m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05234
|
[1] | |||
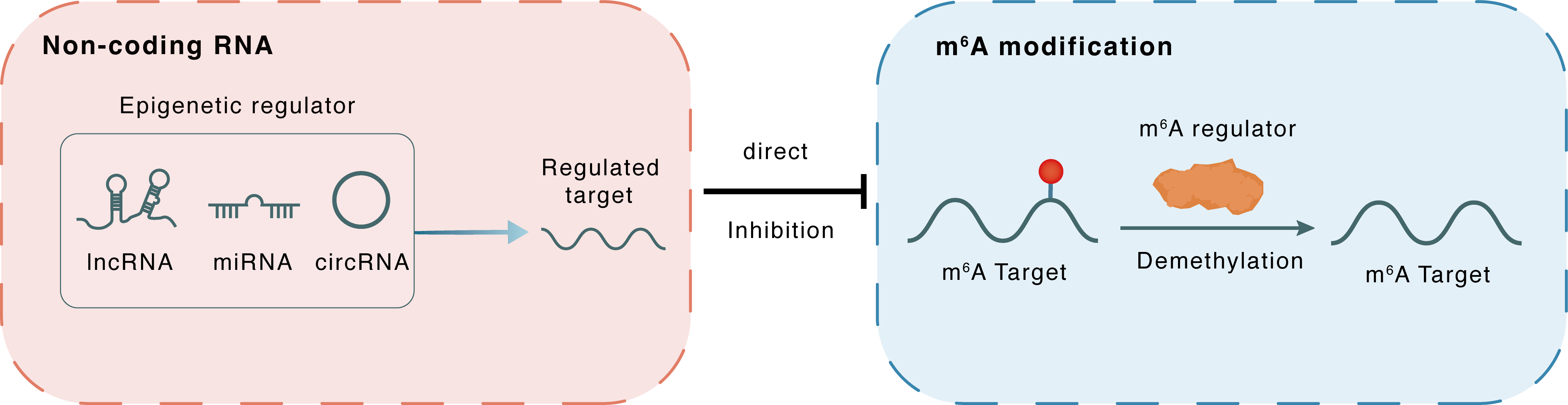 Non-coding RNA
miR-141-3p
ALKBH5
lncRNA miRNA circRNA
Direct
Inhibition
m6A modification
MMP9
MMP9
ALKBH5
Demethylation
Non-coding RNA
miR-141-3p
ALKBH5
lncRNA miRNA circRNA
Direct
Inhibition
m6A modification
MMP9
MMP9
ALKBH5
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | RNA demethylase ALKBH5 (ALKBH5) | ERASER | |||
| m6A Target | Matrix metalloproteinase-9 (MMP9) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | hsa-miR-141-3p | microRNA | View Details | ||
| Regulated Target | RNA demethylase ALKBH5 (ALKBH5) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Inhibition | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through modulating the expression level of m6A regulator | ||||
| Crosstalk Summary | Silencing MALAT1 led to down-regulation of the N6-methyladenosine (m6A) demethylase ALKBH5 via regulating hsa-miR-141-3p expression, which caused a decrease in the expression levels of MMP2 and Matrix metalloproteinase-9 (MMP9) expression, thereby suppressing cell migration and invasion. | ||||
| Responsed Disease | Cervical cancer | ICD-11: 2C77 | |||
In-vitro Model |
C-33 A | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1094 | |
| HeLa | Endocervical adenocarcinoma | Homo sapiens | CVCL_0030 | ||
| Ca Ski | Cervical squamous cell carcinoma | Homo sapiens | CVCL_1100 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Matrix metalloproteinase-9 (MMP9) | 56 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| DP-b99 | Phase 3 | [2] | ||
| Synonyms |
343340-21-6; 222315-88-0; SCHEMBL720046; DP-b 99; DTXSID20187908; DP-BAPTA-99, DP-b99; ZINC162948634; 2,2'-(((ethane-1,2-diylbis(oxy))bis(2,1-phenylene))bis((2-(2-(octyloxy)ethoxy)-2-oxoethyl)azanediyl))diacetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GS-5745 | Phase 3 | [3] | ||
| MOA | Modulator | |||
| External Link | ||||
| Andecaliximab | Phase 3 | [4] | ||
| External Link | ||||
| Curcumin | Phase 3 | [5] | ||
| Synonyms |
458-37-7; Diferuloylmethane; Natural yellow 3; Turmeric yellow; Turmeric; Curcuma; Kacha haldi; Gelbwurz; Indian saffron; Curcumin I; Souchet; Halud; Halad; Haidr; Haldar; Merita earth; Yellow Ginger; Terra Merita; Yellow Root; Safran d'Inde; Yo-Kin; Golden seal; Curcuma oil; Orange Root; Oils, curcuma; CI Natural Yellow 3; Curcumine; Hydrastis; Indian turmeric; Yellow puccoon; Turmeric extract; Diferaloylmethane; Kurkumin [Czech]; (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione; Tumeric yellow; Turmeric oil
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| BLZ-100 | Phase 1/2 | [6] | ||
| Synonyms |
Tozuleristide; UNII-835UH424TU; 835UH424TU; Tozuleristide [INN]; Tozuleristide [USAN]; 1673565-40-6
