m6A-centered Crosstalk Information
Mechanism of Crosstalk between m6A Modification and Epigenetic Regulation
| Crosstalk ID |
M6ACROT05021
|
[1] | |||
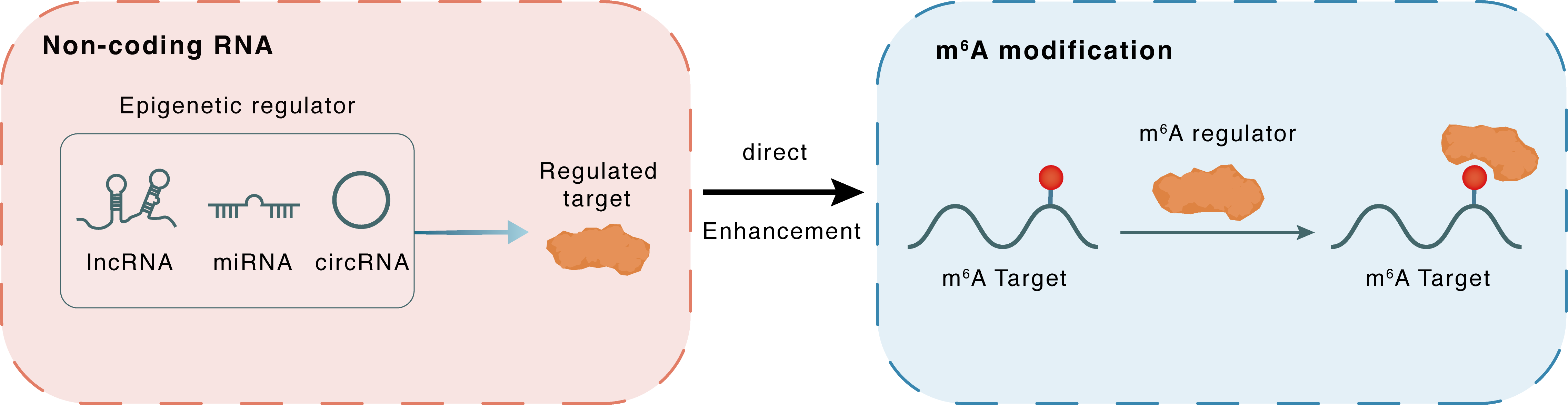 Non-coding RNA
linc01305
IGF2BP2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
Htr3a
Htr3a
IGF2BP2
Non-coding RNA
linc01305
IGF2BP2
lncRNA miRNA circRNA
Direct
Enhancement
m6A modification
Htr3a
Htr3a
IGF2BP2
 : m6A sites : m6A sites
|
|||||
| m6A Modification: | |||||
|---|---|---|---|---|---|
| m6A Regulator | Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2) | READER | |||
| m6A Target | 5-hydroxytryptamine receptor 3A (HTR3A) | ||||
| Epigenetic Regulation that have Cross-talk with This m6A Modification: | |||||
| Epigenetic Regulation Type | Non-coding RNA (ncRNA) | ||||
| Epigenetic Regulator | Long intergenic non-protein coding RNA 1305 (LINC01305) | LncRNA | View Details | ||
| Regulated Target | Insulin like growth factor 2 mRNA binding protein 2 (IGF2BP2) | View Details | |||
| Crosstalk Relationship | ncRNA → m6A | Enhancement | |||
| Crosstalk Mechanism | ncRNAs directly impacts m6A modification through recruiting m6A regulator | ||||
| Crosstalk Summary | LINC01305 promotes metastasis and proliferation of esophageal squamous cell carcinoma through interacting with IGF2BP2 and IGF2BP3 to stabilize 5-hydroxytryptamine receptor 3A (HTR3A) mRNA | ||||
| Responsed Disease | Esophageal Squamous Cell Carcinoma | ICD-11: 2B70.1 | |||
In-vitro Model |
KYSE-180 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1349 | |
| TE-5 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1764 | ||
| KYSE-150 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1348 | ||
| KYSE-510 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1354 | ||
| KYSE-140 | Esophageal squamous cell carcinoma | Homo sapiens | CVCL_1347 | ||
Full List of Potential Compound(s) Related to This m6A-centered Crosstalk
| 5-hydroxytryptamine receptor 3A (HTR3A) | 49 Compound(s) Regulating the Target | Click to Show/Hide the Full List | ||
| Dolasetron | Approved | [2] | ||
| Synonyms |
Dolasetronum; Dolasteron; Anzemet (TN); Dolasetron (INN); Dolasetron [INN:BAN]; Dolasetronum [INN-Latin]; 3-oxooctahydro-2h-2,6-methanoquinolizin-8-yl 1h-indole-3-carboxylate
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Palonosetron | Approved | [3] | ||
| Synonyms |
Aloxi; Onicit; Palonosetron [INN]; Aloxi (TN); RS 25233-197; RS 25259-197; RS-25233-197; RS-25259-197; (S-(R*,R*))-2-(1-Azabicyclo(2.2.2)oct-3-yl)-2,3,3a,4,5,6-hexahydro-1H-benz(de)isoquinolin-1-one
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 0.03162 nM | |||
| External Link | ||||
| Alosetron | Approved | [4] | ||
| Synonyms |
