m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05087
|
[1] | |||
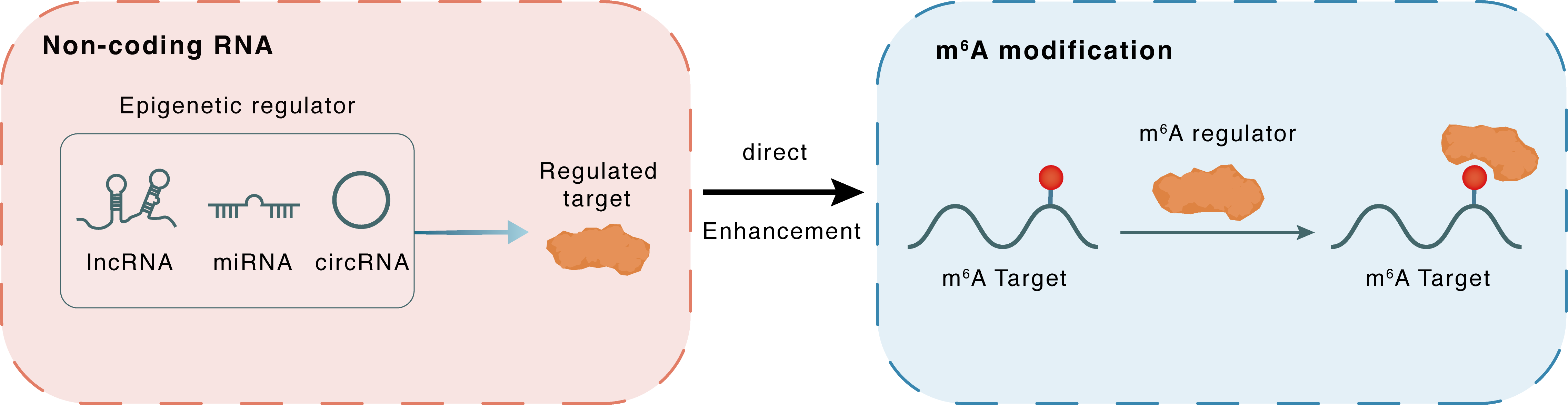 Non-coding RNA
Dubr
YTHDF1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
Tau
Tau
YTHDF1
Non-coding RNA
Dubr
YTHDF1
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
Tau
Tau
YTHDF1
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | YTH domain-containing family protein 1 (YTHDF1) | READER | |||
| m6A Target | Microtubule-associated protein tau (TAU) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | DPPA2 upstream binding RNA (DUBR) | LncRNA | View Details | ||
| Regulated Target | YTH domain-containing family protein 1 (YTHDF1) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | m6A-modified lincRNA DUBR is required for neuronal development by stabilizing YTHDF1 and facilitating mRNA translation.Dubr interacts with m6A-binding proteins, the YTHDF1/3 complex, through its m6A motifs to protect YTHDF1/3 from degradation via the proteasome pathway. Furthermore, Microtubule-associated protein tau (TAU) and Calmodulin are regulated by YTHDF1/3 and m6A-modified Dubr. | ||||
In-vitro Model |
Neuro-2a | Mouse neuroblastoma | Mus musculus | CVCL_0470 | |
|
ND7/23
|
N.A. | Mus musculus | CVCL_4259 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| Microtubule-associated protein tau (TAU) | 21 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Davunetide | Phase 3 | [2] | ||
| Synonyms |
NAP; AL-108; AL-208; Davunetide (intranasal spray), Allon; NAPVSIPQ eight amino acid peptide (intranasal spray), Allon; Davunetide (intravenous-infused), Allon Therapeutics; NAP eight amino acid peptide (neuroprotection/cognitive impairment), Allon; NAPVSIPQ eight amino acid peptide (neuroprotection/cognitive impairment), Allon; Central nervous system therapeutic (Alzheimer's disease/schizophrenia), Allon; Central nervous system therapeutic (post-cardiac artery bypass graft/mild cognitive impairment), Allon; NAPVSIPQ eight amino acid peptide (intravenous-infused/subcutaneous depot formulation), Allon Therapeutics; Davunetide (iv/sc, Alzheimer's disease), Allon Therapeutics
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| TRx0237 | Phase 3 | [3] | ||
| Synonyms |
951131-15-0; UNII-E79ZM68IOZ; E79ZM68IOZ; Leucomethylene Blue dihydrobromide; TRX0237 dihydrobromide; TRX 0237 dihydrobromide; TRX-0237 dihydrobromide; TRx0237(LMTX); TRX-0237 2HBr; Leukomethylene Blue dihydrobromide; Hydromethylthionine HBr(TRX0237); BCP24159; EX-A4299; Reduced methylene Blue dihydrobromide; N3,N3,N7,N7-Tetramethyl-10H-phenothiazine-3,7-diamine dihydrobromide; Leucomethylene Blue 2HBr;TRX0237 dihydrobromide;TRX 0237 dihydrobromide;TRX-0237 dihydrobromide
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LMT-X | Phase 3 | [4] | ||
| Synonyms |
Second-generation tau aggregation inhibitor (Alzheimer's disease); Second-generation tau aggregation inhibitor (Alzheimer's disease), TauRx
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| E2814 | Phase 2/3 | [5] | ||
| External Link | ||||
| PBT-2 | Phase 2 | [6] | ||
| Synonyms |
AD/HD therapy, Prana; Alzheimers/Huntingtons disease therapy (chelating agent), Prana
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| RG6100 | Phase 2 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Tau-binding PET tracer | Phase 2 | [2] | ||
| Synonyms |
T-777; T-807; T-808; Tau-binding PET tracer (Alzheimer disease); Tau-binding PET tracer (Alzheimer disease), Siemens
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BIIB092 | Phase 2 | [7] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| JNJ-63733657 | Phase 2 | [8] | ||
| External Link | ||||
| Semorinemab | Phase 2 | [9] | ||
| Synonyms |
RO7105705
Click to Show/Hide
|
|||
| External Link | ||||
| Bepranemab | Phase 2 | [10] | ||
| Synonyms |
RG6416
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| LY3303560 | Phase 1 | [11] | ||
| External Link | ||||
| PTI-80 | Phase 1 | [12] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| PRX-005 | Phase 1 | [13] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| APNmAb005 | Phase 1 | [14] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| BEY2153 | Phase 1 | [15] | ||
| MOA | Inhibitor | |||
| External Link | ||||
| Lu AF87908 | Phase 1 | [16] | ||
| External Link | ||||
| NI-105 | Investigative | [2] | ||
| Synonyms |
Tau protein modulator (neurodegenerative disease); Tau protein modulator (neurodegenerative disease), Biogen Idec; Tau protein modulator (neurodegenerative disease), Neurimmune Therapeutics
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| AL-408 | Investigative | [2] | ||
| Synonyms |
AL-108 analog (neurodegenerative diseases), Allon
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BLV-0703 | Investigative | [2] | ||
| Synonyms |
BLV-200703; KTP-NH2; TAU-targeting compound (CNS disorders), Bioalvo
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| PMID28766366-Compound-Scheme22Middle | Patented | [17] | ||
| External Link | ||||
References