m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05253
|
[1], [2] | |||
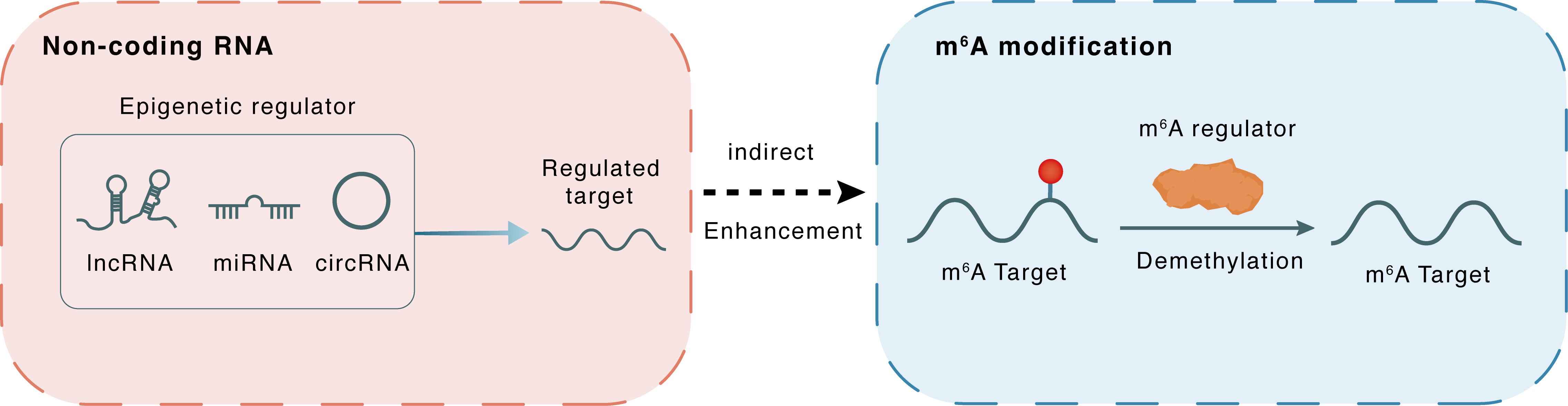 Non-coding RNA
hsa_Circ_0072309
miR-607
lncRNA miRNA circRNA
Indirect
Enhancement
m6A modification
CD274
CD274
FTO
Demethylation
Non-coding RNA
hsa_Circ_0072309
miR-607
lncRNA miRNA circRNA
Indirect
Enhancement
m6A modification
CD274
CD274
FTO
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Fat mass and obesity-associated protein (FTO) | ERASER | |||
| m6A Target | Programmed cell death 1 ligand 1 (CD274/PD-L1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa_circ_0072309 (Circ_LIFR) | circRNA | View Details | ||
| Regulated Target | hsa-miR-607 | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs indirectly impacts m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Circ_LIFR interacted with hsa-miR-607 via its miRNA response element to upregulate the expression of FTO, an m6A demethylase and downstream target of miR-607, thus promoting tumorigenesis of NSCLC.This study revealed that m6A methylation is closely related to the poor prognosis of non-small cell lung cancer patients via interference with the TIME, which suggests that m6A plays a role in optimizing individualized immunotherapy management and improving prognosis. The expression levels of METTL3, FTO and YTHDF1 in non-small cell lung cancer were changed. Patients in Cluster 1 had lower immunoscores, higher Programmed cell death 1 ligand 1 (CD274/PD-L1) expression, and shorter overall survival compared to patients in Cluster 2. The hallmarks of the Myelocytomatosis viral oncogene (MYC) targets, E2 transcription Factor (E2F) targets were significantly enriched. | ||||
| Responsed Disease | Non-small cell lung cancer | ICD-11: 2C25.Y | |||
| Pathway Response | p53 signaling pathway | hsa04115 | |||
| Central carbon metabolism in cancer | hsa05230 | ||||
| PD-L1 expression and PD-1 checkpoint pathway in cancer | hsa05235 | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| CD274 molecule (CD274) | 55 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Avelumab | Approved | [3] | ||
| External Link | ||||
| Durvalumab | Approved | [4] | ||
| MOA | Modulator | |||
| External Link | ||||
| RG-7446 | Approved | [5] | ||
| External Link | ||||
| Bavencio | Approved | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Atezolizumab | Approved | [7] | ||
| External Link | ||||
| Sugemalimab | Approved in China | [8] | ||
| External Link | ||||
| MEDI4736 | Phase 3 | [9] | ||
| MOA | Modulator | |||
| External Link | ||||
| MPDL-3280A | Phase 3 | [10] | ||
| MOA | Modulator | |||
| External Link | ||||
| CS1001 | Phase 3 | [11] | ||
| External Link | ||||
| A167 | Phase 3 | [12] | ||
| Synonyms |
KL-A167
Click to Show/Hide
|
|||
| External Link | ||||
| KN046 | Phase 3 | [13] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Pidilizumab | Phase 2 | [14] | ||
| Synonyms |
CT-011
Click to Show/Hide
|
|||
| External Link | ||||
| KN035 | Phase 2 | [15] | ||
| Synonyms |
Envafolimab
Click to Show/Hide
|
|||
| External Link | ||||
| CX-072 | Phase 2 | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| INCB86550 | Phase 2 | [17] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [18] | ||
| External Link | ||||
