m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05123
|
[1] | |||
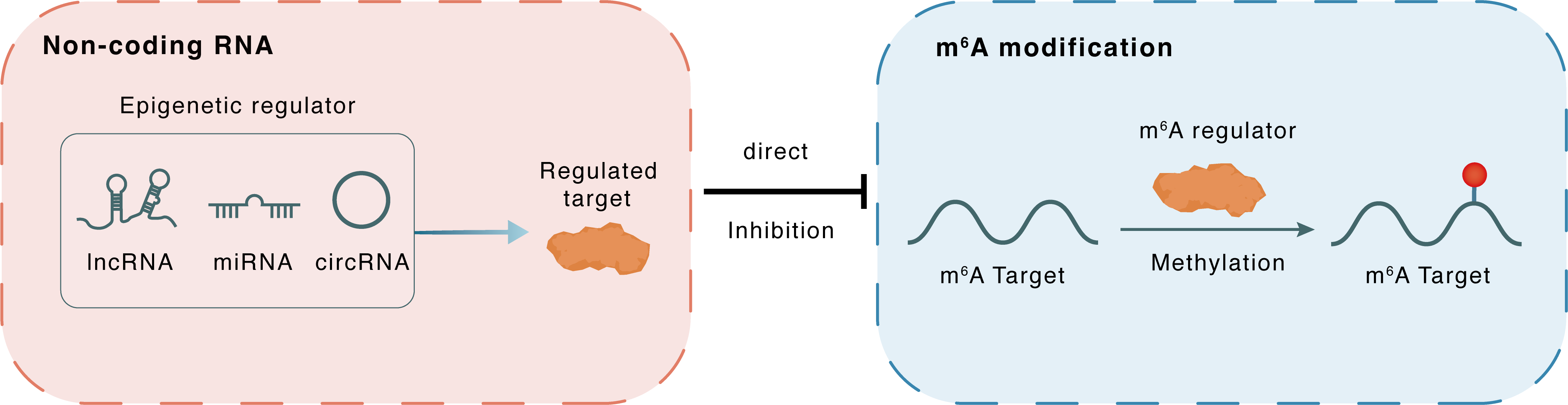 Non-coding RNA
UCA1
METTL14
lncRNA miRNA circRNA
Direct
Inhibition
m6A modification
HIF1A
HIF1A
METTL14
Methylation
Non-coding RNA
UCA1
METTL14
lncRNA miRNA circRNA
Direct
Inhibition
m6A modification
HIF1A
HIF1A
METTL14
Methylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Methyltransferase-like 14 (METTL14) | WRITER | |||
| m6A Target | Hypoxia-inducible factor 1-alpha (HIF-1-Alpha/HIF1A) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Urothelial cancer associated 1 (UCA1) | LncRNA | View Details | ||
| Regulated Target | Methyltransferase-like protein 14 (METTL14) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Inhibition | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | LncRNA UCA1 promotes keratinocyte-driven inflammation via suppressing METTL14 and activating the Hypoxia-inducible factor 1-alpha (HIF-1-Alpha/HIF1A)/NF-kappaB axis in psoriasis | ||||
| Responsed Disease | Psoriasis | ICD-11: EA90 | |||
In-vitro Model |
HaCaT | Normal | Homo sapiens | CVCL_0038 | |
| HUVEC-C | Normal | Homo sapiens | CVCL_2959 | ||
| HEK293 | Normal | Homo sapiens | CVCL_0045 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Hypoxia-inducible factor 1-alpha (HIF-1-Alpha/HIF1A) | 21 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PT2385 | Phase 2 | [2] | ||
| Synonyms |
ONBSHRSJOPSEGS-INIZCTEOSA-N; PT-2385; UNII-6O16716DXP; 1672665-49-4; 6O16716DXP; SCHEMBL16555810; ZINC230453533; AKOS030526641; HY-12867; PT2385,1672665-49-4, PT 2385,PT-2385; Benzonitrile, 3-(((1S)-2,2-difluoro-2,3-dihydro-1-hydroxy-7-(methylsulfonyl)-1H-inden-4-yl)oxy)-5-fluoro-; 3-{[(1s)-2,2-Difluoro-1-Hydroxy-7-(Methylsulfonyl)-2,3-Dihydro-1h-Inden-4-Yl]oxy}-5-Fluorobenzonitrile; 3-(((1S)-2,2-Difluoro-1-hydroxy-7-methanesulfonyl-2,3-dihydro-1hinden-4-yl)oxy)-5-fluorobenzonitrile; 79A
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ISIS 298697 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298744 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298746 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298745 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298743 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298702 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298700 | Investigative | [3] | ||
| External Link | ||||
| ISIS 175510 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298699 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298712 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298711 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298701 | Investigative | [3] | ||
| External Link | ||||
| (5-(1-benzyl-1H-indazol-3-yl)furan-2-yl)methanol | Investigative | [4] | ||
| Synonyms |
Lificiguat; yc-1; 170632-47-0; 3-(5'-Hydroxymethyl-2'-furyl)-1-benzylindazole; YC 1; UNII-515CC1WPTE; Lificiguat(YC-1); 154453-18-6; [5-(1-benzyl-1h-indazol-3-yl)-2-furyl]methanol; 515CC1WPTE; CHEMBL333985; OQQVFCKUDYMWGV-UHFFFAOYSA-N; C19H16N2O2; 3-(5'-Hydroxymethyl-2'-furyl)-1-benzyl indazole; 1-Benzyl-3-(5-hydroxymethyl-2-furyl)indazole; [5-(1-benzyl-1H-indazol-3-yl)furan-2-yl]methanol; 5-[1-(Phenylmethyl)-1H-indazol-3-yl]-2-furanmethanol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HIF-1alpha | Phase 4 | [5] | ||
| Synonyms |
Unii-NA856793UT; 192705-79-6; PD-166866; PD166866; PD 166866; CHEMBL299763; NA856793UT; 1-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-3-tert-butylurea; 1-(2-Amino-6-(3,5-dimethoxyphenyl)-pyrido(2,3-d)pyrimidin-7-yl)-3-tert-butyl urea; Urea,N-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)-; 1-[2-Amino-6-(3,5-dimethoxyphenyl)-pyrido[2,3-d]pyrimidin-7-yl]-3-tert-butyl urea; 6-arylpyrido[2,3-d]pyrimidine deriv 25; AC1NS3U5; SCHEMBL1248489; BDBM3443; CTK4E1060
Click to Show/Hide
|
|||
| External Link | ||||
| IT-101 | Phase 3 | [5] | ||
| External Link | ||||
| 2-Methoxyestradiol | Phase 2 | [5] | ||
| Synonyms |
ESM; Panzem; PulmoLAR; Panzem NCD; M 6383; (17beta)-2-Methoxyestra-1,3,5(10)-triene-3,17-diol; (17beta)-2-methoxyestra-1(10),2,4-triene-3,17-diol; (8R,9S,13S,14S,17S)-2-methoxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; 1,3,5(10)-ESTRATRIEN-2,3,17-BETA-TRIOL 2-METHYL ETHER; 1,3,5(10)-Estratriene-2,3,17-triol 2-methyl ether; 2,3,17beta-Trihydroxy-1,3,5(10)-estratriene 2-methyl ether; 2-Hydroxyestradiol 2-methyl ether; 2-Hydroxyestradol 2-methyl ether; 2-ME2, 2-Methoxyestradiol; 2-Methoxyestra-1,3,5(10)-triene-3,17beta-diol; 2-Methoxyestradiol-17beta; 3,17beta-Dihydroxy-2-methoxy-1,3,5(10)-estratriene
Click to Show/Hide
|
|||
| External Link | ||||
| PX-478 | Phase 1 | [5] | ||
| Synonyms |
685898-44-6; PX-478 2HCl; UNII-T23U22X160; PX478; PX 478; Melphalan N-Oxide Impurity HCl; T23U22X160; 4-[Bis(2-chloroethyl)oxidoamino]-L-phenylalanine; PX-478 dihydrochloride; SCHEMBL18548830; C13H18Cl2N2O3.2ClH; DTXSID00218688; MolPort-035-789-733; 2675AH; s7612; 2-Amino-3-(4'-N,N-bis(2-chloroethyl)amino)phenylpropionic acid N-oxide; AKOS030231369; CS-5164; HY-10231; KB-80169; Z-3209; L-Phenylalanine, 4-(bis(2-chloroethyl)oxidoamino)-, dihydrochloride; (S)-4-(2-amino-2-carboxyethyl)-N,N-bis(2-chloroethyl)aniline oxide di
Click to Show/Hide
|
|||
| External Link | ||||
| EZN-2968 | Phase 1 | [5] | ||
| External Link | ||||
| ENMD-1198 | Phase 1 | [5] | ||
| Synonyms |
