m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05097
|
[1] | |||
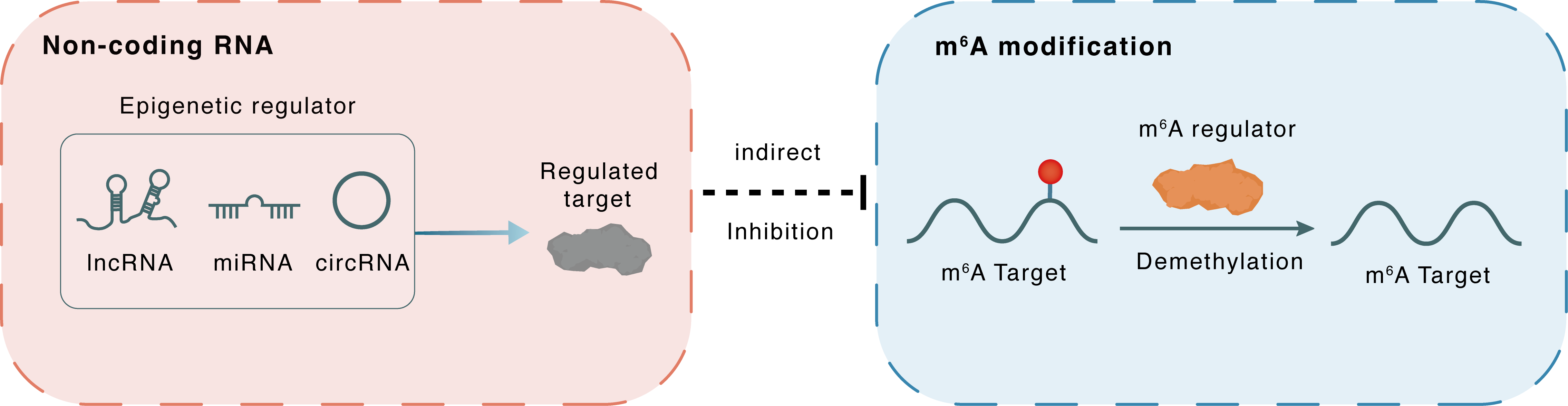 Non-coding RNA
miR-155
HIF1A
lncRNA miRNA circRNA
Indirect
Inhibition
m6A modification
SOX2
SOX2
ALKBH5
Demethylation
Non-coding RNA
miR-155
HIF1A
lncRNA miRNA circRNA
Indirect
Inhibition
m6A modification
SOX2
SOX2
ALKBH5
Demethylation
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | RNA demethylase ALKBH5 (ALKBH5) | ERASER | |||
| m6A Target | Transcription factor SOX-2 (SOX2) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | MicroRNA 155 (MIR155) | microRNA | View Details | ||
| Regulated Target | Hypoxia-inducible factor 1-alpha (HIF-1-Alpha/HIF1A) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Inhibition | |||
| Crosstalk Mechanism | ncRNAs indirectly impacts m6A modification through downstream signaling pathways | ||||
| Crosstalk Summary | Inhibition of MIR155 promoted the expression of Hypoxia-inducible factor 1-alpha (HIF-1-Alpha/HIF1A) and attenuated the m6A modification of Transcription factor SOX-2 (SOX2) mRNA by regulating ALKBH5, thereby promoting the expression of SOX2 and activating the downstream EGFR/MEK/ERK signaling pathway to promote wound healing in an in vitro DFU model. | ||||
| Responsed Disease | Diabetic foot ulcers | ICD-11: BD54 | |||
In-vitro Model |
HaCaT | Normal | Homo sapiens | CVCL_0038 | |
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Hypoxia-inducible factor 1-alpha (HIF-1-Alpha/HIF1A) | 21 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| PT2385 | Phase 2 | [2] | ||
| Synonyms |
ONBSHRSJOPSEGS-INIZCTEOSA-N; PT-2385; UNII-6O16716DXP; 1672665-49-4; 6O16716DXP; SCHEMBL16555810; ZINC230453533; AKOS030526641; HY-12867; PT2385,1672665-49-4, PT 2385,PT-2385; Benzonitrile, 3-(((1S)-2,2-difluoro-2,3-dihydro-1-hydroxy-7-(methylsulfonyl)-1H-inden-4-yl)oxy)-5-fluoro-; 3-{[(1s)-2,2-Difluoro-1-Hydroxy-7-(Methylsulfonyl)-2,3-Dihydro-1h-Inden-4-Yl]oxy}-5-Fluorobenzonitrile; 3-(((1S)-2,2-Difluoro-1-hydroxy-7-methanesulfonyl-2,3-dihydro-1hinden-4-yl)oxy)-5-fluorobenzonitrile; 79A
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| ISIS 298697 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298744 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298746 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298745 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298743 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298702 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298700 | Investigative | [3] | ||
| External Link | ||||
