m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05144
|
[1], [2] | |||
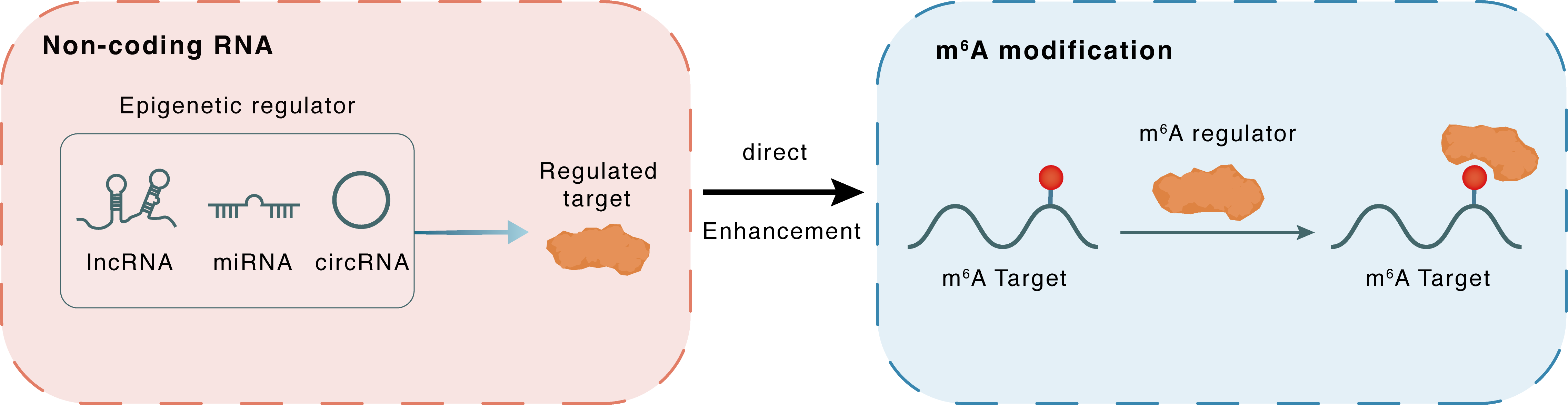 Non-coding RNA
PCAT6
HNRNPA2B1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
MAPK1
MAPK1
hnRNPA2B1
Non-coding RNA
PCAT6
HNRNPA2B1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
MAPK1
MAPK1
hnRNPA2B1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Heterogeneous nuclear ribonucleoproteins A2/B1 (HNRNPA2B1) | READER | |||
| m6A Target | Mitogen-activated protein kinase 1 (MAPK/ERK2/MAPK1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Prostate cancer associated transcript 6 (PCAT6) | LncRNA | View Details | ||
| Regulated Target | Heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | Mechanically, PCAT6 functions as a scaffold between interferon-stimulated gene 15 (ISG15) and heterogeneous nuclear ribonucleoprotein A2/B1 (HNRNPA2B1), leading to ISGylation of hnRNPA2B1, thus protecting hnRNPA2B1 from ubiquitination-mediated proteasomal degradation.In breast cancer, modest stable overexpression of A2B1 in MCF-7 cells (MCF-7-A2B1 cells) resulted in tamoxifen and fulvestrant - resistance whereas knockdown of A2B1 in LCC9 and LY2 cells restored tamoxifen and fulvestrant, endocrine-sensitivity. MCF-7-A2B1 cells have increased ER-alpha and reduced miR-222-3p that targets ER-alpha. MCF-7-A2B1 have activated RAC-alpha serine/threonine-protein kinase (AKT1) and Mitogen-activated protein kinase 1 (MAPK/ERK2/MAPK1) that depend on A2B1 expression and are growth inhibited by inhibitors of these pathways. | ||||
| Responsed Disease | Breast cancer | ICD-11: 2C60 | |||
| Responsed Drug | Tamoxifen | ||||
| Pathway Response | MAPK signaling pathway | hsa04010 | |||
| PI3K-Akt signaling pathway | hsa04151 | ||||
| Cell Process | Cell migration and invasion | ||||
In-vitro Model |
MCF-7 | Invasive breast carcinoma | Homo sapiens | CVCL_0031 | |
| T-47D | Invasive breast carcinoma | Homo sapiens | CVCL_0553 | ||
| MDA-MB-231 | Breast adenocarcinoma | Homo sapiens | CVCL_0062 | ||
| MDA-MB-468 | Breast adenocarcinoma | Homo sapiens | CVCL_0419 | ||
| HCC1806 | Breast squamous cell carcinoma | Homo sapiens | CVCL_1258 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Mitogen-activated protein kinase 1 (MAPK/ERK2/MAPK1) | 27 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| BVD-523 | Phase 2 | [3] | ||
| MOA | Modulator | |||
| Activity | IC50 < 0.3 nM | |||
| External Link | ||||
| HH2710 | Phase 1/2 | [4] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| ASTX029 | Phase 1/2 | [5] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| LY3214996 | Phase 1 | [6] | ||
| Synonyms |
JNPRPMBJODOFEC-UHFFFAOYSA-N; 1951483-29-6; GTPL9975; SCHEMBL17837273; MolPort-046-033-624; EX-A2560; BCP19982; CS-6974; HY-101494; LY 3214996; 6,6-dimethyl-2-[2-[(2-methylpyrazol-3-yl)amino]pyrimidin-4-yl]-5-(2-morpholin-4-ylethyl)thieno[2,3-c]pyrrol-4-one; 6,6-dimethyl-2-(2-((1-methyl-1H-pyrazol-5-yl)amino)pyrimidin-4-yl)-5-(2-morpholinoethyl)-5,6-dihydro-4H-thieno[2,3-c]pyrrol-4-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| VAN-10-4-eluting stent | Phase 1 | [7] | ||
| Synonyms |
INC-105-eluting stent, Inncardio/University of Strathclyde; VAN-10-4-eluting stent, University of Strathclyde; Marigold compound (drug-eluting stent, restenosis), Inncardio/University of Strathclyde
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| GDC-0994 | Phase 1 | [8] | ||
| MOA | Modulator | |||