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Neovastat | Phase 1 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID29130358-Compound-Figure17(13) | Patented | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10000 nM; IC50 > 10 nM | |||
| External Link | ||||
| PMID29130358-Compound-Figure17(12) | Patented | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 < 10000 nM | |||
| External Link | ||||
| PMID29130358-Compound-Figure17(10) | Patented | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 1000 nM; IC50 < 10000 nM | |||
| External Link | ||||
| PMID29130358-Compound-SB-3CT | Patented | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 600 nM | |||
| External Link | ||||
| PMID29130358-Compound-Figure17(11) | Patented | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 > 130 nM; IC50 < 10000 nM | |||
| External Link | ||||
| PMID29130358-Compound-Figure13(4) | Patented | [8] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID29130358-Compound-LonimacranthoideVII | Patented | [8] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID29130358-Compound-Figure18(14a) | Patented | [8] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 2500 nM | |||
| External Link | ||||
| Galarubicin | Discontinued in Phase 2 | [9] | ||
| Synonyms |
DA-125; Metalloprotease inhibitors (cancer), Dong-A
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RS-130830 | Discontinued in Phase 2 | [10] | ||
| Synonyms |
CTS-1027; 193022-04-7; UNII-2QD3F58224; CHEMBL440498; 2QD3F58224; 4-[4-(4-CHLORO-PHENOXY)-BENZENESULFONYLMETHYL]-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HYDROXYAMIDE; 4-[[[4-(4-Chlorophenoxy)phenyl]sulfonyl]methyl]tetrahydro-N-hydroxy-2H-pyran-4-carboxamide; 4-(4-(4-Chloro-phenoxy)-benzenesulfonylmethyl)-tetrahydro-pyran-4-carboxylic acid hydroxyamide; Ro-1130830; AC1MOE9A; SCHEMBL2381112; BDBM11863; DTXSID90172907; 830c; CTS 1027; ZINC1488366; BCP13018; 3563AH; alpha-tetrahydropyran beta-sulfone 1B; AKOS030526690
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.11 nM | |||
| External Link | ||||
| GM6001 | Discontinued in Phase 2 | [11] | ||
| Synonyms |
Ilomastat; Galardin; 142880-36-2; GM 6001; Illomastat; CS 610; GM-6001; UNII-I0403ML141; CHEMBL19611; Ilomastat (GM6001, Galardin); I0403ML141; NCGC00163450-02; 3-(N-HYDROXYCARBOXAMIDO)-2-ISOBUTYLPROPANOYL-TRP-METHYLAMIDE; (R)-N4-Hydroxy-N1-[(S)-2-(1H-indol-3-yl)-1-methylcarbamoyl-ethyl]-2-isobutyl-succinamide; N-[(2R)-2-(Hydroxamidocarbonylmethyl)-4-methylpentanoyl]-L-tryptophan Methylamide; (R)-N(sup 1)-Hydroxy-N-((S)-2-indol-3-yl-1-(methylcarbamoyl)ethyl)-2-isobutylsuccinamide; (S-(R*,S*))-N(sup 4)-Hydroxy-N(sup
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 0.05 nM | |||
| External Link | ||||
| DX-2802 | Preclinical | [12] | ||
| External Link | ||||
| CT-1746 | Terminated | [13] | ||