Lotronex; Lotrpnex; ALOSETRON HYDROCHLORIDE; Alosetron HCl; Alosetron hydrochloride [USAN]; Alosetron monohydrochloride; GR 68755; GR 68755X; GR 68755c; GR68755; Alosetron (INN); Alosetron [INN:BAN]; Alosetron hydrochloride (USAN); GR-68755C; Lotronex (TN); Lotrpnex (TN); 1H-Pyrido(4,3-b)indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2((5-methyl-1H-imidazol-4-yl)methyl)-, monohydrochloride; 1H-Pyrido(4,3-b)indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-((5-methyl-1H-imidazol-4-yl)methyl)-, monohydrochloride; 1H-Pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(4-methyl-1H-imidazol-5-yl)methyl]-, hydrochloride (1:1); 1H-Pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol; 1H-Pyrido[4,3-b]indol-1-one, 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-(9CI); 2,3,4,5-Tetrahydro-5-methyl-2-((5-methyl-1H-imidazol-4-yl)methyl)-1H-pyrido(4,3-b)indol-1-one; 2,3,4,5-Tetrahydro-5-methyl-2-((5-methylimidazol-4-yl)methyl)-1H-pyrido(4,3-b)indol-1-one monohydrochloride; 2,3,4,5-tetrahydro-5-methyl-2-((5-methylimidazol-4-yl)methyl)-1H-pyridol(4,3-b)indol-1-one monohydrochloride; 2,3,4,5-tetrahydro-5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-1H-pyrido[4,3-b]indol-1-one, monohydrochloride; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-2,3,4,5-tetrahydro-1H-pyrido[4,3-b]indol-1-one hydrochloride; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one; 5-methyl-2-[(5-methyl-1H-imidazol-4-yl)methyl]-3,4-dihydropyrido[4,3-b]indol-1-one hydrochloride
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 0.5 nM | |||
| External Link | ||||
| Tropisetron | Approved | [5] | ||
| Synonyms |
Navoban; Novaban; TKT; ICS-205930; Navoban (TN); Tropisetron (INN); [(1R,5S)-8-methyl-8-azabicyclo[3.2.1]octan-3-yl] 1H-indole-3-carboxylate; (3-endo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl 1H-indole-3-carboxylate
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 2.7 nM | |||
| External Link | ||||
| Procaine | Approved | [6] | ||
| Synonyms |
Allocaine; Anticort; Anuject; Duracaine; Gerokit; Gerovital; Jenacain; Jenacaine; Nissocaine; Norocaine; Novocain; Novocaine; Procain; Procaina; Procainum; Scurocaine; Spinocaine; Solution of novocain; Factor H3; SP01; SP01A; Stoff H3; Vitamin H3; Anticort (TM); Diethylaminoethyl p-aminobenzoate; Gerovital H-3; Novocain (TN); P-Aminobenzoyldiethylaminoethanol; P-Aminobenzyoyldiethylaminoethanol; Procaina [INN-Spanish]; Procaine (INN); Procaine [INN:BAN]; Procaine, base; Procainum [INN-Latin]; SP-01A; Solution of novocain (TN); Beta-Diethylaminoethyl 4-aminobenzoate; P-Aminobenzoic acid 2-diethylaminoethyl ester; Beta-(Diethylamino)ethyl 4-aminobenzoate; Beta-(Diethylamino)ethyl p-aminobenzoate; BENZOIC ACID,4-AMINO,2-DIETHYLAMINOETHYL ESTER PROCAIN BASE; Benzoic acid, 4-amino-, 