| M7824 | Phase 2 | [19] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| BGB-A333 | Phase 1/2 | [20] | ||
| External Link | ||||
| GS-4224 | Phase 1/2 | [21] | ||
| Synonyms |
Methyl Pyridazine-4-carboxylate; 34231-77-1; PYRIDAZINE-4-CARBOXYLIC ACID METHYL ESTER; 4-PYRIDAZINECARBOXYLIC ACID, METHYL ESTER; MFCD09953488; ACMC-1AJNN; methyl 4-pyridazinecarboxylate; methylpyridazine-4-carboxylate; SCHEMBL1421640; DTXSID30498310; AMY24958; BCP22435; ANW-50355; ZINC12359421; AKOS015854403; Methyl pyridazine-4-carboxylate, 97%; AC-4414; CS-W003697; PB31452; 4-Pyridazinecarboxylic acid methyl ester; AK-48857; SY004472; AB0024323; DB-030309; FT-0717698; W5569; S-2990; J-522632
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [22] | ||
| MOA | Modulator | |||
| External Link | ||||
| LY3300054 | Phase 1 | [19] | ||
| External Link | ||||
| MSB2311 | Phase 1 | [15] | ||
| External Link | ||||
| Anti-PD-L1 | Phase 1 | [23] | ||
| Synonyms |
BMS-936559
Click to Show/Hide
|
|||
| External Link | ||||
| FAZ053 | Phase 1 | [16] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Anti-PD-L1 CSR T cells | Phase 1 | [24] | ||
| MOA | CAR-T-Cell-Therapy | |||
| External Link | ||||
| BMS-986189 | Phase 1 | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Cosibelimab | Phase 1 | [25] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| CA-170 | Phase 1 | [26] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PD-L1 t-haNK | Phase 1 | [27] | ||
| External Link | ||||
| KD033 | Phase 1 | [28] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| C-Met/PD-L1 CAR-T Cell | Phase 1 | [29] | ||
| MOA | CAR-T-Cell-Therapy(Dual specific) | |||
| External Link | ||||
| CA-170 | Phase 1 | [19] | ||
| Synonyms |
3-Aminopyrrolidine dihydrochloride; 103831-11-4; pyrrolidin-3-amine dihydrochloride; 3-Pyrrolidinamine, dihydrochloride; 3-Aminopyrrolidine 2HCl; 3-Aminopyrrolidine diHCl; SCHEMBL555933; ACMC-2099s1; ACMC-2099s3; ACMC-20989g; 3-pyrrolidinamine dihydrochloride; CTK0H7226; DTXSID00583661; MolPort-002-343-989; NJPNCMOUEXEGBL-UHFFFAOYSA-N; 3-Amino-pyrrolidine dihydrochloride; KS-00000JI6; ACT01710; ANW-14978; SBB003982; ( -3-Aminopyrrolidine dihydrochloride; AKOS015844825; VP60158; AM85320; VP60228; TRA0055207; TRA0000843; TRA0097
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FS118 | Phase 1 | [15] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [30] | ||
| External Link | ||||
| MCLA-145 | Phase 1 | [31] | ||
| MOA | Agonist | |||
| External Link | ||||
| GEN1046 | Phase 1 | [32] | ||
| MOA | Agonist | |||
| External Link | ||||
| ALPN-202 | Phase 1 | [33] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| TAK-252 | Phase 1 | [34] | ||
| Synonyms |
SL-279252
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| INBRX-105 | Phase 1 | [35] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LY3415244 | Phase 1 | [36] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| A337 | Phase 1 | [12] | ||
| External Link | ||||
| IBI318 | Phase 1 | [37] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30107136-Compound-Example2 | Patented | [38] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 150 nM | |||
| External Link | ||||
| PMID30107136-Compound-Example1 | Patented | [38] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 18 nM | |||