| ISIS 175510 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298699 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298712 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298711 | Investigative | [3] | ||
| External Link | ||||
| ISIS 298701 | Investigative | [3] | ||
| External Link | ||||
| (5-(1-benzyl-1H-indazol-3-yl)furan-2-yl)methanol | Investigative | [4] | ||
| Synonyms |
Lificiguat; yc-1; 170632-47-0; 3-(5'-Hydroxymethyl-2'-furyl)-1-benzylindazole; YC 1; UNII-515CC1WPTE; Lificiguat(YC-1); 154453-18-6; [5-(1-benzyl-1h-indazol-3-yl)-2-furyl]methanol; 515CC1WPTE; CHEMBL333985; OQQVFCKUDYMWGV-UHFFFAOYSA-N; C19H16N2O2; 3-(5'-Hydroxymethyl-2'-furyl)-1-benzyl indazole; 1-Benzyl-3-(5-hydroxymethyl-2-furyl)indazole; [5-(1-benzyl-1H-indazol-3-yl)furan-2-yl]methanol; 5-[1-(Phenylmethyl)-1H-indazol-3-yl]-2-furanmethanol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| HIF-1alpha | Phase 4 | [5] | ||
| Synonyms |
Unii-NA856793UT; 192705-79-6; PD-166866; PD166866; PD 166866; CHEMBL299763; NA856793UT; 1-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-3-tert-butylurea; 1-(2-Amino-6-(3,5-dimethoxyphenyl)-pyrido(2,3-d)pyrimidin-7-yl)-3-tert-butyl urea; Urea,N-[2-amino-6-(3,5-dimethoxyphenyl)pyrido[2,3-d]pyrimidin-7-yl]-N'-(1,1-dimethylethyl)-; 1-[2-Amino-6-(3,5-dimethoxyphenyl)-pyrido[2,3-d]pyrimidin-7-yl]-3-tert-butyl urea; 6-arylpyrido[2,3-d]pyrimidine deriv 25; AC1NS3U5; SCHEMBL1248489; BDBM3443; CTK4E1060
Click to Show/Hide
|
|||
| External Link | ||||
| IT-101 | Phase 3 | [5] | ||
| External Link | ||||
| 2-Methoxyestradiol | Phase 2 | [5] | ||
| Synonyms |
ESM; Panzem; PulmoLAR; Panzem NCD; M 6383; (17beta)-2-Methoxyestra-1,3,5(10)-triene-3,17-diol; (17beta)-2-methoxyestra-1(10),2,4-triene-3,17-diol; (8R,9S,13S,14S,17S)-2-methoxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-3,17-diol; 1,3,5(10)-ESTRATRIEN-2,3,17-BETA-TRIOL 2-METHYL ETHER; 1,3,5(10)-Estratriene-2,3,17-triol 2-methyl ether; 2,3,17beta-Trihydroxy-1,3,5(10)-estratriene 2-methyl ether; 2-Hydroxyestradiol 2-methyl ether; 2-Hydroxyestradol 2-methyl ether; 2-ME2, 2-Methoxyestradiol; 2-Methoxyestra-1,3,5(10)-triene-3,17beta-diol; 2-Methoxyestradiol-17beta; 3,17beta-Dihydroxy-2-methoxy-1,3,5(10)-estratriene
Click to Show/Hide
|
|||
| External Link | ||||
| PX-478 | Phase 1 | [5] | ||
| Synonyms |
685898-44-6; PX-478 2HCl; UNII-T23U22X160; PX478; PX 478; Melphalan N-Oxide Impurity HCl; T23U22X160; 4-[Bis(2-chloroethyl)oxidoamino]-L-phenylalanine; PX-478 dihydrochloride; SCHEMBL18548830; C13H18Cl2N2O3.2ClH; DTXSID00218688; MolPort-035-789-733; 2675AH; s7612; 2-Amino-3-(4'-N,N-bis(2-chloroethyl)amino)phenylpropionic acid N-oxide; AKOS030231369; CS-5164; HY-10231; KB-80169; Z-3209; L-Phenylalanine, 4-(bis(2-chloroethyl)oxidoamino)-, dihydrochloride; (S)-4-(2-amino-2-carboxyethyl)-N,N-bis(2-chloroethyl)aniline oxide di