2-(diethylamino)ethyl ester; Benzoic acid, p-amino-, 2-(diethylamino)ethyl ester; 2-(Diethylamino)ethyl 4-aminobenzoate; 2-(Diethylamino)ethyl p-aminobenzoate; 2-(Diethylamino)ethyl-4-aminobenzoate; 2-Diethylaminoethyl 4-aminobenzoate; 2-Diethylaminoethyl p-aminobenzoate; 2-Diethylaminoethylester kyseliny p-aminobenzoove; 2-Diethylaminoethylester kyseliny p-aminobenzoove [Czech]; 4-Aminobenzoesaeure-beta-diethylaminoethylester; 4-Aminobenzoic acid 2-(diethylamino) ethyl ester; 4-Aminobenzoic acid diethylaminoethyl ester
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Levetiracetam | Approved | [5] | ||
| Synonyms |
102767-28-2; Keppra; (S)-2-(2-Oxopyrrolidin-1-yl)butanamide; Keppra XR; Levetiracetamum; ucb L059; (2S)-2-(2-oxopyrrolidin-1-yl)butanamide; UCB-L 059; UCB-L059; Spritam; (S)-alpha-Ethyl-2-oxo-1-pyrrolidineacetamide; (-)-(S)-alpha-Ethyl-2-oxo-1-pyrrolidineacetamide; SIB-S1; UNII-44YRR34555; 1-Pyrrolidineacetamide, alpha-ethyl-2-oxo-, (alphaS)-; UCB-22059; Levetiracetamum [INN-Latin]; Levetiractam; CHEBI:6437; ucb L060; Levetiracetam In Sodium Chloride; 44YRR34555; Levroxa; 1-Pyrrolidineacetamide, alpha-ethyl-2-oxo-,; Leviteracetam; Torleva; Levetiracetam [INN]; Etiracetam levo-isomer; Keppra (TN); L-059; Etiracetam, S-isomer; Keppra, Keppra XR),Levetiracetam; Levetriacetam
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Palonosetron + fosnetupitant | Approved | [7] | ||
| MOA | Antagonist | |||
| External Link | ||||
| Cilansetron | Phase 3 | [8] | ||
| Synonyms |
Calmactin; DU-123265; KC-9946; Cilansetron (USAN/INN)
Click to Show/Hide
|
|||
| MOA | Modulator | |||
| External Link | ||||
| BEMESETRON | Discontinued in Phase 3 | [9] | ||
| Synonyms |
3-Tropanyl-3,5-dichlorobenzoate; MDL 72222; MDL-72222; C15H17Cl2NO2; CHEMBL376379; 40796-97-2; Tropyl 3,5-dichlorobenzoate; 8-Methyl-8-azabicyclo[3.2.1]oct-3-yl 3,5-dichlorobenzoate; Bemesetron [USAN:INN]; Bemesetronum [INN-Latin]; 3,5-Dichloro-benzoic acid 8-methyl-8-aza-bicyclo[3.2.1]oct-3-yl ester; SR-01000075587; Tropanyl 3,5-dichlorobenzoate; 1alphaH,5alphaH-Tropan-3alpha-yl 3,5-dichlorobenzoate; endo-8-Methyl-8-azabicyclo(3.2.1)oct-3-yl 3,5-dichlorobenzoate; (8-methyl-8-azabicyclo[3.2.1]octan-3-yl) 3,5-dichlorobenzo
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 2.5 nM | |||
| External Link | ||||
| YM-114 | Discontinued in Phase 2 | [10] | ||
| Synonyms |
KAE-393; (R)-5-(2,3-Dihydro-1H-indol-1-ylcarbonyl)-4,5,6,7-tetrahydro-1H-benzimidazole hydrochloride
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| Norcisapride | Discontinued in Phase 2 | [11] | ||
| Synonyms |