| MOA | Modulator | |||
| External Link | ||||
| CDP-845 | Terminated | [14] | ||
| MOA | Modulator | |||
| External Link | ||||
| RO-319790 | Terminated | [15] | ||
| Synonyms |
Ro-31-9790; CHEMBL16520; Ro 31-9790; (R)-N1-((S)-3,3-dimethyl-1-(methylamino)-1-oxobutan-2-yl)-N4-hydroxy-2-isobutylsuccinamide; SCHEMBL4633699; QRXOZHSEEGNRFC-ZYHUDNBSSA-N; ZINC1534591; BDBM50063920; CS-6682; HY-101703
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2 nM | |||
| External Link | ||||
| SC-44463 | Terminated | [16] | ||
| Synonyms |
CHEMBL45483; n-[3-(n'-hydroxycarboxamido)-2-(2-methylpropyl)-propanoyl]-o-tyrosine-n-methylamide; 104408-38-0; Ihp-tyr-menh2; HTA; AC1L2U0O; SCHEMBL9284838; sc44463; BDBM50104969; DB07926; N-(2-Isobutyl-3-(N'-hydroxycarbonylamido)propanoyl)-O-methyltyrosinemethylamide; N*4*-Hydroxy-2-isobutyl-N*1*-[2-(4-methoxy-phenyl)-1-methylcarbamoyl-ethyl]-succinamide; (2R)-N-hydroxy-N'-[(1S)-2-(4-methoxyphenyl)-1-(methylcarbamoyl)ethyl]-2-(2-methylpropyl)butanediamide; Butanediamide, N4-hydroxy-N1-(1-((4-methoxyphenyl)meth
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki < 1 nM | |||
| External Link | ||||
| 2-Amino-N,3,3-Trimethylbutanamide | Investigative | [17] | ||
| Synonyms |
AC1MRP6C; SCHEMBL343358; DTXSID30393038; BPKJNEIOHOEWLO-UHFFFAOYSA-N; 2-amino-N,3,3-trimethyl-butanamide; 2-azanyl-N,3,3-trimethyl-butanamide; AKOS022475747; AN-25241
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID15055993C1a | Investigative | [18] | ||
| Synonyms |
GTPL6514; BDBM50131385
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.06 nM | |||
| External Link | ||||
| Carboxylated glucosamine | Investigative | [19] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Methotrexate gamma-L-phenylalaninehydroxamic acid | Investigative | [20] | ||
| Synonyms |
CHEMBL396296
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 15000 nM | |||
| External Link | ||||
| 5-Methyl-5-phenyl-pyrimidine-2,4,6-trione | Investigative | [21] | ||
| Synonyms |
Heptobarbital; Mephebarbital; Eudan; Phenylmethylbarbituric acid; Rutonal; Methylphenylbarbital; 5-Methyl-5-phenylbarbituric acid; 5-Methyl-5-phenylbarbityric acid; 76-94-8; 5-Phenyl-5-methylbarbituric acid; NSC 80543; Barbituric acid, 5-methyl-5-phenyl-; UNII-GFR227X6YY; 5-Methyl-5-phenyl-2,4,6(1H,3H,5H)-pyrimidinetrione; EINECS 200-994-8; BRN 0201825; GFR227X6YY; CHEMBL329617; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-methyl-5-phenyl-; 5-methyl-5-phenyl-barbituric acid; C11H10N2O3; 5-methyl-5-phenyl-1,3-diazinane-2,4,6-trione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 11000 nM | |||
| External Link | ||||
| ARP100 | Investigative | [22] | ||
| Synonyms |
ARP 100; ARP-100
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 200 nM | |||
| External Link | ||||
| Ro 28-2653 | Investigative | [23] | ||