EM-5171; EM-883; EM-900; Hypoxia inducible factor 1 inhibitors, EntreMed; HIF-1 inhibitors, EntreMed; HIF-1 inhibitors (cancer), EntreMed; 2-ME2 analogs (oral, cancer), EntreMed; 2-methoxyestradiol analogs (oral, cancer), EntreMed
Click to Show/Hide
|
|||
| External Link | ||||
| Pyrrolidine carboxamide derivative 1 | Patented | [5] | ||
| Synonyms |
PMID26882240-Compound-22
Click to Show/Hide
|
|||
| External Link | ||||
| EA90: Psoriasis | 166 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Teriparatide | Discontinued in Phase 2 | [6] | ||
| Synonyms |
Forteo; Forteo (TN); Teriparatide (genetical recombination); Teriparatide (USAN/INN); Teriparatide (genetical recombination) (JAN)
Click to Show/Hide
|
|||
| External Link | ||||
| Ponesimod | Phase 2 | [7] | ||
| Synonyms |
854107-55-4; UNII-5G7AKV2MKP; 5G7AKV2MKP; CHEMBL1096146; Ponesimod [USAN:INN]; Ponesimod,ACT-128800; Ponesimod (ACT-128800); GTPL9320; SCHEMBL15477937; SCHEMBL15477934; DTXSID50234631; MolPort-035-681-391; MolPort-046-033-541; EX-A1417; ZINC34509627; s8241; BDBM50316768; AKOS022180266; DB12016; Compound 8bo [PMID:20446681]; HY-10569; KB-72962; AS-35140; AJ-89002; (2Z,5Z)-5-(3-Chloro-4-((2R)-2,3-dihydroxypropoxy)phenylmethylidene)-3-(2-methylphenyl)-2-(propylimino)-1,3-thiazolidin-
Click to Show/Hide
|
|||
| External Link | ||||
| Spesolimab | Approved | [8] | ||
| External Link | ||||
| Lestaurtinib | Approved (orphan drug) | [9] | ||
| Synonyms |
A 1544750; CEP 701; KT 5555; KT5555; SP 924; CEP-701; KT-5555; SPM-924; Lestaurtinib (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| Belumosudil | Approved | [10] | ||
| External Link | ||||
| Deucravacitinib | Approved | [11] | ||
| External Link | ||||
| Dithranol | Approved | [12] | ||
| Synonyms |
Anthralin; Micanol; Dithranol cream (Crystalip); Dithranol cream (Crystalip), Bioglan
Click to Show/Hide
|
|||
| External Link | ||||
| Ciclosporin | Approved | [13] | ||
| Synonyms |
Cyclosporine A; CSA; Antibiotic S 7481F1; BMT-ABA-SAR-MLE-VAL-MLE-ALA-ALA-MLE-MLE-MVA; BMT-ABA-SAR-MLE-VAL-MLE-ALA-DAL-MLE-MLE-MVA; C 3662; CB-01-09 MMX; CYCLOSPORIN A (SEE ALSO TRANSGENIC MODEL EVALUATION (CYCLOSPORIN A)); Cicloral (TN); Ciclosporin (JP15); Cipol N; Cipol-N; Consupren; Consupren S; CsA & IFN-alpha; Cyclokat; Cyclophorine; Cyclosporin; Cyclosporin A; Cyclosporin A & IFN-alpha; Cyclosporin A Implant; Cyclosporin A, Tolypocladium inflatum; Cyclosporine (USP); Cyclosporine [USAN]; DE-076; Equoral; From Tolypocladium inflatum (Trichoderma polysporin); GNF-Pf-2808; Gengraf; Gengraf (TN); Helv Chim Acta 60: 1568 (1977); Mitogard; Modusik-A; Neoplanta; Neoral; Neoral (TN); NeuroSTAT; Nova-22007; OL 27-400; OL-27400; OLO-400; Papilock; Pulminiq; RamihyphinA; Restasis; Restasis (TN); S-Neoral; SDZ-OXL 400; ST-603; Sandimmun; Sandimmun Neoral; Sandimmune; Sandimmune (TN); Sandimmune, Gengraf, Restasis, Atopica, Sangcya, Cyclosporine; Sang-2000; Sang-35; SangCyA; Sigmasporin; Sigmasporin Microoral; TRANSGENIC MODEL EVALUATION (CYCLOSPORIN A); Vekacia; Zyclorin
Click to Show/Hide
|
|||
| External Link | ||||
| Xp-828l | Approved | [12] | ||
| Synonyms |
Dermylex (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Voclosporin | Phase 3 | [14] | ||
| Synonyms |
Luveniq; ISAtx-247; ISA-247; UNII-2PN063X6B1; ISATX247; 515814-01-4; ISA247; 2PN063X6B1; ISA 247; trans-ISA-247; LX211; Voclosporin (USAN/INN); Voclosporin [USAN:INN]; Voclera; 3odi; LX-211; LX-214; ISA247, Luveniq; AC1OCFHS; R-1524; SCHEMBL12632344; CHEBI:135957; (E)-ISA-247; DB11693; 515814-00-3; HY-106638; CS-0026210; D09033; Cyclosporin A, 6-((2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6,8-nonadienoic acid)-; 368455-04-3; Voclosporin [USAN]; R 1524; Trans-ISA 247; TrkA-IgG
Click to Show/Hide
|
|||
| External Link | ||||
| Verteporfin | Approved | [12] | ||
| Synonyms |
Visudyne (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Mirikizumab | Approved | [10] | ||
| External Link | ||||
| Tazarotene | Approved | [15] | ||
| Synonyms |
Avage; Suretin; Tazaroteno; Tazarotenum; Tazorac; Tazoral; Zorac; AGN 190168; AGN-190168; Avage (TN); Tazarotene [USAN:INN]; Tazorac (TN); Zorac (TN); Tazarotene (JAN/USAN/INN); Tazorac, Avage, Zora, Tazarotene; Ethyl 6-((4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate; Ethyl 6-(2-(4,4-dimethylthiochroman-6-yl)ethynyl)nicotinate; Ethyl 6-[(4,4-dimethyl-3,4-dihydro-2H-thiochromen-6-yl)ethynyl]nicotinate; Ethyl 6-[2-(4,4-dimethyl-2,3-dihydrothiochromen-6-yl)ethynyl]pyridine-3-carboxylate; 3-Pyridinecarboxylic acid, 6-((3,4-dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl)-, ethyl ester; 3-Pyridinecarboxylic acid, 6-((3,4-dihydro-4,4-dimethyl-2H-1-benzothiopyran-6-yl)ethynyl)-,ethyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| Ustekinumab | Phase 3 | [16] | ||
| Synonyms |
6,6-Dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-1,6-dihydro-1,3,5-triazine-2,4-diamine; WR99210; WR-99210; 47326-86-3; BRN 0629517; WR 99210; 1,3,5-Triazine-2,4-diamine, 1,6-dihydro-6,6-dimethyl-1-[3-(2,4,5-trichlorophenoxy)propoxy]-; WR-99,210; CHEMBL129788; BRL 6231; 1,6-Dihydro-6,6-dimethyl-1-(3-(2,4,5-trichlorophenoxy)-propoxy)-1,3,5-triazine-2 ,4-diamine; 1-(2',4',5'-Trichlorophenoxypropoxy)-1,2-dihydro-2,2-dimethyl-4,6-diamino-s-triazine; Stelara (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Azaribine | Approved | [12] | ||
| Synonyms |