84946-16-7; CHEMBL1748; 4-Amino-5-chloro-2-methoxy-N-(3-methoxy-4-piperidyl)benzamide; 4-amino-5-chloro-2-methoxy-N-(3-methoxy-4-piperidinyl)benzamide; (4-Amino-5-chloro-2-methoxy)-N-[3-methoxy(4-piperidyl)]benzamide; 4-amino-5-chloro-2-methoxy-N-(3-methoxypiperidin-4-yl)benzamide; Benzamide,4-amino-5-chloro-2-methoxy-N-[(3S,4R)-3-methoxy-4-piperidinyl]-, hydrochloride(1:1); EINECS 284-619-3; AC1MI81F; SCHEMBL593405; CTK4E8653; OMLDMGPCWMBPAN-UHFFFAOYSA-N; BDBM50301927; AKOS030254741; API0006151; DB-076176
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 2300 nM | |||
| External Link | ||||
| ATI-17000 | Preclinical | [12] | ||
| Synonyms |
CompB; J-113397; UNII-00M5444DIY; CHEMBL357076; 00M5444DIY; J113397; 1-[(3R,4R)-1-(cyclooctylmethyl)-3-(hydroxymethyl)piperidin-4-yl]-3-ethylbenzimidazol-2-one; 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one; AC1NSK6N; J-113,397; 1-(1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl)-3-ethyl-1,3-dihydro-2H-benzimidazol-2-one; 256640-45-6; SCHEMBL875219; J 113397; GTPL1691; (+)-J-113397; ZINC1483900; BDBM50083230; NCGC00344513-02; 2H-Benzimidazol-2-one,
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| AS-8112 | Terminated | [13] | ||
| MOA | Antagonist | |||
| External Link | ||||
| BP4.879a | Terminated | [14] | ||
| MOA | Antagonist | |||
| External Link | ||||
| 5-hydroxyindole | Investigative | [15] | ||
| Synonyms |
1H-Indol-5-ol; 1953-54-4; INDOL-5-OL; 5-Hydroxy-1H-indole; Hydroxy-5 indole; 5-Indolol; UNII-320UN7XZYN; Hydroxy-5-indole; Hydroxy-5 indole [French]; CCRIS 4422; 5-Hydroxyindole, 97%; EINECS 217-782-6; MFCD00005677; NSC 87503; BRN 0112349; 320UN7XZYN; CHEBI:89649; LMIQERWZRIFWNZ-UHFFFAOYSA-N; NSC-87503; 5-hydroxy-indole; Hydroxy-5 indole [French]; 5-hydroxylindole; 5-hydroxy-indol; 5-hydroxy indole; 3fuh; 5H1; zlchem 359; 5Hydroxy-1H-indole; PubChem7263; 1-H-indol-5-ol; 1H-indol-5-ol.; 4b3c; ACMC-1BQT3; WLN: T56 BMJ GQ
Click to Show/Hide
|
|||
| MOA | Modulator (allosteric modulator) | |||
| External Link | ||||
| TMB-8 | Investigative | [16] | ||
| Synonyms |
8-(Diethylamino)octyl 3,4,5-trimethoxybenzoate; TMB 8; 57818-92-5; TM-8; 8-(N,N-Diethylamino)octyl-3,4,5-trimethoxybenzoate; 3,4,5-Trimethoxybenzoic acid, 8-(diethylamino)octyl ester; C22H37NO5; AC1L1KGS; AC1Q67GP; Lopac-861804; Lopac0_000022; BSPBio_001503; GTPL4323; CHEMBL258764; SCHEMBL2173737; CTK5A7496; DTXSID70206532; CHEBI:107633; HMS1989L05; HMS1791L05; HMS3402L05; 53464-72-5 (hydrochloride); ZINC3875139; EI-110; AKOS030559942; MCULE-5343453436; CCG-204118; NCGC00162047-03; NCGC00162047-01; NCGC00014998-06
Click to Show/Hide
|
|||
| MOA | Blocker (channel blocker) | |||
| External Link | ||||
| 1-(biphenyl-4-yl)-3-(4-(piperidin-1-yl)butyl)urea | Investigative | [17] | ||
| Synonyms |
CHEMBL1086332; SCHEMBL13527422
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 7900 nM | |||
| External Link | ||||
