m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05098
|
[1] | |||
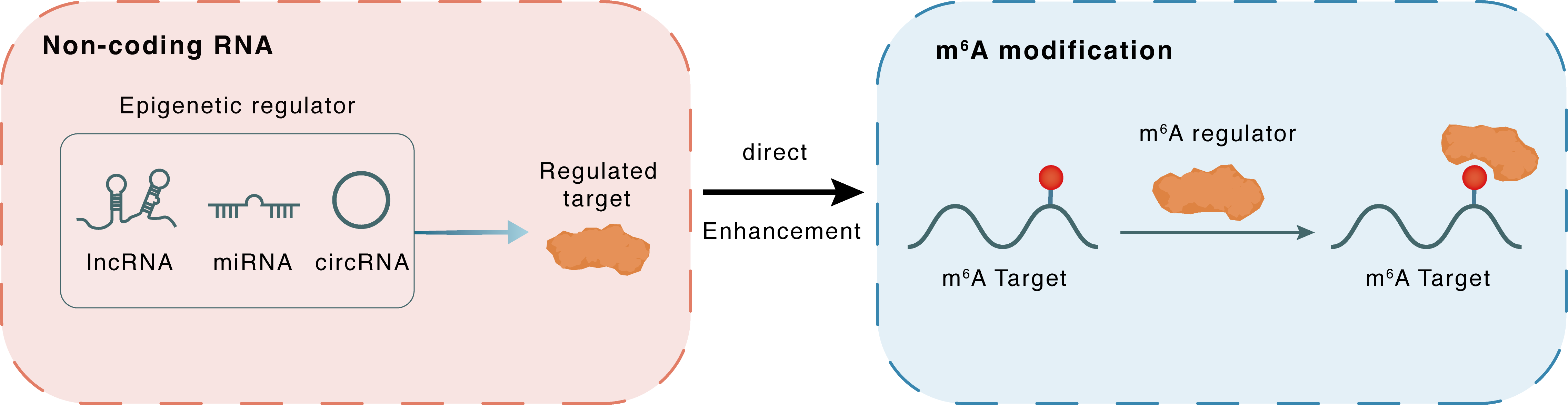 Non-coding RNA
HNF1A-AS1
IGF2BP2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
CCND1
CCND1
IGF2BP2
Non-coding RNA
HNF1A-AS1
IGF2BP2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
CCND1
CCND1
IGF2BP2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) | READER | |||
| m6A Target | G1/S-specific cyclin-D1 (CCND1) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | HNF1A antisense RNA 1 (HNF1A-AS1) | LncRNA | View Details | ||
| Regulated Target | Insulin like growth factor 2 mRNA binding protein 2 (IGF2BP2) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | m6A Modification of Long Non-Coding RNA HNF1A-AS1 Facilitates Cell Cycle Progression in Colorectal Cancer via IGF2BP2-Mediated G1/S-specific cyclin-D1 (CCND1) mRNA Stabilization | ||||
| Responsed Disease | Colorectal cancer | ICD-11: 2B91 | |||
In-vitro Model |
HCT 116 | Colon carcinoma | Homo sapiens | CVCL_0291 | |
| HT-29 | Colon adenocarcinoma | Homo sapiens | CVCL_0320 | ||
| SW480 | Colon adenocarcinoma | Homo sapiens | CVCL_0546 | ||
| SW620 | Colon adenocarcinoma | Homo sapiens | CVCL_0547 | ||
| DLD-1 | Colon adenocarcinoma | Homo sapiens | CVCL_0248 | ||
| LoVo | Colon adenocarcinoma | Homo sapiens | CVCL_0399 | ||
| HUVEC-C | Normal | Homo sapiens | CVCL_2959 | ||
| HCoEpiC (Healthy colon epithelial HCoEpiC cells) | |||||
| In-vivo Model | Ten BALB/C nude mice (4 weeks old, female) were injected in 1 × 106 HCT116 cells in 100 uL PBS at each side. The tumor size was detected every four days after the injection of cells and calculated according to the formula. | ||||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| G1/S-specific cyclin-D1 (CCND1) | 8 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| ABT-263 | Phase 3 | [2] | ||
| Synonyms |
Navitoclax; ABT 263; S1001_Selleck; ABT263, Navitoclax; 4-(4-{[2-(4-chlorophenyl)-5,5-dimethylcyclohex-1-en-1-yl]methyl}piperazin-1-yl)-N-({4-({(1R)-3-morpholin-4-yl-1-[(phenylsulfanyl)methyl]propyl}amino)-3-[(trifluoromethyl)sulfonyl]phenyl}sulfonyl)benzamide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| Briciclib | Phase 1 | [3] | ||
| Synonyms |
865783-99-9; ON-014185; UNII-WG93X96336; WG93X96336; ON 014185; Briciclib [USAN:INN]; Briciclib (USAN/INN); SCHEMBL1634579; SCHEMBL1634581; CHEMBL1206245; MolPort-046-033-539; LXENKEWVEVKKGV-BQYQJAHWSA-N; EX-A2492; BCP17292; ZINC28965775; AKOS027439966; DB12004; CS-5589; HY-16366; KB-79924; Briciclib(ON 013105 ON 014185); ON-013105; D10614; (2-methoxy-5-{[(E)-2-(2,4,6-trimethoxyphenyl)ethenesulfonyl]methyl}phenoxy)phosphonic acid; (e)-5-((2,4,6-trimethoxystyrylsulfonyl)methyl)-2-methoxyphenyl dihydro
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-hydroxycoumarin | Investigative | [4] | ||
| Synonyms |