| External Link | ||||
| PMID30247903-Compound-General structure7 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound7
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CA-327 | Investigative | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure8 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound8
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure5 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound5
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure12 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound12
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure9 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound9
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure6 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID30247903-Compound-General structure10 | Investigative | [15] | ||
| Synonyms |
PMID30247903Compound10
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| YH011 | Investigative | [39] | ||
| External Link | ||||
| YH010 | Investigative | [40] | ||
| External Link | ||||
| RG6084 | Phase 1 | [41] | ||
| External Link | ||||
| 2C25: Lung cancer | 52 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Adagrasib | Approved | [42] | ||
| Synonyms |
2326521-71-3; MRTX-849; UNII-8EOO6HQF8Y; 8EOO6HQF8Y; 2-((S)-4-(7-(8-Chloronaphthalen-1-yl)-2-(((S)-1-methylpyrrolidin-2-yl)methoxy)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-4-yl)-1-(2-fluoroacryloyl)piperazin-2-yl)acetonitrile; CHEMBL4594350; SCHEMBL20974691; GTPL10888; Kras G12C inhibitor MRTX849; BCP31538; EX-A3258; MRTX 849; MFCD32263433; s8884; compound 20 [PMID: 32250617]; BS-16211; HY-130149; CS-0105265; 2-Piperazineacetonitrile, 4-(7-(8-chloro-1-naphthalenyl)-5,6,7,8-tetrahydro-2-(((2S)-1-methyl-2-pyrrolidinyl)methoxy)pyrido(3,4-d)pyrimidin-4-yl)-1-(2-fluoro-1-oxo-2-propen-1-yl)-, (2S)-
Click to Show/Hide
|
|||
| External Link | ||||
| Amivantamab | Approved | [43] | ||
| External Link | ||||
| Mobocertinib | Approved | [44] | ||
| Synonyms |
1847461-43-1; TAK-788; TAK788; AP32788; UNII-39HBQ4A67L; 39HBQ4A67L; propan-2-yl 2-[4-{[2-(dimethylamino)ethyl](methyl)amino}-2-methoxy-5-(prop-2-enamido)anilino]-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Mobocertinib [INN]; Mobocertinib [USAN]; AP-32788; Mobocertinib (TAK788); Mobocertinib(TAK-788); SCHEMBL17373133; GTPL10468; BDBM368374; BCP31045; EX-A3392; US10227342, Example 10; MFCD32669806; NSC825519; s6813; TAK-788;AP32788; WHO 11183; NSC-825519; example 94 [WO2015195228A1]; HY-135815; CS-0114256; TAK-788;TAK 788; AP 32788; 5-Pyrimidinecarboxylic acid, 2-((4-((2-(dimethylamino)ethyl)methylamino)-2-methoxy-5-((1-oxo-2-propen-1-yl)amino)phenyl)amino)-4-(1-methyl-1H-indol-3-yl)-, 1-methylethyl ester; C(C=C)(=O)NC=1C(=CC(=C(C=1)NC1=NC=C(C(=N1)C1=CN(C2=CC=CC=C12)C)C(=O)OC(C)C)OC)N(C)CCN(C)C; Isopropyl 2-((5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenyl)amino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Isopropyl 2-(5-acrylamido-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyphenylamino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; Propan-2-yl 2-(5-(acryloylamino)-4-((2-(dimethylamino)ethyl)(methyl)amino)-2-methoxyanilino)-4-(1-methyl-1H-indol-3-yl)pyrimidine-5-carboxylate; propan-2-yl 2-[4-[2-(dimethylamino)ethyl-methylamino]-2-methoxy-5-(prop-2-enoylamino)anilino]-4-(1-methylindol-3-yl)pyrimidine-5-carboxylate