Click to Show/Hide
|
|||
| External Link | ||||
| EZN-2968 | Phase 1 | [5] | ||
| External Link | ||||
| ENMD-1198 | Phase 1 | [5] | ||
| Synonyms |
EM-5171; EM-883; EM-900; Hypoxia inducible factor 1 inhibitors, EntreMed; HIF-1 inhibitors, EntreMed; HIF-1 inhibitors (cancer), EntreMed; 2-ME2 analogs (oral, cancer), EntreMed; 2-methoxyestradiol analogs (oral, cancer), EntreMed
Click to Show/Hide
|
|||
| External Link | ||||
| Pyrrolidine carboxamide derivative 1 | Patented | [5] | ||
| Synonyms |
PMID26882240-Compound-22
Click to Show/Hide
|
|||
| External Link | ||||
| BD54: Diabetic foot ulcers | 47 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Pexiganan | Approved | [6] | ||
| Synonyms |
UNII-TVF29Q70Q1; TVF29Q70Q1; Pexiganan [INN]; 147664-63-9; SCHEMBL476400; CHEMBL1275802; Glycyl-L-isoleucylglycyl-L-lysyl-L-phenylalanyl-L-leucyl-L-lysyl-L-lysyl-L-alanyl-L-lysyl-L-lysyl-L-phenylalanylglycyl-L-lysyl-L-alanyl-L-phenylalanyl-L-valyl-L-lysyl-L-isoleucyl-L-leucyl-L-lysyl-L-lysinamide; Magainin I, 7-L-lysine-8-L-lysine-10-L-lysine-18-L-lysine-19-de-L-glutamic acid-21-L-leucine-23-L-lysinamide; L-Lysinamide, glycyl-L-isoleucylglycyl-L-lysyl-L-phenylalanyl-L-leucyl-L-lysyl-L-lysyl-L-alanyl-L-lys
Click to Show/Hide
|
|||
| External Link | ||||
| CT-102 | Approved | [7] | ||
| External Link | ||||
| Doxycycline | Phase 2 | [8] | ||
| Synonyms |
Atridox; Azudoxat; DOXY; Deoxymykoin; Dossiciclina; Doxiciclina; Doxitard; Doxivetin; Doxycen; Doxychel; Doxycin; Doxycyclin; Doxycyclinum; Doxysol; Doxytec; Doxytetracycline; Hydramycin; Investin; Jenacyclin; Liviatin; Monodox; Oracea; Ronaxan; Spanor; Supracyclin; Vibramycin; Vibramycine; Vibravenos; DOXCYCLINE ANHYDROUS; DOXYCYCLINE CALCIUM; DOXYCYCLINE MONOHYDRATE; Dossiciclina [DCIT]; Doxiciclina [Italian]; Doxycycline anhydrous; Doxycycline hyclate; Vibramycin Novum; Alpha-Doxycycline; Alti-Doxycycline; Apo-Doxy; BMY-28689; BU-3839T; Doxiciclina [INN-Spanish]; Doxy-Caps; Doxy-Puren; Doxy-Tabs; Doxychel (TN); Doxycycline (INN); Doxycycline (TN); Doxycycline (anhydrous); Doxycycline (internal use); Doxycycline-Chinoin; Doxycyclinum [INN-Latin]; Novo-Doxylin; Nu-Doxycycline; Periostat (TN); Vibra-tabs; Alpha-6-Deoxyoxytetracycline; DMSC (*Fosfatex); Doxycycline (200mg/day) or Placebo; Monodox (*monohydrate); Vibramycin (*monohydrate); Vivox (*Hyclate); GS-3065 (*monohydrate); Alpha-6-Deoxy-5-hydroxytetracycline; (2E,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2Z)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2Z,4S,4aR,5S,5aR,6R)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (2Z,4S,4aR,5S,5aR,6R,12aS)-2-[amino(hydroxy)methylidene]-4-(dimethylamino)-5,10,11,12a-tetrahydroxy-6-methyl-4a,5,5a,6-tetrahydro-4H-tetracene-1,3,12-trione; (4S,4aR,5S,5aR,6R,12aS)-4-(dimethylamino)-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-1,4,4a,5,5a,6,11,12a-octahydrotetracene-2-carboxamide; 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-, (4S,4aR,5S,5aR,6R,12aS); 5-Hydroxy-alpha-6-deoxytetracycline; 6-Deoxyoxytetracycline; 6-Deoxytetracycline; 6-alpha-Deoxy-5-oxytetracycline; 6alpha-Deoxy-5-oxytetracycline
Click to Show/Hide
|
|||
| External Link | ||||
| Nemonoxacin | Phase 3 | [9] | ||
| Synonyms |
378746-64-6; UNII-P94L0PVO94; TG-873870; P94L0PVO94; Nemonoxacin [INN]; 7-((3S,5S)-3-amino-5-methylpiperidin-1-yl)-1-cyclopropyl-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; 7-[(3S,5S)-3-Amino-5-methylpiperidin-1-yl]-1-cyclopropyl-8-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid; SCHEMBL1006373; CHEMBL1213456; CHEBI:136053; ZINC40435195; SB16570; FT-0700967; 7-[(3S,5S)-3-amino-5-methylpiperidin-1-yl]-1-cyclopropyl-8-methoxy-4- oxo-1,4-dihydroquinoline-3-carboxylic acid
Click to Show/Hide
|
|||
| External Link | ||||
| Alprostadil | Approved | [7] | ||
| Synonyms |
Prostaglandin E1; 745-65-3; PGE1; Muse; Edex; Caverject; Prostin VR; Alprostadilum; Femprox; Befar; Vitaros; Prostandin; Liprostin; PGE-1; Prink (TN); Befar (TN); Prostavasin; Vasaprostan; MR-256; Minprog; UNII-F5TD010360; Alprostadil(Caverject); CHEMBL495; (11alpha,13E,15S)-11,15-dihydroxy-9-oxoprost-13-en-1-oic acid; Prostivas; Lipoprost; 11alpha,15alpha-Dihydroxy-9-oxo-13-trans-prostenoic acid; (13E)-(15S)-11alpha,15-Dihydroxy-9-oxoprost-13-enoate; FemLife; RayVa; Sugiran; Viridal; MR 256; PGE1 Oligomer; BML1-F06; Caverject (TN); Edex (TN); HEI-507; Muse (TN); Prostin VR pediatric (TN); U-10136; Alprostadil (JP15/USP/INN); Prost-13-en-1-oic acid, 11,15-dihydroxy-9-oxo-, (11alpha,13E,15S)-, homopolymer; (11alpha,13E,15S)-11,15-Dihydroxy-9-oxoprost-13-enoic acid; (13E,15S)-11alpha,15-dihydroxy-9-oxoprost-13-en-1-oic acid; 7-[(1R,2R,3R)-3-hydroxy-2-[(E,3S)-3-hydroxyoct-1-enyl]-5-oxocyclopentyl]heptanoic acid; Lipo-alprostadil
Click to Show/Hide
|
|||
| External Link | ||||
| Daprodustat | Phase 1 | [10] | ||
| Synonyms |