Triazure; Triacetyl-6-azauridine; Azaribinum; Azaribina; 2169-64-4; 6-Azaribine; TA-Azur; Azaribin; 2',3',5'-Tri-O-acetyl-6-azauridine; 6-Azauridine triacetate; 6-Azauridin-triacetat; 6-Azauridine 2',3',5'-triacetate; Azaribinum [INN-Latin]; 6-AzUR-TA; Azaribina [INN-Spanish]; 2',3',5'-Triacetyl-6-azauridine; CB-304; UNII-K1U80DO9EB; 6-Azauracilribosid-triacetat; K1U80DO9EB; Azaribine [USAN:INN:BAN]; MLS000069488; EINECS 218-515-6; Azauridine triacetate; 2-beta-D-Ribofuranosyl-as-triazine-3,5(2H,4H)-dione 2',3',5'-triace
Click to Show/Hide
|
|||
| External Link | ||||
| Ammoniated mercury | Approved | [12] | ||
| Synonyms |
White precipitate; Mercuric chloride, ammoniated; Mercury ammoniated; Ammoniated mercuric chloride; Mercury, ammoniated; Aminomercury chloride; Aminomercuric chloride; Hydrargyrum ammoniatum; Mercury amine chloride; MERCURIC AMMONIUM CHLORIDE; White mercuric precipitate; White mercury precipitated; Quecksilber(II)-amid-chlorid; Hydrargyrum precipitatum album; Hydrargyrum praecipitatum album; UNII-JD546Z56F0; Mercury(II) chloride ammonobasic; Mercury, ammonobasic (HgNH2Cl); HSDB 1175; JD546Z56F0; EINECS 233-3
Click to Show/Hide
|
|||
| External Link | ||||
| Tacalcitol | Approved | [17] | ||
| Synonyms |
Bonalfa (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Methoxsalen | Approved | [18] | ||
| Synonyms |
Ammodin; Ammoidin; Deltasoralen; Dermox; Geroxalen; Meladinin; Meladinina; Meladinine; Meladoxen; Meloxine; Methoxalen; Methoxaten; Oxoralen; Oxsoralen; Oxypsoralen; Puvalen; Puvamet; Ultramop; Uvadex; XANTHOTOXIN; Xanthotoxine; Zanthotoxin; Boehringer Ingelheim Brand of Methoxsalen; Canderm Brand of Methoxsalen; Chinoin Brand of Methoxsalen; DB Brand of Methoxsalen; Delta Brand of Methoxsalen; Dermatech Brand of Methoxsalen; Galderma Brand of Methoxsalen; Methoxa Dome; Methoxsalen Canderm Brand; Methoxsalen Chinoin Brand; Methoxsalen Delta Brand; Methoxsalen Dermatech Brand; Methoxsalen plus ultraviolet radiation; Mex America Brand of Methoxsalen; Oxsoralen Ul tra; Oxsoralen Ultra; Oxsoralen lotion; Sanofi Synthelabo Brand of Methoxsalen; Ultra Mop; Ultramop Lotion; ICN Brand 1 of Methoxsalen; ICN Brand 2 of Methoxsalen; ICN Brand 3 of Methoxsalen; X0009; An 8-methoxyfurocoumarin; Meladinin (VAN); Methoxa-Dome; Methoxsalen Mex-America Brand; Methoxsalen Sanofi-Synthelabo Brand; Methoxsalen [BAN:JAN]; Methoxsalen with ultra-violet A theraphy; Mex-America Brand of Methoxsalen; New-Meladinin; O-methylxanthotoxol; OXSORALEN (TN); Oxsoralen-ultra; Proralone-mop; Psoralen-mop; Psoralon-MOP; Sanofi-Synthelabo Brand of Methoxsalen; Methoxsalen (JP15/USP); Methoxy-8-psoralen; 5-Benzofuranacrylic acid, 6-hydroxy-7-methoxy-, .delta.-lactone; 5-Benzofuranacrylic acid, 6-hydroxy-7-methoxy-, delta-lactone; 6-Hydroxy-7-methoxy-5-benzofuranacrylic acid delta-lactone; 7-Furocoumarin; 8 Methoxypsoralen; 8-METHOXYPSORALEN + UVA (SEE ALSO C55903); 8-MOP; 8-Methoxy; 8-Methoxy(furano-3'.2':6.7-coumarin); 8-Methoxy-(furano-3'.2':6.7-coumarin); 8-Methoxy-2',3',6,7-furocoumarin; 8-Methoxy-4',5',6,7-furocoumarin; 8-Methoxy-4',5':6,7-furocoumarin; 8-Methoxy-[furano-3'.2':6.7-coumarin]; 8-Methoxyfuranocoumarin; 8-Methoxypsoralen; 8-Methoxypsoralen with ultraviolet A therapy; 8-Methoxypsoralene; 8-methoxyfuranocoumarins; 8-methoxyfurocoumarins; 8MO; 8MOP; 9-(methyloxy)-7H-furo[3,2-g]chromen-7-one; 9-Methoxy-7H-furo(3,2-g)(1)benzopyran-7-one; 9-Methoxy-7H-furo(3,2-g)benzopyran-7-one; 9-Methoxy-7H-furo[3,2-g][1]benzopyran-7-one; 9-Methoxy-7H-furo[3,2-g]chromen-7-one; 9-Methoxyfuro(3,2-g)chromen-7-one; 9-Methoxyfuro[3,2-g][1]benzopyran-7-one; 9-Methoxypsoralen; 9-metho xy-7H-furo(3,2-g)benzopyran-7-one; 9-methoxyfuro[3,2-g]chromen-7-one
Click to Show/Hide
|
|||
| External Link | ||||
| Valrubicin | Approved | [12] | ||
| Synonyms |
Valstar; Valrubicin [USAN]; Valstar Preservative Free; AD 32; Antibiotic AD 32; Valstar (TN); N-Trifluoroacetyladriamycin 14-valerate; N-Trifluoroacetyldoxorubicin 14-valerate; Trifluoroacetyladriamycin-14-valerate; Valrubicin (USP/INN); N-Trifluoroacetyladriamycin-14-valerate; Adriamycin, trifluoroacetyl-, 14-valerate; [2-oxo-2-[(2S,4S)-2,5,12-trihydroxy-4-[5-hydroxy-6-methyl-4-[(2,2,2-trifluoroacetyl)amino]oxan-2-yl]oxy-7-methoxy-6,11-dioxo-3,4-dihydro-1H-tetracen-2-yl]ethyl] pentanoate; (2S-cis)-2-(1,2,3,4,6,11-Hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetyl)amino)-alpha-L-lyxo-hexopyranosyl)oxy)-2-naphthacenyl)-2-oxoethyl pentanoate; (2S-cis)-Pentanoic acid, 2-(1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetyl)amino)-alpha-L-lyxo-hexopyranosyl)oxy)-2-naphth acenyl)-2-oxoethyl ester; (8S,10S)-8-Glycoloyl-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-10-((2,3,6-trideoxy-3-(2,2,2-trifluoroacetamido)-alpha-L-lyxo-hexopyranosyl)oxy)-5,12-naphthacenedione 8(sup 2)-valerate; Pentanoic acid, 2-((2S,4S)-1,2,3,4,6,11-hexahydro-2,5,12-trihydroxy-7-methoxy-6,11-dioxo-4-((2,3,6-trideoxy-3-((trifluoroacetylamino)-, alpha-L-lysohexopyranoxyl)oxy)-2-naphthacenyl)-2-oxoethyl ester
Click to Show/Hide
|
|||
| External Link | ||||
| Apremilast | Approved | [19] | ||
| Synonyms |
Apremilast (USAN); CC-10004; N-[2-[1-(3-ethoxy-4-methoxy-phenyl)-2-methylsulfonyl-ethyl]-1,3-dioxo-isoindol-4-yl]acetamide
Click to Show/Hide
|
|||
| External Link | ||||
| Acitretin | Approved | [20] | ||
| Synonyms |