Click to Show/Hide
|
|||
| External Link | ||||
| Sugemalimab | Approved in China | [8] | ||
| External Link | ||||
| Sacituzumab govitecan | Approved | [45] | ||
| Synonyms |
1491917-83-9; 1535963-91-7; 1796566-95-4; CYSTEINYL CL2A-SN-38; DA64T2C2IO; DTXSID401335985; EX-A4354; F82944; GOVITECAN CYSTEINYL CONJUGATE; hRS 7SN38; hRS7-SN38; IMMU 132; IMMU-132; M9BYU8XDQ6; Sacituzumab govitecan; Sacituzumab govitecan [USAN]; sacituzumab-govitecan; Satralizumab linker; SN-38 CYSTEINYL CONJUGATE; UNII-M9BYU8XDQ6
Click to Show/Hide
|
|||
| External Link | ||||
| Atezolizumab | Approved | [7] | ||
| External Link | ||||
| Tepotinib | Approved | [46] | ||
| Synonyms |
1100598-32-0; EMD 1214063; UNII-1IJV77EI07; Tepotinib (EMD 1214063); EMD1214063; 1IJV77EI07; MSC-2156119J; Benzonitrile, 3-[1,6-dihydro-1-[[3-[5-[(1-methyl-4-piperidinyl)methoxy]-2-pyrimidinyl]phenyl]methyl]-6-oxo-3-pyridazinyl]-; C29H28N6O2; Tepotinib [INN]; Benzonitrile, 3-[1,6-Dihydro-1-[[3-[5-[(1-Methyl-4-Piperidinyl)Methoxy]-2-PyriMidinyl]Phenyl]Methyl]-6-Oxo-3-Pyridazinyl]; Benzonitrile, 3-(1,6-dihydro-1-((3-(5-((1-methyl-4-piperidinyl)methoxy)-2-pyrimidinyl)phenyl)methyl)-6-oxo
Click to Show/Hide
|
|||
| External Link | ||||
| Sotorasib | Approved | [47] | ||
| Synonyms |
AMG-510; AMG510; AMG-510 racemate; 2252403-56-6; AMG 510; Kras G12C inhibitor 9; 2296729-00-3; UNII-2B2VM6UC8G; 2B2VM6UC8G; CHEMBL4535757; 2296729-00-3 (racemate); 4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-[(2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl]pyrido[2,3-d]pyrimidin-2-one; Sotorasib [INN]; 6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-propan-2-ylpyridin-3-yl)-4-((2S)-2-methyl-4-prop-2-enoylpiperazin-1-yl)pyrido(2,3-d)pyrimidin-2-one; AMG510 racemate; Sotorasib [USAN]; AMG-510(racemate); Kras mutant-targeting AMG 510; SCHEMBL20560375; GTPL10678; AMG 510 pound>>AMG-510; AMY16918; BCP30452; BCP33368; EX-A3538; BDBM50514402; NSC818433; s8830; WHO 11370; DB15569; NSC-818433; BS-16684; HY-114277; CS-0081316; compound (R)-38 [PMID: 31820981]; (1m)-6-Fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(propan-2-yl)pyridin-3-yl)-4-((2S)-2-methyl-4-(prop-2-enoyl)piperazin-1-yl)pyrido(2,3-d)pyrimidin-2(1H)-one; (1S)-4-((S)-4-Acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(2-isopropyl-4-methylpyridin-3-yl)pyrido[2,3-d]pyrimidin-2(1H)-one; 2296729-66-1; Pyrido(2,3-d)pyrimidin-2(1H)-one, 6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-(4-methyl-2-(1-methylethyl)-3-pyridinyl)-4-((2S)-2-methyl-4-(1-oxo-2-propen-1-yl)-1-piperazinyl)-
Click to Show/Hide
|
|||
| External Link | ||||
| Entrectinib | Approved | [48] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Repotrectinib | Approved | [49] | ||
| Synonyms |
FIKPXCOQUIZNHB-RRKGBCIJSA-N; SCHEMBL20438940; TPX 0005; BCP19778
Click to Show/Hide
|
|||
| External Link | ||||
| MYL-1402O | Phase 3 | [50] | ||
| Synonyms |
bevacizumab biosimilar
Click to Show/Hide
|
|||
| External Link | ||||
| AB154 | Phase 3 | [51] | ||
| Synonyms |
Domvanalimab
Click to Show/Hide
|
|||
| External Link | ||||
| Datopotamab deruxtecan | Phase 3 | [52] | ||
| External Link | ||||