Daprodustat; 960539-70-2; UNII-JVR38ZM64B; GSK-1278863; JVR38ZM64B; N-((1,3-Dicyclohexylhexahydro-2,4,6-trioxopyrimidin-5-yl)carbonyl)glycine; Daprodustat [USAN:INN]; GSK 1278863; Daprodustat(GSK1278863); Daprodustat (JAN/USAN/INN); GTPL8455; Daprodustat (GSK1278863); CHEMBL3544988; KS-00000M8Z; EX-A1121; BCP16766; s8171; AKOS027439964; Glycine, N-((1,3-dicyclohexylhexahydro-2,4,6-trioxo-5-pyrimidinyl)carbonyl)-; ZINC231226004; CS-5453; SB19761; DB11682; HY-17608; J3.560.573H; D10874
Click to Show/Hide
|
|||
| External Link | ||||
| Prezatide copper acetate | Approved | [6] | ||
| Synonyms |
Iamin gel (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| Nitroglycerin | Approved | [7] | ||
| Synonyms |
Glyceryl trinitrate; Trinitroglycerin; Nitroglycerol; Nitrostat; Nitroglycerine; Nitro-dur; Minitran; Trinitroglycerol; 55-63-0; Nitroderm; Nitroglyn; Nitrospan; Nitronet; Tridil; Glycerol trinitrate; Transderm-nitro; Transderm Nitro; Epinitril; Susadrin; Perlinganit; Nitrolingual; Nitrolan; Sustonit; Nitrocine; Trinitrin; Natispray; Lenitral; Nitrong; Niong; Nitro-Bid; Nitroderm TTS; Trinitrosan; Rectogesic; Nitroglicerina; Cordipatch; Perglottal; Nitrorectal; Gilustenon; Nitroretard; Nitroplast; Corditrine; Trinitrolong; Adesitrin; Aldonitrin; Angibid; Angiolingual; Angiplex; Anglix; Angonist; Angorin; Anogesic; Buccal; Buccard; Cardabid; Cardamist; Cardinit; Cardiodisco; Cellegesic; Chitamite; Colenitral; Dauxona; Deponit; Diafusor; Discotrine; Dynamite; GTN; Glonoin; Glycerintrinitrate; Glyceroltrinitraat; Glyceryl; Glycerylnitrat; Glytrin; Herwicard; Herzer; Klavikordal; Lentonitrina; Millisrol; Minitram; Minitro; Mionitrat; Myocon; Myoglycerin; Myovin; NTG; Niglin; Niglycon; Nirmin; Nitora; Nitradisc; Nitrangin; Nitrek; Nitriderm; NitroBid; NitroCor; NitroDur; NitroMist; NitroQuick; NitroQuik; Nitroard; Nitrobaat; Nitrobukal; Nitrocap; Nitrocard; Nitrocerin; Nitroclyn; Nitrocontin; Nitrocot; Nitrodisc; Nitrodyl; Nitrogard; Nitrogliceryna; Nitroglin; Nitroject; Nitroletten; Nitrolin; Nitrolowe; Nitromel; Nitromex; Nitromint; Nitropatch; Nitropen; Nitropercuten; Nitroperlinit; Nitroprol; Nitropront; Nitroprontan; Nitrorex; Nitrostabilin; Nitrovis; Nysconitrine; Percutol; Perganit; Plastranit; Polnitrin; Ratiopharm; Soup; Suscard; Sustac; Sustak; TNG; Temponitrin; Trinalgon; Trinipatch; Triniplas; Trinitrol; Trinitron; Turicard; Vascana; Vasoglyn; Vasolator; Vernies; Willong; Blasting gelatin; Blasting oil; Gepan Nitroglicerin; Gilucor nitro; Glycerin trinitrate; Glycerine trinitrate; Glyceryl nitrate; NITRO IV; Neos nitro OPT; Niong Retard; Nitradisc Pad; Nitradisc TTS; Nitriderm TTS; Nitro Bid; Nitro Dur; Nitro Dur TTS; Nitro Mack Retard; Nitro Retard; Nitro Rorer; Nitrobid Oint; Nitrocontin Continus; Nitroderm TTS Ext; Nitrodyl TTS; Nitrol Ointment; Nitrolingual