| Activity | IC50 = 3.1 nM | |||
| External Link | ||||
| JSI-1187 | Phase 1 | [9] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| CHIR-99021 | Patented | [10] | ||
| Synonyms |
CHIR99021; CHIR 99021; CT-99021; CT99021
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 > 10000 nM | |||
| External Link | ||||
| COR-D | Preclinical | [11] | ||
| MOA | Activator | |||
| External Link | ||||
| SB220025 | Terminated | [12] | ||
| Synonyms |
3erk; sb 220025; SB-220025; CHEMBL274064; 165806-53-1; CHEBI:82713; 4-[4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl]pyrimidin-2-amine; 4-(4-FLUOROPHENYL)-1-(4-PIPERIDINYL)-5-(2-AMINO-4-PYRIMIDINYL)-IMIDAZOLE; SB4; 5-(2-Amino-4-pyrimidinyl)-4-(4-fluorophenyl)-1-(4-piperidinlyl)imidazole; 4-[5-(4-fluorophenyl)-3-(4-piperidyl)imidazol-4-yl]pyrimidin-2-amine; SB-220025-A; 1-(4-piperidinyl)-4-(4-fluorophenyl)-5-(2-(amino)-4-pyrimidinyl)imidazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 19000 nM | |||
| External Link | ||||
| AEZS-131 | Investigative | [13] | ||
| Synonyms |
ERK inhibitor (cancer), AEterna
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| SCH772984 | Investigative | [14] | ||
| Synonyms |
SCH-772984
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 < 0.3 nM | |||
| External Link | ||||
| ERK inhibitor III | Investigative | [15] | ||
| Synonyms |
AC1NSSSU; 1-nitro-2-[(Z)-[5-(3-nitrophenyl)furan-2-yl]methylideneamino]guanidine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| (4-Fluoro-phenyl)-(9-methyl-9H-purin-6-yl)-amine | Investigative | [16] | ||
| Synonyms |
SCHEMBL6659391
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FR-180204 | Investigative | [17] | ||
| Synonyms |
865362-74-9; FR 180204; FR180204; ERK Inhibitor II, FR180204; 5-(2-Phenyl-pyrazolo[1,5-a]pyridin-3-yl)-1H-pyrazolo[3,4-c]pyridazin-3-ylamine; CHEMBL259551; 5-(2-PHENYLPYRAZOLO[1,5-A]PYRIDIN-3-YL)-1H-PYRAZOLO[3,4-C]PYRIDAZIN-3-AMINE; C18H13N7; 5-{2-phenylpyrazolo[1,5-a]pyridin-3-yl}-1H-pyrazolo[3,4-c]pyridazin-3-amine; 5-{2-phenylpyrazolo[1,5-a]pyridin-3-yl}-2H-pyrazolo[3,4-c]pyridazin-3-amine; 5-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-2H-pyrazolo[3,4-c]pyridazin-3-amine; ERK inhibitor II; MLS002607685
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 310 nM | |||
| External Link | ||||
| DEBROMOHYMENIALDISINE | Investigative | [18] | ||
| MOA | Inhibitor | |||
| Activity | IC50 = 824 nM | |||
| External Link | ||||
| Phosphonothreonine | Investigative | [12] | ||
| Synonyms |
phosphothreonine; O-phospho-L-threonine; 1114-81-4; L-Threonine O-phosphate; (2S,3R)-2-amino-3-(phosphonooxy)butanoic acid; O-Phosphothreonine; L-Threonine phosphate; Threoninium dihydrogen phosphate; O-phosphono-L-threonine; L-Threonine O-3-phosphate; O3-phosphothreonine; 27530-80-9; threonine phosphate ester; (2S,3R)-2-amino-3-hydroxybutanoic acid 3-phosphate; Threonine, O-phosphono-; H-Thr(PO3H2)-OH; C4H10NO6P; phospho-l-threonine; EINECS 214-217-5; Synonyms Sources; (S)-2-Amino-3-hydroxybutanoic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| KT-5720 | Investigative | [19] | ||
| Synonyms |
KT 5720; KT5720; 108068-98-0; GTPL337; ZINC3873013; KT 5720, > hexyl (15R,16R,18S)-16-hydroxy-15-methyl-3-oxo-28-oxa-4,14,19-triazaoctacyclo[12.11.2.1^{15,18}.0^{2,6}.0^{7,27}.0^{8,13}.0^{19,26}.0^{20,25}]octacosa-1(26),2(6),7(27),8,10,12,20,22,24-nonaene-16-carboxylate; (9S,10S,12R)-2,3,9,10,11,12-Hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3 inverted exclamation marka,2 inverted exclamation marka,1 inverted exclamation marka-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-c