| CS1001 | Phase 3 | [53] | ||
| External Link | ||||
| JDQ443 | Phase 3 | [54] | ||
| Synonyms |
(S)-JDQ-443; 1-(6-((4S)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl)-2-azaspiro[3.3]heptan-2-yl)prop-2-en-1-one; 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-inda zol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]- 2-Propen-1-one; 1-[6-[(4R)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-2-propen-1-one; 1-[6-[4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methylindazol-5-yl)pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl]prop-2-en-1-one; 1-{6-[(4M)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5- methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2- azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 1-{6-[(4M)-4-(5-Chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]heptan-2-yl}prop-2-en-1-one; 2653994-08-0; 2653994-10-4; 2-Propen-1-one, 1-[6-[(4R)-4-(5-chloro-6-methyl-1H-indazol-4-yl)-5-methyl-3-(1-methyl-1H-indazol-5-yl)-1H-pyrazol-1-yl]-2-azaspiro[3.3]hept-2-yl]-; AKOS040757949; AT36708; BDBM50579985; CHEMBL5077861; compound 5 [PMID: 35404998]; CS-0226220; CS-0311034; EX-A5693; example 1a [WO2021120890A1]; GLXC-25533; GTPL11715; HY-139612A; JDQ 443; JDQ 443 [WHO-DD]; JDQ443; JDQ-443; MS-29737; NSC846146; NSC-846146; NVP-JDQ443; NVP-JDQ-443; Opnurasib; opnurasib [INN]; -PROPEN-1-ONE, 1-(6-((4R)-4-(5-CHLORO-6-METHYL-1H-INDAZOL-4-YL)-5-METHYL-3-(1-METHYL-1H-INDAZOL-5-YL)-1H-PYRAZOL-1-YL)-2-AZASPIRO(3.3)HEPT-2-YL)-; Q3W0H3V1LQ; SCHEMBL23533580
Click to Show/Hide
|
|||
| External Link | ||||
| TRS003 | Phase 3 | [55] | ||
| External Link | ||||
| Tusamitamab ravtansine | Phase 3 | [56] | ||
| Synonyms |
SAR408701
Click to Show/Hide
|
|||
| External Link | ||||
| RG6058 | Phase 3 | [57] | ||
| Synonyms |
Tiragolumab
Click to Show/Hide
|
|||
| External Link | ||||
| GSK4069889 | Phase 2 | [58] | ||
| Synonyms |
TSR-022
Click to Show/Hide
|
|||
| External Link | ||||
| APL-101 | Phase 2 | [59] | ||
| Synonyms |
Bozitinib; PLB-1001; 1440964-89-5; Vebreltinib; Vebreltinib [USAN]; UNII-2WZP8A9VFN; 2WZP8A9VFN; Bozitinib (PLB-1001); SCHEMBL15594471; BDBM107096; CBI-3103; s6762; WHO 11677; HY-125017; CS-0088607; US9695175, 44; 1,2,4-Triazolo(4,3-b)pyridazine, 6-(1-cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6- fluoro-2-methyl-2H-indazol-5-yl)methyl)-; 6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5- yl)methyl)-1,2,4-triazolo(4,3-b)pyridazine
Click to Show/Hide
|
|||
| External Link | ||||
| SGN-LIV1A | Phase 2 | [60] | ||
| Synonyms |
Ladiratuzumab Vedotin
Click to Show/Hide
|
|||
| External Link | ||||
| BNT116 | Phase 2 | [61] | ||
| External Link | ||||
| AZD7789 | Phase 2 | [62] | ||
| External Link | ||||
| L-DOS47 | Phase 1/2 | [63] | ||
| External Link | ||||
| NC318 | Phase 2 | [64] | ||
| External Link | ||||
| Vorolanib | Phase 2 | [65] | ||
| Synonyms |
UNII-YP8G3I74EL; YP8G3I74EL; 1013920-15-4; (S,Z)-N-(1-(Dimethylcarbamoyl)pyrrolidin-3-yl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; Vorolanib [INN]; SCHEMBL2439528; CHEMBL4297587; N-((3S)-1-(dimethylcarbamoyl)pyrrolidin-3-yl)-5-((Z)-(5-fluoro-2-oxo-1H-indol-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxamide; s6843; DB15247; HY-109019; CS-0030517; Q27294638; 1H-Pyrrole-3-carboxamide, N-((3S)-1-((dimethylamino)carbonyl)-3-pyrrolidinyl)-5-((Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidene)methyl)-2,4-dimethyl-