Umbelliferone; 93-35-6; 7-Hydroxy-2H-chromen-2-one; Skimmetin; Hydrangin; 7-hydroxycoumarine; 7-Oxycoumarin; Umbelliferon; Skimmetine; Hydrangine; 2H-1-Benzopyran-2-one, 7-hydroxy-; 7-Hydroxy-2H-1-benzopyran-2-one; Coumarin, 7-hydroxy-; beta-Umbelliferone; 7-hydroxychromen-2-one; 7 HC; UNII-60Z60NTL4G; 7-hydroxy-coumarin; NSC 19790; CCRIS 3591; NSC19790; EINECS 202-240-3; 7H-1-Benzopyran-7-one, 2-hydroxy-; BRN 0127683; CHEMBL51628; AI3-38054; 7-hydroxy-1-benzopyran-2-one; 7-HC; 60Z60NTL4G; CHEBI:27510
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(indole-3-yl)-4-phenyl-1H-pyrrole-2,5-dione | Investigative | [5] | ||
| Synonyms |
CHEMBL380598; SCHEMBL3148490; HVQJGNALTWNDMX-UHFFFAOYSA-N; BDBM50375058; 2-(1H-Indole-3-yl)-3-phenylmaleimide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3,4-di-(4-methoxyphenyl)-1H-pyrrole-2,5-dione | Investigative | [5] | ||
| Synonyms |
1H-Pyrrole-2,5-dione, 3,4-bis(4-methoxyphenyl)-; 108774-82-9; ACMC-20mbs9; CHEMBL381099; CTK0G2626; DTXSID90449388
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3,4-diphenyl-1H-pyrrole-2,5-dione | Investigative | [5] | ||
| Synonyms |
2,3-diphenylmaleimide; 1H-Pyrrole-2,5-dione, 3,4-diphenyl-; 31295-36-0; AC1MBL6S; SCHEMBL114611; CHEMBL201949; CTK1B9880; 3,4-diphenylpyrrole-2,5-dione; DTXSID70372903; ZINC3847556
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 3-(4-methoxyphenyl)-4-phenyl-1H-pyrrole-2,5-dione | Investigative | [5] | ||
| Synonyms |
CHEMBL372076; SCHEMBL3822337
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-[(3,5-diamino-1H-pyrazol-4-yl)diazenyl]phenol | Investigative | [6] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| 2B91: Colorectal cancer | 25 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Retifanlimab | Approved | [7] | ||
| Synonyms |
INCMGA0012; Retifanlimab
Click to Show/Hide
|
|||
| External Link | ||||
| Aflibercept | Approved | [8] | ||
| Synonyms |
Ziv-Aflibercept; Zaltrap (TN); VEGF Trap; VEGF Trap-Eye
Click to Show/Hide
|
|||
| External Link | ||||
| Regorafenib | Approved | [9] | ||
| Synonyms |
755037-03-7; BAY 73-4506; Regorafenibum; Stivarga; 4-(4-(3-(4-Chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide; BAY73-4506; Regorafenib (BAY 73-4506); UNII-24T2A1DOYB; 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methylpyridine-2-carboxamide; BAY-73-4506; 24T2A1DOYB; CHEMBL1946170; CHEBI:68647; Stivarga (TN); BAY73-4506 hydrochloride; Regorafenib [USAN:INN]
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [2] | ||
| Synonyms |
Bevacizumab (ophthalmic slow-release tissue tablet)
Click to Show/Hide
|
|||
| External Link | ||||
| SYM-004 | Phase 3 | [2] | ||
| Synonyms |
Chimeric IgG1 antibody 1024 (cancer), Symphogen; Chimeric IgG1 antibody 992 (cancer), Symphogen; Chimeric IgG1 antibodies992 + 1024 (cancer), Symphogen
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab + Erlotinib | Phase 3 | [10] | ||
| External Link | ||||
