-{bicyclo[221]heptan-2-yl}-2-({4-[4-(3-hydroxypropyl)piperidin-1-yl]phenyl}amino)-7H,8H-pyrido[2,3-d]pyrimidin-7-one; 8-(3-bicyclo[221]heptanyl)-2-[4-[4-(3-hydroxypropyl)piperidin-1-yl]anilino]pyrido[2,3-d]pyrimidin-7-one; PD0183813
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| AV-370 | Investigative | [13] | ||
| Synonyms |
Anti-FGF3 receptor antibody (cancer), AVEO Pharmaceuticals
Click to Show/Hide
|
|||
| External Link | ||||
| SU5402 | Investigative | [3] | ||
| Synonyms |
215543-92-3; SU 5402; SU-5402; 3-[3-(2-Carboxyethyl)-4-methylpyrrol-2-methylidenyl]-2-indolinone; (Z)-3-(4-methyl-2-((2-oxoindolin-3-ylidene)methyl)-1H-pyrrol-3-yl)propanoic acid; CHEMBL89363; 3-[(3-(2-CARBOXYETHYL)-4-METHYLPYRROL-2-YL)METHYLENE]-2-INDOLINONE; J-502595; 3-{[3-(2-carboxyethyl)-4-methylpyrrol-2-yl]methylene}-2-indolinone; 3-[4-methyl-2-[(Z)-(2-oxo-1H-indol-3-ylidene)methyl]-1H-pyrrol-3-yl]propanoic acid; (Z)-3-(4-Methyl-2-((2-oxoindolin-3-ylidene)-methyl)-1H-pyrrol-3-yl)propanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 5,11-Dimethyl-6H-pyrido[4,3-b]carbazol-9-ol | Investigative | [14] | ||
| Synonyms |
9-Hydroxyellipticine; 9-Hydroxyellipticin; 51131-85-2; Hydroxyellipticine; ELLIPTICINE, 9-HYDROXY-; UNII-9G4A3ET6XG; IGIG 929; Hydroxy-9 ellipticine [French]; EINECS 257-000-0; NSC 237070; NSC 210717; 9G4A3ET6XG; CHEMBL26559; CHEBI:88297; C17H14N2O; 5,11-Dimethyl-6H-pyrido(4,3-b)carbazol-9-ol; 6H-Pyrido(4,3-b)carbazol-9-ol, 5,11-dimethyl-; 6H-Pyrido[4,3-b]carbazol-9-ol, 5,11-dimethyl-; 9-hydroxy-5,11-dimethyl-6H-pyrido[4,3-b]carbazole; Hydroxy-9 ellipticine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 400 nM | |||
| External Link | ||||
| ACTB-1003 | Investigative | [13] | ||
| Synonyms |
Multi-mode kinase inhibitor (oral, cancer), ACT Biotech; Multi-mode kinase inhibitor (oral, cancer), Bayer
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID21493067C1d | Investigative | [15] | ||
| Synonyms |
GTPL8123; BDBM50343726
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 115 nM | |||
| External Link | ||||
| Ro-4396686 | Investigative | [16] | ||
| Synonyms |
SCHEMBL5809947; CHEMBL606964
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ND56: Unspecific body region injury | 1 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| ONO-4819 | Discontinued in Phase 2 | [17] | ||
| Synonyms |
Rivenprost; AE1-734; ONO-4819CD; ONO-4819.CD; ONO-AE1-734
Click to Show/Hide
|
|||
| External Link | ||||
References