| Synonyms |
Ro-28-2653; 261956-22-3; CHEMBL456911; SCHEMBL726759; SCHEMBL8471189; EX-A2614; BDBM50363130; ZINC33975062; Ro28-2653; KB-275261; 5-biphenyl-4-yl-5-[4-(4-nitro-phenyl)-piperazin-1-yl]-pyrimidine-2,4,6-trione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7 nM | |||
| External Link | ||||
| Methotrexate gamma-L-proline-hydroxamic acid | Investigative | [20] | ||
| Synonyms |
CHEMBL388879
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| PMID23631440C29e | Investigative | [24] | ||
| Synonyms |
GTPL8578; BDBM50433865
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1.5 nM | |||
| External Link | ||||
| 5-(4-Phenoxy-phenyl)-pyrimidine-2,4,6-trione | Investigative | [21] | ||
| Synonyms |
CHEMBL176517; 219311-20-3; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-(4-phenoxyphenyl)-; SCHEMBL6380971; CTK0J6997; DTXSID60470422; NNRYJLARUIVRRO-UHFFFAOYSA-N; 5-(4'-phenoxyphenyl)barbituric acid; 5-(4-phenoxyphenyl)pyrimidine-2,4,6-trione
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Methotrexate gamma-hydroxamic acid | Investigative | [20] | ||
| Synonyms |
CHEMBL244883
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4'-chloro-biphenyl-4-sulfonyl)-pentanoic acid | Investigative | [25] | ||
| Synonyms |
CHEMBL378740; BDBM50185874; 2-(4''-chloro-biphenyl-4-sulfonyl)-pentanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10005 nM | |||
| External Link | ||||
| 5-Hexyl-5-phenyl-pyrimidine-2,4,6-trione | Investigative | [21] | ||
| Synonyms |
5-Hexyl-5-phenylbarbituric acid; BRN 0297956; BARBITURIC ACID, 5-HEXYL-5-PHENYL-; 67051-21-2; CHEMBL173075; AC1L2LKK; 4-24-00-02103 (Beilstein Handbook Reference); CTK8J9653; DTXSID20217355; 5-hexyl-5-phenyl barbituric acid; BDBM50099125; LS-24512
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 330 nM | |||
| External Link | ||||
| 5-Biphenyl-4-yl-5-hexyl-pyrimidine-2,4,6-trione | Investigative | [21] | ||
| Synonyms |
CHEMBL175282; BDBM50099116
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 863 nM | |||
| External Link | ||||
| Roche 28-2653 | Investigative | [23] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| N-hydroxy-3-(2-oxo-2H-chromen-3-yl)propanamide | Investigative | [26] | ||
| Synonyms |
CHEMBL252674; 2H-1-Benzopyran-3-propanamide, N-hydroxy-2-oxo-; BDBM50224963
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 1410 nM | |||
| External Link | ||||
| 2-(biphenyl-4-ylsulfonamido)pentanedioic acid | Investigative | [27] | ||
| Synonyms |
CHEMBL475540; 2-(Biphenyl-4-sulfonylamino)-pentanedioic acid; SR-01000365325; BAS 07869980; AC1LD9LY; MLS000075919; CHEBI:91838; HMS2373B21; ML034; BDBM50247207; AKOS000737926; AKOS024284065; MCULE-3770968562; ST072428; SMR000014780; 2-[(4-phenylphenyl)sulfonylamino]pentanedioic acid; SR-01000365325-1; SR-01000365325-3; 2-{[(4-phenylphenyl)sulfonyl]amino}pentanedioic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 14000 nM | |||