Acitretina; Acitretine; Acitretinum; Etretin; Isoacitretin; Isoetretin; Neotigason; Soriatane; TMMP; Acitretina [Spanish]; Acitretine [French]; Acitretinum [Latin]; Retinoid etretin; U0279; Ro 10-1670; Ro 13-7652; Soriatane (TN); Soriatane, Acitretin; Acitretin (USAN/INN); Acitretin [USAN:INN:BAN]; All-trans-Acitretin; Ro 10-1670/000; Ro-10-1670; Ro-13-7652; Ro-10-1670/000; All-trans-3,7-Dimethyl-9-(4-methoxy-2,3,6-trimethylphenyl)-2,4,6,8-nonatetraenoic acid; (2E,4E,6E,8E)-3,7-dimethyl-9-[2,3,6-trimethyl-4-(methyloxy)phenyl]nona-2,4,6,8-tetraenoic acid; (2E,4E,6E,8E)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid; (all-E)-9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid; 13-cis-Acitretin; 9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid; 9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Brodalumab | Approved | [21] | ||
| Synonyms |
AMG 827
Click to Show/Hide
|
|||
| External Link | ||||
| Coal tar | Approved | [12] | ||
| External Link | ||||
| Efalizumab | Approved | [22] | ||
| Synonyms |
Raptiva; Raptiva (TN); Efalizumab (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| Tapinarof | Approved | [23] | ||
| Synonyms |
GSK-2894512
Click to Show/Hide
|
|||
| External Link | ||||
| Calcipotriol | Approved | [24] | ||
| Synonyms |
Calcipotriene; Divonex; Dovonex; BMS-181161; Daivonex (TN); Dovonex (TN); MC-903; (1R,3S,5Z)-5-[(2E)-2-[(1R,3aS,7aR)-1-[(E,2R,5S)-5-cyclopropyl-5-hydroxypent-3-en-2-yl]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol; (1S,5Z,7Z,17ALPHA,22E)-24-CYCLOPROPYL-9,10-SECOCHOLA-5,7,10,22-TETRAENE-1,3,24-TRIOL; 1-ALPHA,24S-(OH)2-22-ENE-26,27-DEHYDROVITAMIN D3
Click to Show/Hide
|
|||
| External Link | ||||
| NPS-31807 | Phase 4 | [25] | ||
| Synonyms |
PP-05; TNF alpha modulator (inflammation), Piramal Life Sciences; TNF alpha modulator (inflammation), NPIL Research & Development
Click to Show/Hide
|
|||
| External Link | ||||
| LEO 80185 | Phase 4 | [26] | ||
| Synonyms |
Taclonex
Click to Show/Hide
|
|||
| External Link | ||||
| ARQ-151 | Phase 3 | [27] | ||
| External Link | ||||
| IB-MECA | Phase 3 | [28] | ||
| Synonyms |
152918-18-8; piclidenoson; CF-101; 3-IB-Meca; N(6)-Ibamu; CF 101; Cf101; N(6)-(3-iodobenzyl)-5'-N-methylcarboxamidoadenosine; UNII-30679UMI0N; N(6)-(3-Iodobenzyl)adenosine-5'-N-methyluronamide; 1-Deoxy-1-(6-(((3-iodophenyl)methyl)amino)-9H-purin-9-yl)-N-methyl-beta-D-ribofuranuronamide; CHEMBL119709; CHEBI:73286; 30679UMI0N; RPR-113090; 3-iodobenzyl-5'-N-methylcarboxamidoadenosine; N(6)-(3-iodo-benzyl)adenosine-5'-N-methyluronamide
Click to Show/Hide
|
|||
| External Link | ||||
| E-0116 | Phase 3 | [29] | ||
| External Link | ||||
| 5-methoxypsoralen | Phase 3 | [30] | ||
| Synonyms |
5-MOP
Click to Show/Hide
|
|||
| External Link | ||||
| RAVAX | Phase 3 | [31] | ||
| External Link | ||||
| MK-3222 | Phase 3 | [32] | ||
| External Link | ||||
| Serlopitant | Phase 2 | [10] | ||
| Synonyms |
VPD-737; UNII-277V92K32B; CHEMBL447955; 860642-69-9; 277V92K32B; 3-((3aR,4R,5S,7aS)-5-((R)-1-(3,5-bis(trifluoromethyl)phenyl)ethoxy)-4-(4-fluorophenyl)hexahydro-1H-isoindol-2(3H)-yl)cyclopent-2-enone; Serlopitant [USAN:INN]; VPD 737; Serlopitant (USAN); SCHEMBL3183159; GTPL9280; mk-0594; FLNYCRJBCNNHRH-OIYLJQICSA-N; BDBM50277511; compound 17 (Jiang et al. 2009); DB12973; D09378; 3-[(3aR,4R,5S,7aS)-5-{(1R)-1-[3,5-Bis(trifluoromethyl)phenyl]ethoxy}-4-(4-fluorophenyl)octahydro-1H-isoindol-2-yl]cyclopent-2-en-1-one
Click to Show/Hide
|
|||
| External Link | ||||
| M-518101 | Phase 3 | [33] | ||
| External Link | ||||
| ASP-015K | Phase 3 | [34] | ||
| Synonyms |
Peficitinib; ASP015K; UNII-HPH1166CKX; 944118-01-8; HPH1166CKX; 4-[[(1R,3S)-5-hydroxy-2-adamantyl]amino]-1H-pyrrolo[2,3-b]pyridine-5-carboxamide; 4-[[(1S,3R)-5-oxidanyl-2-adamantyl]amino]-1H-pyrrolo[2,3-b]pyridine-5-carboxamide; Peficitinib [USAN:INN]; ASP 015K; JNJ-54781532; 9T6; Peficitinib (USAN/INN); SCHEMBL1154421; SCHEMBL9990248; SCHEMBL4447032; GTPL8315; SCHEMBL9990240; SCHEMBL1154418; CHEMBL3137308; SCHEMBL17645135; BCP18465; BDBM50124208; SB16834; DB11708; SC-17960; D10653; Peficitinib pound A
Click to Show/Hide
|
|||
| External Link | ||||
| Fumaric acid | Phase 3 | [35] | ||
| Synonyms |
Fum; Fumarsaeure; Acidum fumaricum; Allomaleic acid; Allomalenic acid; Ammonium fumarate; Boletic acid; Butenedioic acid; Kyselina fumarova; Kyselina fumarova [Czech]; Lichenic acid; Tumaric acid; F0067; OR17920; FC 33 (acid); Fumarate, 10; Fumaric acid (8CI); Fumaric acid (NF); Lichenic acid (VAN); S04-0167; Trans-Butenedioic acid; U-1149; E-2-Butenedioic acid; Trans-2-Butenedioic acid; USAF EK-P-583; Trans-1,2-Ethylenedicarboxylic acid; Trans-but-2-enedioic acid; (2E)-2-butenedioic acid; (2E)-but-2-enedioic acid; (E)-2-Butenedioic acid; 1,2-Ethylenedicarboxylic acid, (E); 2-(E)-Butenedioic acid; 2-Butenedioic acid; 2-Butenedioic acid (2E-(9CI)
Click to Show/Hide
|
|||
| External Link | ||||
| Bimosiamose | Phase 2a | [36] | ||
| Synonyms |
TBC 1269; TBC-1269
Click to Show/Hide
|
|||
| External Link | ||||
| JNJ-77242113 | Phase 2 | [37] | ||
| Synonyms |
JNJ-2113
Click to Show/Hide
|
|||
| External Link | ||||
| PF-07038124 | Phase 2 | [38] | ||
| Synonyms |
(R)-4-(5-(4-Methoxy-3-propoxyphenyl)pyridin-3-yl)-1,2-oxaborolan-2-ol; 2415085-44-6; 3-[(4R)-2-hydroxyoxaborolan-4-yl]-5-(4-methoxy-3-propoxyphenyl)pyridine; BDBM589740; CS-0433935; Example 4 [US2020108083A1]; GLXC-25702; GTPL11950; HY-144683; M6ZU548FWD; PF 07038124 [WHO-DD]; PF07038124; PF-07038124; Pyridine, 3-((4R)-2-hydroxy-1,2-oxaborolan-4-yl)-5-(4-methoxy-3-propoxyphenyl)-; UNII-M6ZU548FWD; US11559538, Example 4