| 10,11-dihydro-5H-dibenzo[b,f]azepine | Investigative | [18] | ||
| Synonyms |
Iminodibenzyl; 494-19-9; 10,11-Dihydro-5H-dibenz[b,f]azepine; Iminobibenzyl; 2,2'-Iminodibenzyl; 2,2'-Iminobibenzyl; 5H-Dibenz[b,f]azepine, 10,11-dihydro-; RP 23669; UNII-262BX7OE3U; NSC 72110; 10,11-Dihydro-5-dibenz(b,f)azepine; 6,11-dihydro-5H-benzo[b][1]benzazepine; 10,11-Dihydrodibenz(b,f)azepine; EINECS 207-787-1; 10,11-Dihydro-5H-dibenz(b,f)azepine; BRN 0152732; CHEMBL63054; 5H-Dibenz(b,f)azepine, 10,11-dihydro-; AI3-39165; 262BX7OE3U; ZSMRRZONCYIFNB-UHFFFAOYSA-N
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-Benzoxazo-2-yl-1,4-diazabicyclo[3.2.2]nonane | Investigative | [19] | ||
| Synonyms |
CHEMBL611082; SCHEMBL373021; CXJLWJAYGMWLRR-UHFFFAOYSA-N; BDBM50309862
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 13 nM | |||
| External Link | ||||
| 3alpha-(2'-Indolecarbonyloxy)-nortropane | Investigative | [20] | ||
| Synonyms |
CHEMBL596256; BDBM50304333
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| FLUPENTIXOLE | Investigative | [18] | ||
| Synonyms |
(E)-Flupenthixol; beta-Flupenthixol; trans-flupenthixol; trans-(E)-Flupentixol; trans-Flupentixol; 53772-85-3; EINECS 258-759-0; UNII-895OJP78MJ; Flupentiol; 2709-56-0; 895OJP78MJ; Flupenthixol, Beta; FLUPENTHIXOL, Alpha; 1-Piperazineethanol, 4-(3-(2-(trifluoromethyl)-9H-thioxanthen-9-ylidene)propyl)-, (E)-; (E)-4-(3-(2-(Trifluoromethyl)-9H-thioxanthen-9-ylidene)propyl)-1-piperazineethanol; 2-[4-[(3E)-3-[2-(trifluoromethyl)thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethanol
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| [3H]ramosetron | Investigative | [21] | ||
| MOA | Antagonist | |||
| External Link | ||||
| 3alpha-(1'-Methyl-2'-Indolecarbonyloxy)-tropane | Investigative | [20] | ||
| Synonyms |
CHEMBL593963; BDBM50304334
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| [3H]GR65630 | Investigative | [22] | ||
| Synonyms |
[3H]-GR65630
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| [3H](S)-zacopride | Investigative | [23] | ||
| Synonyms |
GTPL4074
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| External Link | ||||
| BRL-24682 | Investigative | [24] | ||
| Synonyms |
Brl 24682; SCHEMBL7292676; CHEMBL301039; BDBM82519; PDSP2_001249; PDSP1_001265; 76272-78-1; CAS_76272-78-1; 4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicyclo[3.2.1]octan-3-yl)benzamide; Benzamide, 4-amino-5-chloro-2-methoxy-N-(8-methyl-8-azabicyclo(3.2.1)oct-3-yl)-, endo-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 6-(4-Methyl-piperazin-1-yl)-phenanthridine | Investigative | [25] | ||
| Synonyms |
CHEMBL43193; 23441-13-6; 6-(4-methylpiperazin-1-yl)phenanthridine; CTK0I7937; DTXSID50433889; ZINC13778637; BDBM50063258; 6-(4-Methylpiperazino)phenanthridine; Phenanthridine, 6-(4-methyl-1-piperazinyl)-