| External Link | ||||
| N-hydroxy-2,3-bis(phenylsulfonamido)propanamide | Investigative | [28] | ||
| Synonyms |
CHEMBL496717
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 227 nM | |||
| External Link | ||||
| N-hydroxy-3-(6-methoxy-2-oxo-2H-chromen-3-yl) | Investigative | [26] | ||
| Synonyms |
CHEMBL402990
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5660 nM | |||
| External Link | ||||
| 3-(4-Phenylethynylbenzoyl)nonanoic acid | Investigative | [29] | ||
| Synonyms |
CHEMBL201298
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5410 nM | |||
| External Link | ||||
| Folate gamma-hydroxamic acid | Investigative | [20] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-amino-3-(4-(hexyloxy)phenyl)-4-oxobutanoic acid | Investigative | [27] | ||
| Synonyms |
CHEMBL448246; 3-(4-Hexyloxy-phenyl)-succinamic acid; AC1LCKXS; SMR000008837; MLS000073581; cid_651854; HMS2162F22; HMS3313H20; BDBM50247490; AKOS000505159; AKOS030483668; BAS 00404306; 4-amino-3-(4-hexoxyphenyl)-4-oxobutanoic acid; SR-01000514729
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 160 nM | |||
| External Link | ||||
| 5-Biphenyl-4-yl-5-ethyl-pyrimidine-2,4,6-trione | Investigative | [21] | ||
| Synonyms |
CHEMBL367524; 94209-48-0; 2,4,6(1H,3H,5H)-Pyrimidinetrione, 5-[1,1'-biphenyl]-4-yl-5-ethyl-; ACMC-20lyhc; AC1M3WRE; Oprea1_691960; MLS001000776; NIOSH/CQ0532020; CTK3G9325; DTXSID20367041; MolPort-002-095-507; HMS2827O03; BDBM50099119; ZINC96299878; STK760260; AKOS005616072; MCULE-2406616532; Acido 5-etil 5-(p-difenilil)barbiturici; NCGC00245692-01; 5-(4-Biphenylyl)-5-ethylbarbituric acid; SMR000498104; LS-23825; M.G. 3419; CQ05320200; Barbituric acid, 5-(4-biphenylyl)-5-ethyl-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 696 nM | |||
| External Link | ||||
| (+/-)5-(biphenyl-4-yl)-3-hydroxypentanoic acid | Investigative | [30] | ||
| Synonyms |
CHEMBL572982; SCHEMBL5613354; ILGSIIFHQGOKKV-UHFFFAOYSA-N; BDBM50300465; 5-biphenyl-4-yl-3-hydroxy-pentanoic acid; 5-(biphenyl-4-yl)-3-hydroxypentanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 16500 nM | |||
| External Link | ||||
| 3-(4-(2-phenylethynyl)benzoyl)pentanoic acid | Investigative | [29] | ||
| Synonyms |
CHEMBL202875
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 3600 nM | |||
| External Link | ||||
| UK-356618 | Investigative | [31] | ||
| Synonyms |
UK 356618; 230961-08-7; CHEMBL117225; UK-356,618; SCHEMBL6437730; GTPL6528; CHEBI:94305; DTXSID50438778; MolPort-023-277-089; ZINC3924338; BDBM50097263; AKOS024458021; FT-0675728; PF 03890101; PF-03890101; UK-356618, > J-014983; BRD-K57011718-001-01-5; (R)-N*1*-[(S)-2,2-Dimethyl-1-((R)-1-phenyl-ethylcarbamoyl)-propyl]-N*4*-hydroxy-2-[3-(2-methyl-biphenyl-4-yl)-propyl]-succinamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 840 nM | |||