Click to Show/Hide
|
|||
| External Link | ||||
| IR502 | Phase 2 | [39] | ||
| Synonyms |
Zorcell
Click to Show/Hide
|
|||
| External Link | ||||
| VTP-43742 | Phase 2 | [28] | ||
| External Link | ||||
| Bertilimumab | Phase 2 | [28] | ||
| Synonyms |
CAT-213; ICo-008
Click to Show/Hide
|
|||
| External Link | ||||
| LEO 90100 | Phase 2 | [40] | ||
| External Link | ||||
| PF-06700841 | Phase 2 | [28] | ||
| Synonyms |
BUWBRTXGQRBBHG-RUXDESIVSA-N; 2140301-96-6; PF-06700841 free base; EX-A2762; 1883299-62-4; ((S)-2,2-difluorocyclopropyl)(3-(2-((1-methyl-1H-pyrazol-4-yl)amino)pyrimidin-4-yl)-3,8-diazabicyclo[3.2.1]octan-8-yl)methanone
Click to Show/Hide
|
|||
| External Link | ||||
| LEO-32731 | Phase 1 | [41] | ||
| Synonyms |
LP0058
Click to Show/Hide
|
|||
| External Link | ||||
| Ascrolimus | Phase 2 | [42] | ||
| Synonyms |
A-86281; ABT-281; ZK-248258
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-582949 | Phase 2 | [43] | ||
| Synonyms |
623152-17-0; BMS 582949; UNII-CR743OME9E; BMS582949; CR743OME9E; PS540446; CHEMBL1230065; PS-540446; 4-{[5-(cyclopropylcarbamoyl)-2-methylphenyl]amino}-5-methyl-N-propylpyrrolo[2,1-f][1,2,4]triazine-6-carboxamide; 3mvl; SCHEMBL254996; GTPL7838; DTXSID90211380; MolPort-044-560-326; EX-A1265; BCP14356; ZINC36475284; s8124; BDBM50327009; AKOS030573299; DB12696; Pyrrolo(2,1-f)(1,2,4)triazine-6-carboxamide, 4-((5-((cyclopropylamino)carbonyl)-2-methylphenyl)amino)-5-methyl-n-propyl-; BMS582949 free base; PS 540446
Click to Show/Hide
|
|||
| External Link | ||||
| BT-061 | Phase 2 | [44] | ||
| External Link | ||||
| VB-201 | Phase 2 | [45] | ||
| External Link | ||||
| MK-0873 | Phase 2 | [46] | ||
| Synonyms |
MK-0873 (dermatological, psoriasis); MK-0873 (dermatological, psoriasis), Merck & Co
Click to Show/Hide
|
|||
| External Link | ||||
| GSK2982772 | Phase 1 | [28] | ||
| Synonyms |
LYPAFUINURXJSG-AWEZNQCLSA-N; 1622848-92-3; UNII-T5W3M0VO9B; T5W3M0VO9B; GSK-2982772; (S)-5-benzyl-N-(5-methyl-4-oxo-2,3,4,5-tetrahydrobenzo[b][1,4]oxazepin-3-yl)-1H-1,2,4-triazole-3-carboxamide; GTPL9554; SCHEMBL15956219; MolPort-044-830-634; s8484; AKOS030528033; compound 5 [PMID: 28151659]; ACN-041458; CS-6899; GSK 2982772; AS-35128; AC-29894; HY-101760; J3.650.802G; 5-benzyl-N-[(3S)-5-methyl-4-oxo-2,3-dihydro-1,5-benzoxazepin-3-yl]-1H-1,2,4-triazole-3-carboxamide; 3-Benzyl-N-[(3s)-5-Methyl-4-Oxo-2,3,4,5-Tetrahydr
Click to Show/Hide
|
|||
| External Link | ||||
| PH-10 | Phase 2 | [28] | ||
| Synonyms |
Rose Bengal sodium salt; Bengal Rose B sodium salt; CI Acid red 94; NCGC00159508-02; disodium 2,3,4,5-tetrachloro-6-(2,4,5,7-tetraiodo-3-oxido-6-oxoxanthen-9-yl)benzoate; DSSTox_CID_2814; AC1L1NH9; AC1MC45U; DSSTox_RID_76740; DSSTox_GSID_22814; DTXSID4022814; CHEMBL2359093; CTK8G2989; Rose bengal, Dye content 95 %; UWBXIFCTIZXXLS-UHFFFAOYSA-L; Tox21_111727; MFCD00005043; GT2559; AKOS015902615; AKOS024319604; MCULE-2782240614; CAS-632-69-9; Tetrachlorotetraiodofluorescein disodium salt; ST50411638; F000
Click to Show/Hide
|
|||
| External Link | ||||
| ANB-019 | Phase 2 | [47] | ||
| External Link | ||||
| IP10 C8 | Phase 2 | [48] | ||
| Synonyms |
IP10C8
Click to Show/Hide
|
|||
| External Link | ||||
| BMS-986020 | Phase 1 | [28] | ||
| Synonyms |
GQBRZBHEPUQRPL-LJQANCHMSA-N; 1257213-50-5; AP-3152 free acid; UNII-38CTP01B4L; 38CTP01B4L; SCHEMBL344742; GTPL9498; EX-A866; MolPort-044-616-249; ZINC113624125; AKOS030631907; CS-5844; AS-35060; HY-100619; FT-0700148; J-690107; Cyclopropanecarboxylic acid, 1-(4'-(3-methyl-4-((((1R)-1-phenylethoxy)carbonyl)amino)-5-isoxazolyl)(1,1'-biphenyl)-4-yl)-; 1-{4'-[3-Methyl-4-((R)-1-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}-cyclopropanecarboxylic acid; 1-[4-[4-[3-methyl-4-[[(1R)-1-phenylethoxy]carbonyl
Click to Show/Hide
|
|||
| External Link | ||||
| CMI-392 | Phase 2 | [49] | ||
| Synonyms |
LDP-392; 3-[2-[2-(4-Chlorophenylsulfanyl)ethoxy]-3-methoxy-5-[trans-5-(3,4,5-trimethoxyphenyl)tetrahydrofuran-2-yl]benzyl]-1-hydroxy-1-methylurea
Click to Show/Hide
|
|||
| External Link | ||||
| Santalum album | Phase 2 | [28] | ||
| External Link | ||||
| CT 327 | Phase 2 | [50] | ||
| External Link | ||||
| RVT-505 | Phase 2 | [10] | ||
| External Link | ||||
| Rambazole | Phase 2 | [14] | ||
| Synonyms |
Talarozole; 201410-53-9; R-115866; N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1-yl)butyl)phenyl)benzo[d]thiazol-2-amine; R115866; CHEMBL459505; C21H23N5S; Rambazole (TN); Talarozole (USAN/INN); Talarozole [USAN:INN]; R 115866; N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1-yl)butyl)phenyl)-2-benzothiazolamine; Talarozole pound> SCHEMBL721201; CHEBI:102167; MolPort-018-666-712; SNFYYXUGUBUECJ-UHFFFAOYSA-N; BCP28256; BCP21218; BDBM50253810; 0328AB; AKOS005067289; DB13083; CS-1343; NCGC00378894-01; HY-14531; AX8224298; D09385; W-5674; MEN13510
Click to Show/Hide
|
|||
| External Link | ||||
| Siplizumab | Discontinued in Phase 2 | [51] | ||
| Synonyms |
Siplizumab (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| IMO-3100 | Phase 2 | [52] | ||
| External Link | ||||
| LLL-3348 | Phase 2 | [53] | ||
| Synonyms |
Desoris; LL-3348; Herbal anti-inflammatory (psoriasis), Lupin
Click to Show/Hide
|
|||
| External Link | ||||