Spray; Nitromack Retard; Nitromint Aerosol; Nitromint Retard; Nitronal Aqueous; Nitrong Retard; Nitrong parenteral; Nitrozell retard; Percutol Oint; Spirit of glonoin; Tridil sublin; Trinitrin Tablets; Trinitrina Erba; Deponit 5; Deponit TTS 10; Deponit TTS 5; Nitrocine 5; Aquo-Trimitrosan; Coro-Nitro; Deponit-5; GTN-Pohl; IMX-150; MED-2002; MQX-503; Mi-Trates; Minitran (TN); NK-843; Natispray (TN); Nit-Ret; Nitrine-TDC; Nitro-Dur 10; Nitro-Dur 5; Nitro-Gesanit Retard; Nitro-Mack Retard; Nitro-Par; Nitro-Pflaster; Nitro-Span; Nitro-Time; Nitro-lent; NitrocapTD; Nitroderm TTS-5; Nitrogard-SR; Nitroglycerin (NG); Nitroglycerin-ACC; Nitrolingual Pump Spray (TN); Nitromist (TN); Nitrong-SR; Nitrospan (TN); Nitrostat (TN); SDM No 17; SK-106N; SK-866; SK-878; Top-Nitro; Transderm Nitro (TN); Transderm-N TTS; Transderm-Nitro TTS; Transiderm-nitro; Tridil (TN); Trinipatch (TN); Trinitrate, Glyceryl; Nitro-Dur (TN); Nitro-M-Bid; Nitro-bid (TN); Transderm-nitro (TN); DWP-401
Click to Show/Hide
|
|||
| External Link | ||||
| Apligraf | Approved | [6] | ||
| Synonyms |
Graftskin
Click to Show/Hide
|
|||
| External Link | ||||
| HEGF | Approved | [7] | ||
| External Link | ||||
| Donaperminogene seltoplasmid | Phase 3 | [11] | ||
| Synonyms |
VM202
Click to Show/Hide
|
|||
| External Link | ||||
| ALLO-ASC-DFU | Phase 3 | [12] | ||
| External Link | ||||
| Granexin | Phase 3 | [13] | ||
| External Link | ||||
| White blood cell therapy | Phase 3 | [14] | ||
| External Link | ||||
| ICX-PRO | Phase 3 | [15] | ||
| Synonyms |
Cyzact; ProtoDerm; ICXP-007; Allogeneic human dermal fibroblasts (wound healing), Healthpoint; Allogeneic human dermal fibroblasts (wound healing), Intercytex
Click to Show/Hide
|
|||
| External Link | ||||
| CureXcell | Phase 3 | [16] | ||
| External Link | ||||
| INL-002 | Phase 3 | [10] | ||
| External Link | ||||
| Talactoferrin | Phase 3 | [17] | ||
| Synonyms |
Talactoferrin (topical)
Click to Show/Hide
|
|||
| External Link | ||||
| VM-202 | Phase 3 | [18] | ||
| External Link | ||||
| Ch14.18 mab | Phase 3 | [19] | ||
| External Link | ||||
| PDGF-BB | Phase 3 | [20] | ||
| Synonyms |
PDGF-BB (BioChaperone technology, diabetic foot ulcer/venous ulcers/burns)
Click to Show/Hide
|
|||
| External Link | ||||
| DSC-127 | Phase 3 | [21] | ||
| Synonyms |
aclerastide; UNII-YYD6UT8T47; YYD6UT8T47; Aclerastide [INN]; Norleu3-a(1-7); Asp-arg-nle-tyr-ile-his-pro; DSC127; USB-001; 227803-63-6; 1-7-Angiotensin II, 3-L-norleucine-5-L-isoleucine
Click to Show/Hide
|
|||
| External Link | ||||
| Granexin gel | Phase 3 | [18] | ||
| Synonyms |
alphaCT1
Click to Show/Hide