| External Link | ||||
| Ro-37-9790 | Investigative | [32] | ||
| Synonyms |
CHEMBL306412; BDBM50290086
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 10.4 nM | |||
| External Link | ||||
| 2-(Biphenyl-4-ylsulfonyl)N-hydroxybenzamide | Investigative | [33] | ||
| Synonyms |
CHEMBL574589
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7700 nM | |||
| External Link | ||||
| SL422 | Investigative | [34] | ||
| Synonyms |
analogue 55f [PMID: 11472217]
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.9 nM | |||
| External Link | ||||
| [2-(Biphenyl-4-sulfonyl)phenyl]acetic Acid | Investigative | [33] | ||
| Synonyms |
CHEMBL573935
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7300 nM | |||
| External Link | ||||
| N-Hydroxy-2-(4-phenoxy-benzenesulfonyl)benzamide | Investigative | [33] | ||
| Synonyms |
CHEMBL575896
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 690 nM | |||
| External Link | ||||
| SR-973 | Investigative | [35] | ||
| Synonyms |
CHEMBL204357; SCHEMBL4486832; ZINC34801833; BDBM50182403; (2S,3R)-N1-hydroxy-3-isobutyl-N4-((S)-2-oxo-1-(3-phenoxybenzyl)azepan-3-yl)-2-propylsuccinamide; (2R,3S)-N'-Hydroxy-N-[1-(3-phenoxybenzyl)-2,3,4,5,6,7-hexahydro-2-oxo-1H-azepine-3beta-yl]-2-isobutyl-3-propylbutanediamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 5 nM | |||
| External Link | ||||
| IK-862 | Investigative | [36] | ||
| Synonyms |
YDMIPBHQKFOFQW-NSYGIPOTSA-N; (2R)-N-HYDROXY-2-[(3S)-3-METHYL-3-{4-[(2-METHYLQUINOLIN-4-YL)METHOXY]PHENYL}-2-OXOPYRROLIDIN-1-YL]PROPANAMIDE; CHEMBL148169; CHEBI:40083; (2R)-N-hydroxy-2-[(3S)-3-methyl-3-[4-[(2-methylquinolin-4-yl)methoxy]phenyl]-2-oxopyrrolidin-1-yl]propanamide; IK862; 2fv5; AC1OCAC0; BMCL181958 Compound 1; GTPL8680; SCHEMBL5966106; BDBM26526; IK682; DB07145; C468787000; (R)-2-[(3S)-2-Oxo-3alpha-[4-(2-methyl-4-quinolinylmethoxy)phenyl]-3-methylpyrrolizino]propanehydroximic; IK 862; compound 32; IK-682
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 10340 nM | |||
| External Link | ||||
| MMI270 | Investigative | [37] | ||
| Synonyms |
CGS-27023A; CGS-27023; UNII-80AXY59IT2; 80AXY59IT2; N-HYDROXY-2(R)-[[(4-METHOXYPHENYL)SULFONYL](3-PICOLYL)AMINO]-3-METHYLBUTANAMIDE HYDROCHLORIDE; CHEMBL514138; (2R)-N-hydroxy-2-[(4-methoxyphenyl)sulfonyl-(pyridin-3-ylmethyl)amino]-3-methylbutanamide; CGS; 1eub; MMI270B free base; hydroxamate analogue 1; 2w0d; 1bm6; MMI-270B free base; AC1L9JQY; 3MP-HAV-MSB; CGS-27023A free base; BMCL16311 Compound 1a; BDBM8465; SCHEMBL3468445; GTPL8846; CHEMBL267178; BSIZUMJRKYHEBR-QGZVFWFLSA-N; CGS 27023; BDBM50066658; DB07556; 161314-70-1
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 4.3 nM | |||
| External Link | ||||
References