| Elisidepsin | Phase 2 | [54] | ||
| Synonyms |
Irvalec; Elisidepsin trifluoroacetate; PM-02734; Erbb3 tyrosine kinase receptor inhibitor (cancer), PharmaMar; Kahalalide therapy (solid tumor), PharmaMar
Click to Show/Hide
|
|||
| External Link | ||||
| Santalum | Phase 2 | [10] | ||
| External Link | ||||
| Apo805K1 | Phase 2 | [55] | ||
| External Link | ||||
| BTT-1023 | Phase 2 | [56] | ||
| Synonyms |
SI-3106; SI-636; Fully human VAP-1 mAbs (inflammation), BioTie/Seikagaku; Fully human VAP-1 monoclonal antibody (inflammation), BioTie/Seikagaku; VAP-1 antibody (rheumatoid arthritis/psoriasis), BioTie
Click to Show/Hide
|
|||
| External Link | ||||
| BFH-772 | Phase 2 | [57] | ||
| Synonyms |
NVP-BFH-772
Click to Show/Hide
|
|||
| External Link | ||||
| LL-4218 | Phase 2 | [58] | ||
| Synonyms |
Desoside-P
Click to Show/Hide
|
|||
| External Link | ||||
| BIM23A760 | Discontinued in Phase 3 | [59] | ||
| Synonyms |
Lonapalene; RS-43179; 91431-42-4; UNII-WIF31Q54NJ; WIF31Q54NJ; CHEMBL36648; Lonapalenum; Lonapaleno; Lonapalenum [Latin]; Lonapaleno [Spanish]; Lonapalene (USAN); Lonapalene [USAN:INN]; RS 43179; 6-Chloro-2,3-dimethoxy-1,4-naphthalenediol diacetate; (4-acetyloxy-6-chloro-2,3-dimethoxynaphthalen-1-yl) acetate; AC1L1KN9; SCHEMBL120134; ZINC1630; DTXSID00238563; IFWMVQUGSGWCRP-UHFFFAOYSA-N; HY-U00156; BDBM50004677; CS-7197; ZB000377; D04770; 6-chloro-1,4-diacetoxy-2,3-dimethoxynaphthalene
Click to Show/Hide
|
|||
| External Link | ||||
| AN0128 | Phase 2 | [60] | ||
| Synonyms |
Bis(3-chloro-4-methyl-phenyl)boranyl 3-hydroxypyridine-2-carboxylate; 2-Pyridinecarboxylic acid, 3-hydroxy-, anhydride with bis(3-chloro-4-methylphenyl)borinic acid; 3-Hydroxypyridine-2-carbonyloxy-bis(3-chloro-4-methylphenyl)borane
Click to Show/Hide
|
|||
| External Link | ||||
| DPS-102 | Phase 2 | [61] | ||
| Synonyms |
Non-steroidal anti-inflammatory (topical dermatological, scalp psoriasis), DermiPsor
Click to Show/Hide
|
|||
| External Link | ||||
| LEO 22811 | Phase 2 | [62] | ||
| External Link | ||||
| E6201 | Phase 2 | [63] | ||
| Synonyms |
novel MEK inhibitors
Click to Show/Hide
|
|||
| External Link | ||||
| Fezakinumab | Phase 2 | [64] | ||
| Synonyms |
Fezakinumab (IV), fezakinumab (SC), ILV-094 (IV), ILV-094 (SC), PF-5212367 (IV) PF-5212367(SC)
Click to Show/Hide
|
|||
| External Link | ||||
| NN-8226 | Phase 2 | [65] | ||
| Synonyms |
NNC-109-0012; IL-20 monoclonal antibody (inflammation), Novo Nordisk; Anti-IL20 (inflammation), ZymoGenetics/Novo Nordisk; Anti-IL20mAb (RA), ZymoGenetics/Novo Nordisk; Anti-interleukin-20 (rheumatoid arthritis), ZymoGenetics/Novo Nordisk
Click to Show/Hide
|
|||
| External Link | ||||
| Lunacalcipol | Phase 2 | [66] | ||
| Synonyms |
CTA-018; MT-2832; Lunacalcipol (intravenous, secondary hyperparathyroidism); Lunacalcipol (intravenous, secondary hyperparathyroidism), Cytochroma; Vitamin D analogs (secondary hyperparathyroidism), Johns Hopkins/Cytochroma; CTA-018 (intravenous, secondary hyperparathyroidism), Cytochroma; CYP24 inhibitors (intravenous, secondary hyperparathyroidism), Johns Hopkins/Cytochroma
Click to Show/Hide
|
|||
| External Link | ||||
| Dalazatide | Phase 1b/2a | [67] | ||
| Synonyms |
Dalazatide [INN]; UNII-6U0259J807; 1081110-69-1; o-PHOSPHONO-L-Tyrosyl-2-(2-(2-aminoethoxy)ethoxy)acetyl(potassium channel toxin kappa-stichotoxin-shela stoichactis helianthus (caribbean sea anemone)) peptidamide; 6U0259J807
Click to Show/Hide
|
|||
| External Link | ||||
| Debio-0824 | Phase 1b/2a | [67] | ||
| Synonyms |
Shk-192; ShK analogs (Subcutaneous formulation, autoimmune disease), Kineta; Stichodactyla helianthus-derived Kv1.3 potassium channel inhibitors (injectable peptide, autoimmune disease); Stichodactyla helianthus-derived Kv1.3 potassium channel inhibitor (Subcutaneous formulation, autoimmune disease), Airmid; Stichodactyla helianthus-derived Kv1.3 potassium channel inhibitor (injectable peptide, autoimmune disease), Davis; Stichodactyla helianthus-derived Kv1.3 potassium channel inhibitors (injectable peptide, autoimmune disease), Kineta One
Click to Show/Hide
|
|||
| External Link | ||||
| COVA322 | Phase 1/2a | [68] | ||
| External Link | ||||
| AS-210 | Phase 1/2 | [69] | ||
| Synonyms |
Psoraxine; AS-1001; AS-1002; AS-200; Psoriasis immunotherapy, Astralis/SkyePharma
Click to Show/Hide
|
|||
| External Link | ||||
| ART621 | Phase 1/2 | [14] | ||
| External Link | ||||
| JNJ-1459 | Phase 1 | [70] | ||
| External Link | ||||
| XCUR17 | Phase 1 | [10] | ||
| External Link | ||||
| CM2489 | Phase 1 | [71] | ||
| External Link | ||||
| EDP1066 | Phase 1 | [10] | ||
| External Link | ||||
| BAY1834845 | Phase 1 | [10] | ||
| External Link | ||||
| LEO-27989 | Phase 1 | [72] | ||
| External Link | ||||
| SNA-125 | Phase 1 | [10] | ||
| External Link | ||||
| ONS-3010 | Phase 1 | [28] | ||
| External Link | ||||
| CC-90006 | Phase 1 | [10] | ||
| External Link | ||||
| Pc4 | Phase 1 | [73] | ||
| Synonyms |
Pc4 (topical formulation, cancer/psoriasis/dermatological disease) Fluence/CWRU/UHCMC
Click to Show/Hide
|
|||
| External Link | ||||
| PRX003 | Phase 1 | [28] | ||
| External Link | ||||
| JNJ-3534 | Phase 1 | [10] | ||
| External Link | ||||
| BBI-6000 | Phase 1 | [10] | ||
| External Link | ||||
| PF-06263276 | Phase 1 | [74] | ||
| External Link | ||||
| ALX-0761 | Phase 1 | [75] | ||
| External Link | ||||