|
|||
| External Link | ||||
| Dermacyn | Phase 2 | [22] | ||
| External Link | ||||
| MBN-101 | Phase 2 | [23] | ||
| External Link | ||||
| ACT-1 | Phase 2 | [24] | ||
| External Link | ||||
| CVBT-141B | Phase 2 | [10] | ||
| External Link | ||||
| IL22-Fc | Phase 2 | [18] | ||
| External Link | ||||
| HO/03/03 | Phase 2 | [25] | ||
| Synonyms |
PKC modulator (topical peptide, DU/VU/PU), HealOr
Click to Show/Hide
|
|||
| External Link | ||||
| RG7880 | Phase 2 | [26] | ||
| External Link | ||||
| GAM-501 | Phase 2 | [27] | ||
| Synonyms |
Excellarate; Diabetic ulcer product, Selective Genetics; AdPDGF-B/GAM; Adenoviral gene therapy (PDGF-B), Selective Genetics; PDGF-B adenoviral gene therapy (collagen gel, diabetic foot ulcer), Tissue Repair Company
Click to Show/Hide
|
|||
| External Link | ||||
| Nexagon | Phase 2 | [28] | ||
| External Link | ||||
| MRE-0094 | Phase 2 | [7] | ||
| Synonyms |
SCHEMBL13010610; MRE 0094
Click to Show/Hide
|
|||
| External Link | ||||
| CVBT-141B topical | Phase 2 | [18] | ||
| External Link | ||||
| PDA-002 | Phase 2 | [10] | ||
| External Link | ||||
| DCB-WH1 | Phase 2 | [29] | ||
| Synonyms |
PAF4 + S1 (wound healing), Development Centre for Biotechnology; PAF4 + S1 (wound healing), Microbio
Click to Show/Hide
|
|||
| External Link | ||||
| Galnobax | Phase 1/2 | [10] | ||
| External Link | ||||
| Nu-3 | Phase 1/2 | [10] | ||
| External Link | ||||
| ATG002 | Phase 1/2 | [30] | ||
| External Link | ||||
| Nu-3 | Phase 1/2 | [18] | ||
| Synonyms |
Topical Nubiotics (bacterial infection), Lakewood Amedex
Click to Show/Hide
|
|||
| External Link | ||||
| ExpressGraft | Phase 1 | [31] | ||
| External Link | ||||
| BRX-005 | Phase 1 | [32] | ||
| Synonyms |
Arimoclomol maleate; BRX-220; Bimoclomol derivatives, Biorex
Click to Show/Hide
|
|||
| External Link | ||||
| WPP-201 | Phase 1 | [33] | ||
| External Link | ||||
| Telbermin | Discontinued in Phase 2 | [7] | ||
| Synonyms |
Telbermin (USAN/INN)
Click to Show/Hide
|
|||
| External Link | ||||
| Rusalatide acetate | Discontinued in Phase 2 | [34] | ||
| Synonyms |
Chrysalin; TP508; Chrysalin (gel); TP-508; TP508 (gel); TRAP-508
Click to Show/Hide
|
|||
| External Link | ||||
| PLR-15 | Terminated | [35] | ||
| External Link | ||||
| PDGF gene therapy | Investigative | [7] | ||
| Synonyms |
UNII-54FF09J702; 54FF09J702; Ancriviroc besylate; SCH-351125 Besylate; 565428-86-6; (1,4'-Bipiperidine)-4-methanimine, alpha-(4-bromophenyl)-1'-((2,4-dimethyl-1-oxido-3-pyridinyl)carbonyl)-N-ethoxy-4'-methyl-, (alphaZ)-, benzenesulfonate
Click to Show/Hide
|
|||
| External Link | ||||
References