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro31-8220 | Investigative | [19] | ||
| Synonyms |
Bisindolylmaleimide IX; ro 31-8220; 125314-64-9; Ro 31 8220; Ro 318220; UNII-W9A0B5E78O; Ro-318220; Ro-31-8220; CHEMBL6291; W9A0B5E78O; CHEBI:38912; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl carbamimidothioate; 3-{3-[4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-2,5-dihydro-1H-pyrrol-3-yl]-1H-indol-1-yl}propyl imidothiocarbamate; CHEMBL1591531; Carbamimidothioic acid, 3-(3-(2,5-dihydro-4-(1-methyl-1H-indol-3-yl)-2,5-dioxo-1H-pyrrol-3-yl)-1H-indol-1-yl)propyl; bisindolymaleimide IX
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Ro-4396686 | Investigative | [20] | ||
| Synonyms |
SCHEMBL5809947; CHEMBL606964
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 12600 nM | |||
| External Link | ||||
| BMS-536924 | Investigative | [21] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| KN-62 | Investigative | [19] | ||
| Synonyms |
KN-62 (non-isomeric); GTPL6001; HMS3229A04; CCG-206863
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CI-1040 | Investigative | [19] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 4,5,6,7-tetrabromo-1H-benzo[d][1,2,3]triazole | Investigative | [22] | ||
| Synonyms |
4,5,6,7-tetrabromobenzotriazole
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [23] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Bisindolylmaleimide-I | Investigative | [19] | ||
| Synonyms |
Bisindolylmaleimide i; 133052-90-1; GF 109203X; GF109203X; Go 6850; GF-109203X; RBT205 INHIBITOR; Go-6850; UNII-L79H6N0V6C; Bisindolylmaleimide I (GF 109203X); CHEMBL7463; 3-{1-[3-(DIMETHYLAMINO)PROPYL]-1H-INDOL-3-YL}-4-(1H-INDOL-3-YL)-1H-PYRROLE-2,5-DIONE; 3-(1-(3-(Dimethylamino)propyl)-1H-indol-3-yl)-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione; L79H6N0V6C; QMGUOJYZJKLOLH-UHFFFAOYSA-N; 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl)maleimide; GF-109203; Go6850
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| RO-316233 | Investigative | [19] | ||
| Synonyms |
119139-23-0; bisindolylmaleimide iv; 3,4-di(1H-indol-3-yl)-1H-pyrrole-2,5-dione; Arcyriarubin A; 3,4-Bis(3-indolyl)maleimide; 3,4-Di-1H-indol-3-yl-1H-pyrrole-2,5-dione; UNII-MBK3OO5K8T; BIM IV; 3,4-bis(1H-indol-3-yl)pyrrole-2,5-dione; MBK3OO5K8T; CHEMBL266487; 3,4-bis(1H-indol-3-yl)-2,5-dihydro-1H-pyrrole-2,5-dione; DQYBRTASHMYDJG-UHFFFAOYSA-N; 2,3-bis(1H-Indol-3-yl)maleimide; 1H-Pyrrole-2,5-dione, 3,4-di-1H-indol-3-yl-; Ro-31-6233; AK-15401; 3,4-bis(3-indolyl)-1H-pyrrole-2,5-dione; Bisindoylmaleimide; Bisindolyl deriv. 3
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2C60: Breast cancer | 2 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Entrectinib | Approved | [24] | ||
| Synonyms |
1108743-60-7; RXDX-101; UNII-L5ORF0AN1I; Entrectinib (RXDX-101); L5ORF0AN1I; Benzamide, N-[5-[(3,5-difluorophenyl)methyl]-1H-indazol-3-yl]-4-(4-methyl-1-piperazinyl)-2-[(tetrahydro-2H-pyran-4-yl)amino]-; Benzamide, N-(5-((3,5-difluorophenyl)methyl)-1H-indazol-3-yl)-4-(4-methyl-1-piperazinyl)-2-((tetrahydro-2H-pyran-4-yl)amino)-; Entrectinib [USAN:INN]; YMX; Kinome_2659; Entrectinib(rxdx-101); Entrectinib (USAN/INN); SCHEMBL3512601; GTPL8290; CHEMBL1983268; KS-00000TSK
Click to Show/Hide
|
|||
| External Link | ||||
| Everolimus | Approved | [25] | ||
| External Link | ||||
References