| ILV-095 | Phase 1 | [76] | ||
| Synonyms |
PF-05212368, WAY-264095
Click to Show/Hide
|
|||
| External Link | ||||
| ARN-6039 | Phase 1 | [28] | ||
| Synonyms |
BOS172767
Click to Show/Hide
|
|||
| External Link | ||||
| LY3316531 | Phase 1 | [10] | ||
| External Link | ||||
| SAFINGOL | Phase 1 | [77] | ||
| Synonyms |
Kynac; Kynacyte; SPC-100270; Safingol < Rec INN; L-threo-Dihydrosphingosine; SPC-100271 (HCl); (2S,3S)-2-Aminooctadecane-1,3-diol
Click to Show/Hide
|
|||
| External Link | ||||
| BCT-194 | Phase 1 | [78] | ||
| External Link | ||||
| SB414 | Phase 1 | [10] | ||
| External Link | ||||
| AST-005 | Phase 1 | [10] | ||
| External Link | ||||
| Quinazoline derivative 12 | Patented | [79] | ||
| Synonyms |
PMID26936077-Compound-23
Click to Show/Hide
|
|||
| External Link | ||||
| Quinazoline derivative 10 | Patented | [79] | ||
| Synonyms |
PMID26936077-Compound-21
Click to Show/Hide
|
|||
| External Link | ||||
| Quinazoline derivative 11 | Patented | [79] | ||
| Synonyms |
PMID26936077-Compound-22
Click to Show/Hide
|
|||
| External Link | ||||
| PMID27998201-Compound-5 | Patented | [80] | ||
| External Link | ||||
| VX-148 | Patented | [81] | ||
| Synonyms |
SCHEMBL12343182; BDBM248095; VX-148 (3)
Click to Show/Hide
|
|||
| External Link | ||||
| IRX-4310 | Discontinued in Phase 3 | [82] | ||
| Synonyms |
AGN-194310; AGN-4310; ALRT-4310; LGD-4310; NRX-4310; RARa antagonist (oral, chemotherapy-induced neutropenia), Io Therapeutics; Retinoic acid receptor alpha antagonist (oral, chemotherapy-induced neutropenia), Io Therapeutics
Click to Show/Hide
|
|||
| External Link | ||||
| Etarotene | Discontinued in Phase 3 | [83] | ||
| Synonyms |
Ro-15-1570; Ro-15-1570/000
Click to Show/Hide
|
|||
| External Link | ||||
| KC706 | Discontinued in Phase 2 | [84] | ||
| External Link | ||||
| VTP-201227 | Discontinued in Phase 2 | [85] | ||
| External Link | ||||
| IC-747 | Discontinued in Phase 2 | [86] | ||
| Synonyms |
LFA-1 antagonists, ICOS/Biogen
Click to Show/Hide
|
|||
| External Link | ||||
| CRx-191 | Discontinued in Phase 2 | [87] | ||
| External Link | ||||
| LAS-37779 | Discontinued in Phase 2 | [88] | ||
| External Link | ||||
| TU-2100 | Discontinued in Phase 2 | [89] | ||
| External Link | ||||
| ASF-1075 | Discontinued in Phase 2 | [90] | ||
| External Link | ||||
| BMS-587101 | Discontinued in Phase 2 | [91] | ||
| Synonyms |
BMS-688521
Click to Show/Hide
|
|||
| External Link | ||||
| R-68151 | Discontinued in Phase 2 | [92] | ||
| Synonyms |
1-Ethyl-3-{4-[4-(4-hydroxy-phenyl)-piperazin-1-yl]-phenyl}-5,5-dimethyl-2-thioxo-imidazolidin-4-one; 5-Lipoxygenase-In-1; R 68151; CHEMBL85599; 138331-04-1; AC1L30HG; SCHEMBL13696698; DTXSID10160583; BDBM50006793; HY-U00308; CS-7300; (1-Ethyl-3(4-(4-(4-hydroxyphenyl)-1-piperazinyl)phenyl)-5-dimethyl)-2-thioxo-4-imidazolidinone; 1-ethyl-3-[4-[4-(4-hydroxyphenyl)piperazin-1-yl]phenyl]-5,5-dimethyl-2-sulfanylideneimidazolidin-4-one; M55551
Click to Show/Hide
|
|||
| External Link | ||||
| T487 | Discontinued in Phase 2 | [93] | ||
| Synonyms |
AC1MVK5U; 2-(2,4-diethyloctoxy)-N-[2-(2,4-diethyloctoxy)ethyl]-N-methyl-ethanamine; 2-(2,4-diethyloctoxy)-N-[2-(2,4-diethyloctoxy)ethyl]-N-methylethanamine
Click to Show/Hide
|
|||
| External Link | ||||
| SDZ-LAP-977 | Discontinued in Phase 2 | [94] | ||
| Synonyms |
SDZ-281-977
Click to Show/Hide
|
|||
| External Link | ||||
| VML-262 | Discontinued in Phase 2 | [95] | ||
| Synonyms |
Marigold compound (topical, psoriasis), University of Strathclyde; VAN-10-4 (topical, psoriasis), University of Strathclyde/Vanguard Medica
Click to Show/Hide
|
|||
| External Link | ||||
| SB-201993 | Discontinued in Phase 2 | [96] | ||
| Synonyms |
UNII-4TWC061LPM; 4TWC061LPM; CHEMBL422598; SB 201993; AC1O5KJ7; SCHEMBL1893839; SCHEMBL1893835; sb201993; BDBM50037385; (E)-3-((((6-(2-Carboxyethenyl)-5-((8-(4-methoxyphenyl)octyl)oxy)-2-pyridinyl)methyl)thio)methyl)benzoic acid; L009321; 3-[[6-[(E)-3-hydroxy-3-oxoprop-1-enyl]-5-[8-(4-methoxyphenyl)octoxy]pyridin-2-yl]methylsulfanylmethyl]benzoic acid; 3-{6-((E)-2-Carboxy-vinyl)-5-[8-(4-methoxy-phenyl)-octyloxy]-pyridin-2-ylmethylsulfanylmethyl}-benzoic acid; Benzoic acid, 3-((((6-(2-carboxyethenyl)-5-((8-(4-m
Click to Show/Hide
|
|||
| External Link | ||||
| PVAC | Discontinued in Phase 2 | [97] | ||
| Synonyms |
DDMV, Corixa/ Genesis/ Zenyaku Kogyo; Delipidated, deglycolipidated Mycobacterium vaccae, Corixa/ Genesis/ Zenyaku Kogyo
Click to Show/Hide
|
|||
| External Link | ||||
| Tisocalcitate | Discontinued in Phase 2 | [98] | ||
| Synonyms |
SH-597; ZK-156942; Calcitriol analog (topical formulation), Schering AG
Click to Show/Hide
|
|||
| External Link | ||||
| BAL-2299 | Discontinued in Phase 2 | [99] | ||
| Synonyms |
Cyclohexanediol, Basilea
Click to Show/Hide
|
|||
| External Link | ||||
| Atocalcitol | Discontinued in Phase 2 | [100] | ||
| Synonyms |
KH-1650
Click to Show/Hide
|
|||
| External Link | ||||
| FPL-64170 | Discontinued in Phase 2 | [101] | ||
| External Link | ||||
| BIRT 2584 | Discontinued in Phase 2 | [91] | ||
| Synonyms |
QSNSVPRLHYSSQG-AMXDTQDGSA-N; UNII-24P4O1J71F; 24P4O1J71F; SCHEMBL4017930; BIRT-2584; (S)-2-[(R)-7-(3,5-Dichloro-phenyl)-5-methyl-6-oxo-5-(4-trifluoromethoxy-benzyl)-6,7-dihydro-5H-imidazo[1,2-a]imidazole-3-sulfonylamino]-propionamide; 688756-00-5
Click to Show/Hide
|
|||
| External Link | ||||