| CPI-613 | Phase 3 | [2] | ||
| Synonyms |
95809-78-2; 6,8-bis(benzylthio)octanoic acid; CPI 613; MLS006010202; SCHEMBL1062218; 6,8-Bis(benzylsulfanyl)octanoic acid; Octanoic acid, 6,8-bis((phenylmethyl)thio)-; Octanoic acid, 6,8-bis[(phenylMethyl)thio]-; 6,8-Bis[(phenylmethyl)thio]octanoic acid; CPI613; CHEMBL3186849; QCR-193; AOB1058; MolPort-023-219-128; HMS3656L06; C22H28O2S2; BCP04663; EX-A2043; s2776; AKOS025142095; BCP9000552; DB12109; RL06062; CS-0961; NCGC00344764-01; SMR004701300; AS-16613; BC261916; AK174899; HY-15453; BCP0726000030; KB-293127; AB0035874
Click to Show/Hide
|
|||
| External Link | ||||
| Bevacizumab | Approved | [8] | ||
| External Link | ||||
| AlloStim | Phase 2/3 | [11] | ||
| Synonyms |
AlloStim (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Sibrotuzumab | Phase 2 | [12] | ||
| External Link | ||||
| CV301 | Phase 2 | [13] | ||
| External Link | ||||
| Efatutazone | Phase 2 | [14] | ||
| Synonyms |
Inolitazone; 223132-37-4; 5-[[4-[[6-(4-amino-3,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-yl]methoxy]phenyl]methyl]-2,4-Thiazolidinedione; Efatutazone [INN]; RS5444; CS-7017; SCHEMBL3246054; CHEMBL3545280; JCYNMRJCUYVDBC-UHFFFAOYSA-N; Efatutazone;CS-7017;RS5444; BCP07478; AKOS030526729; DB11894; CS-0778; KB-77905; DA-07988; HY-14792; QC-10456; 4CA-1384; FT-0737589; 5-[4-[6-(4-amino-3 ,5-dimethylphenoxy)-1-methyl-1H-benzimidazol-2-ylmethoxy]benzyl]thiazolidine-2,4-dione
Click to Show/Hide
|
|||
| External Link | ||||
| LOR-2040 | Phase 2 | [15] | ||
| External Link | ||||
| RG7221 | Phase 2 | [16] | ||
| External Link | ||||
| PEG-SN38 | Phase 2 | [17] | ||
| Synonyms |
EZN-2208
Click to Show/Hide
|
|||
| External Link | ||||
| MEGF0444A | Phase 2 | [18] | ||
| External Link | ||||
| Encapsulated cell therapy | Phase 1/2 | [19] | ||
| External Link | ||||
| AB928 | Phase 1/2 | [20] | ||
| External Link | ||||
| MGD007 | Phase 1 | [16] | ||
| External Link | ||||
| BNC-101 | Phase 1 | [21] | ||
| External Link | ||||
| Navicixizumab | Phase 1 | [2] | ||
| External Link | ||||
| RG7160 | Discontinued in Phase 2 | [22] | ||
| External Link | ||||
| Nimesulide | Terminated | [23] | ||
| Synonyms |
51803-78-2; N-(4-Nitro-2-phenoxyphenyl)methanesulfonamide; Mesulid; Flogovital; Sulidene; Nimed; R-805; 4-NITRO-2-PHENOXYMETHANESULFONANILIDE; Nisulid; Nimesulidum [INN-Latin]; Nimesulida [INN-Spanish]; R 805; UNII-V4TKW1454M; 4-Nitro-2-phenoxy-methanesulfonanilide; 4'-Nitro-2'-phenoxymethanesulfonanilide; Methanesulfonamide, N-(4-nitro-2-phenoxyphenyl)-; EINECS 257-431-4; 4'-Nitro-2'-phenoxymethansulfonanilid; BRN 2421175; CHEMBL56367; MLS000069680; V4TKW1454M; Methanesulfonanilide, 4'-nitro-2'-phenoxy-; CHEBI:44445; Dulanermin
Click to Show/Hide
|
|||
| External Link | ||||
| Saracatinib | Phase 2 | [24] | ||
| External Link | ||||
| G3139 + Irinotecan | Investigative | [25] | ||
| External Link | ||||
References