Click to Show/Hide
|
|||
| External Link | ||||
| Xofigo | Phase 2 | [66] | ||
| External Link | ||||
| Bintrafusp alfa | Phase 2 | [67] | ||
| External Link | ||||
| Voyager-V1 | Phase 2 | [68] | ||
| Synonyms |
VSV-IFNBeta-NIS
Click to Show/Hide
|
|||
| External Link | ||||
| AB-106 | Phase 2 | [69] | ||
| Synonyms |
DS6051b; GTPL11198; AB106
Click to Show/Hide
|
|||
| External Link | ||||
| RO-5126766 | Phase 2 | [70] | ||
| Synonyms |
VS-6766; CH-5126766; Dual Raf/MEK protein kinase inhibitor (cancer), Roche
Click to Show/Hide
|
|||
| External Link | ||||
| TC-210 | Phase 1/2 | [71] | ||
| External Link | ||||
| EMB-01 | Phase 1/2 | [72] | ||
| External Link | ||||
| NM21-1480 | Phase 1/2 | [73] | ||
| External Link | ||||
| MRx0518 | Phase 1/2 | [74] | ||
| External Link | ||||
| DZD9008 | Phase 1/2 | [75] | ||
| External Link | ||||
| BGB-A425 | Phase 1/2 | [76] | ||
| External Link | ||||
| Rilvegostomig | Phase 1/2 | [77] | ||
| Synonyms |
AZD2936
Click to Show/Hide
|
|||
| External Link | ||||
| IK-007 | Phase 1/2 | [78] | ||
| Synonyms |
grapiprant
Click to Show/Hide
|
|||
| External Link | ||||
| IBI318 | Phase 1 | [79] | ||
| External Link | ||||
| GEN-011 | Phase 1 | [80] | ||
| External Link | ||||
| ENV-105 | Phase 1 | [81] | ||
| Synonyms |
Carotuximab
Click to Show/Hide
|
|||
| External Link | ||||
| AMG 160 | Phase 1 | [82] | ||
| External Link | ||||
| ADP-A2M10 | Phase 1 | [83] | ||
| External Link | ||||
| MEDI5752 | Phase 1 | [84] | ||
| External Link | ||||
| PF-07104091 | Phase 1 | [85] | ||
| External Link | ||||
| PF-06936308 | Phase 1 | [86] | ||
| External Link | ||||
| GEM3PSCA | Phase 1 | [87] | ||
| External Link | ||||
| PF-07257876 | Phase 1 | [30] | ||
| External Link | ||||
| Cosibelimab | Phase 1 | [88] | ||
| Synonyms |
CK-301/TG-1501
Click to Show/Hide
|
|||
| External Link | ||||
| Gemcitabine | Approved | [89] | ||
| External Link | ||||
| SMI-4a | Investigative | [90] | ||
| Synonyms |
438190-29-5; SMI 4a; TCS PIM-1 4a; (Z)-SMI-4a; (Z)-5-(3-(trifluoromethyl)benzylidene)thiazolidine-2,4-dione; (5Z)-5-[3-(trifluoromethyl)benzylidene]-1,3-thiazolidine-2,4-dione; CHEMBL183906; (5Z)-5-[[3-(TRIFLUOROMETHYL)PHENYL]METHYLENE]-2,4-THIAZOLIDINEDIONE; (5Z)-5-[[3-(trifluoromethyl)phenyl]methylidene]-1,3-thiazolidine-2,4-dione; 327033-36-3; C11H6F3NO2S; (Z)-5-(3-(Trifluoromethyl)benzylidene)-thiazolidine-2,4-dione; (5Z)-5-{[3-(trifluoromethyl)phenyl]methylidene}-1,3-thiazolidine-2,4-dione; Pim inhibitor 4a; 3vc4; SMI-4q; TCS PIM-1-4a; 5-(3-(Trifluoromethyl)benzylidene)thiazolidine-2,4-dione; 5-[3-(Trifluoromethyl)benzylidene]thiazolidine-2,4-dione; cc-717; thiazolidine-2,4-dione, 4a; SCHEMBL2541382; SCHEMBL2541388; BDBM26626; AOB6260; EX-A111; SYN1113; BDBM138364; HMS3229J21; 2720AH; HY-16576A; MFCD01152003; s8005; ZINC12576047; AKOS001314163; SMI-4a, >=98% (HPLC); CCG-265027; NCGC00345836-02; NCGC00345836-14; AC-32861; HY-15474; AB0165836; EC-000.2291; J3.561.866J; A11945; W-5256; US8877795, 12; Q27451064; 5-[[3-(trifluoromethyl)phenyl]methylene]-2,4-thiazolidinedione
Click to Show/Hide
|
|||
| External Link | ||||
References