| SB 235699 | Discontinued in Phase 1 | [102] | ||
| Synonyms |
4-(4-(4-Fluorophenyl)-1-(piperidin-4-yl)-1H-imidazol-5-yl)pyridine; VK-19911; CHEMBL279416; UNII-NP7J08ZRYY; NP7J08ZRYY; 180869-32-3; SCHEMBL140202; HEP 689; sb235699; BDBM50099331; SB-235699; 4-(5-(4-Fluorophenyl)-3-(4-piperidyl)imidazol-4-yl)pyridine; Pyridine, 4-(4-(4-fluorophenyl)-1-(4-piperidinyl)-1H-imidazol-5-yl)-
Click to Show/Hide
|
|||
| External Link | ||||
| PD-153035 | Discontinued in Phase 1 | [103] | ||
| Synonyms |
183322-45-4; PD153035 hydrochloride; N-(3-Bromophenyl)-6,7-dimethoxyquinazolin-4-amine hydrochloride; PD153035 HCl; PD 153035 HYDROCHLORIDE; PD-153035 hydrochloride; PD153035 (Hydrochloride); Tyrphostin AG 1517; AG 1517 hydrochloride; SU-5271 hydrochloride; UNII-AHJ252P69N; ZM 252868; SU 5271; AG 1517; 4-[(3-BROMOPHENYL)AMINO]-6,7-DIMETHOXYQUINAZOLINE HYDROCHLORIDE; AHJ252P69N; pd 153035; PD153035.HCl; CHEMBL1204168; 6,7-Dimethoxy-4-[N-(3-bromophenyl)amino]quinazoline hydrochloride; C16H15BrClN3O2; 4-Quinazolinamine, N-(3-bromo
Click to Show/Hide
|
|||
| External Link | ||||
| ALS-00T2-0501 | Discontinued in Phase 1 | [104] | ||
| Synonyms |
TNF Blocker (Intradel, psoriasis), Apollo Life Sciences; TNF Blocker (TransD, psoriasis), Apollo Life Sciences; TNF Blocker (transdermal cream, psoriasis), Apollo Life Sciences; TNF receptor antagonist (Intradel, psoriasis), Apollo Life Sciences; TNF receptor antagonist (TransD, psoriasis), Apollo Life Sciences; TNF receptor antagonist (transdermal cream, psoriasis), Apollo Life Sciences
Click to Show/Hide
|
|||
| External Link | ||||
| CCX-832 | Discontinued in Phase 1 | [105] | ||
| Synonyms |
ChemR2 receptor antagonist (inflammation), ChemoCentryx/GlaxoSmithKline
Click to Show/Hide
|
|||
| External Link | ||||
| CLS008 | Application submitted | [10] | ||
| External Link | ||||
| DYV024 | Preclinical | [106] | ||
| External Link | ||||
| VCB102 | Preclinical | [107] | ||
| External Link | ||||
| RAP-160 | Preclinical | [108] | ||
| External Link | ||||
| ATL-1101 | Preclinical | [109] | ||
| External Link | ||||
| Hu Dreg 55 | Terminated | [110] | ||
| Synonyms |
SMART Anti-L-Selectin
Click to Show/Hide
|
|||
| External Link | ||||
| DB-200 | Terminated | [111] | ||
| Synonyms |
DB-200 series (topical, psoriasis); CPT-1 inhibitor (topical, psoriasis), DARA; DB-200 series (topical, psoriasis), DARA BioSciences
Click to Show/Hide
|
|||
| External Link | ||||
| MOR-102 | Terminated | [112] | ||
| Synonyms |
MOR-101; MOR-101); ICAM-1 antibody (inflammation), MorphoSys
Click to Show/Hide
|
|||
| External Link | ||||
| YP-008 | Terminated | [113] | ||
| Synonyms |
RPL-228; Cyclopentenone prostaglandin mimetic (PGM) (topical, psoriasis), York Pharma; Natural-defence-prostanoid mimetic (topical, psoriasis), York Pharma
Click to Show/Hide
|
|||
| External Link | ||||
| CRA-028129 | Terminated | [114] | ||
| External Link | ||||
| SB-209247 | Terminated | [115] | ||
| Synonyms |
Ticolubant; LTB4 antagonist, SB
Click to Show/Hide
|
|||
| External Link | ||||
| AD-177 | Terminated | [116] | ||
| External Link | ||||
| R348 | Terminated | [14] | ||
| External Link | ||||
| FM-301 | Investigative | [117] | ||
| External Link | ||||
| AZ-17 | Investigative | [117] | ||
| External Link | ||||
| Hairpin RNA | Investigative | [117] | ||
| Synonyms |
Hairpin RNA (psoriasis); Hairpin RNA (psoriasis), SomaGenics; ShRNA (psoriasis), SomaGenics
Click to Show/Hide
|
|||
| External Link | ||||
| KM-133 | Investigative | [117] | ||
| External Link | ||||
| PU-2049 | Investigative | [117] | ||
| Synonyms |
N-[2-(5-Methoxyindol-3-yl)ethyl]-10-undecenamide; 5-Methoxy-N-(10-undecenoyl)tryptamine
Click to Show/Hide
|
|||
| External Link | ||||
| ASB-16165 | Investigative | [117] | ||
| Synonyms |
PDE7 inhibitor (topical, psoriasis), Asubio
Click to Show/Hide
|
|||
| External Link | ||||
| Amphiregulin targeting human mabs | Investigative | [117] | ||
| Synonyms |
Amphiregulin targeting human mAbs (psoriasis)
Click to Show/Hide
|
|||
| External Link | ||||
| GSK-2263167 | Investigative | [117] | ||
| Synonyms |
S1P1 agonists (psoriasis), GlaxoSmithKline; Sphingosine 1 phosphate-1 agonists (psoriasis); Sphingosine 1 phosphate-1 agonists (psoriasis), GlaxoSmithKline
Click to Show/Hide
|
|||
| External Link | ||||
| DPS-151 | Investigative | [117] | ||
| External Link | ||||
| LPD-1050 | Investigative | [117] | ||
| External Link | ||||
| CXCL8 | Investigative | [117] | ||
| Synonyms |
Interleukin-8; CHEMBL411250; IL-8
Click to Show/Hide
|
|||
| External Link | ||||
| DM-512 | Investigative | [117] | ||
| External Link | ||||
| KIN-4050 | Investigative | [117] | ||
| Synonyms |
ERK inhibitor (topical, psoriasis), Kinentia; MAP kinase inhibitor (topical, psoriasis), Kinentia
Click to Show/Hide
|
|||
| External Link | ||||
| Ankinara | Investigative | [118] | ||
| External Link | ||||
| BL-7020 | Investigative | [117] | ||
| Synonyms |
Recombinant IGFBP (intradermal, psoriasis), BioLineRx
Click to Show/Hide
|
|||
| External Link | ||||
| X-083-NAB | Investigative | [117] | ||
| Synonyms |
NATHMAB (psoriasis/Crohns disease/type 1 diabetes), XBiotech; Unspecified cytokine receptor antagonist (human monoclonal antibody, psoriasis/Crohns disease/type 1 diabetes), XBiotech
Click to Show/Hide
|
|||
| External Link | ||||
| LEO-90110 | Investigative | [117] | ||
| External Link | ||||
References