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| trichloroethanol | Investigative | [26] | ||
| Synonyms |
2,2,2-TRICHLOROETHANOL; 115-20-8; Trichlorethanol; Trichloroethyl alcohol; Ethanol, 2,2,2-trichloro-; 2,2,2-Trichloro-1-ethanol; (Hydroxymethyl)trichloromethane; 2,2,2-Trichloroethyl alcohol; 2,2,2-Trichloroethan-1-ol; C2H3Cl3O; UNII-AW835AJ62N; NSC 66407; beta-trichloroethanol; CCRIS 6763; CCl3CH2OH; EINECS 204-071-0; 2,2,2-trichloro-ethanol; 2,2,2-tris-chloroethanol; BRN 1697495; AW835AJ62N; CHEBI:28094; KPWDGTGXUYRARH-UHFFFAOYSA-N; .beta.,.beta.,.beta-Trichloroethanol; 2,2,2-Trichloroethanol, 99%; 4yas
Click to Show/Hide
|
|||
| MOA | Modulator (allosteric modulator) | |||
| External Link | ||||
| (4-Quinolin-2-ylpiperazin-1-yl)acetic Acid | Investigative | [27] | ||
| Synonyms |
CHEMBL468498; AC1LEONH; SCHEMBL13780710; A3329/0141355; BDBM50258497; AKOS009544966; 2-(4-quinolin-2-ylpiperazin-1-yl)acetic acid
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 1-phenylbiguanide | Investigative | [28] | ||
| Synonyms |
phenylbiguanide; Phenyl biguanide; 102-02-3; Phenyldiguanide; Phenylguanide; phenyl diguanide; N-Phenyl-N'-guanylguanidine; N-phenylimidodicarbonimidic diamide; Biguanide, phenyl-; Imidodicarbonimidic diamide, N-phenyl-; UNII-W8PKA3T2I3; BIGUANIDE, 1-PHENYL-; C8H11N5; N-Phenyl-imidocarbonimidic diamide; EINECS 202-998-5; W8PKA3T2I3; CHEMBL13791; CHEBI:75377; P 1426; Imidodicarbonimidicdiamide, N-phenyl-; 1-(diaminomethylidene)-2-phenylguanidine; SR-01000075565; N-phenylbiguanide; N'-phenylbiguanide
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| External Link | ||||
| (S)-zacopride | Investigative | [29] | ||
| Synonyms |
CHEMBL28992; Tocris-1795; NCGC00025295-01; AC1O7H1O; SCHEMBL5373467; ZINC3961; GTPL2289; SCHEMBL16233195; PDSP2_001618; PDSP1_001634; BDBM50056419; UNII-9GN3OT4156 component FEROPKNOYKURCJ-CYBMUJFWSA-N; 4-Amino-N-(S)-1-aza-bicyclo[2.2.2]oct-3-yl-5-chloro-2-methoxy-benzamide; 4-amino-N-[(3S)-1-azabicyclo[2.2.2]octan-3-yl]-5-chloro-2-methoxybenzamide; 4-amino-N-[(8S)-1-azabicyclo[2.2.2]octan-8-yl]-5-chloro-2-methoxybenzamide
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 0.182 nM | |||
| External Link | ||||
| PH-709829 | Investigative | [30] | ||
| Synonyms |
PTGWFYYEAUFEAS-ZYHUDNBSSA-N; CHEMBL403858; PHA-709829; SCHEMBL844377; GTPL3997; BDBM50377050
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 2-(4-Benzyl-piperazin-1-yl)-benzothiazole | Investigative | [31] | ||
| Synonyms |
2-(4-benzylpiperazin-1-yl)-1,3-benzothiazole; CHEMBL282234; 2-(4-benzylpiperazino)-1,3-benzothiazole; 35463-75-3; AC1LSALZ; Oprea1_030694; MLS001166194; SCHEMBL7760900; KS-00003DHQ; MolPort-002-878-239; HMS2852D05; ZINC20405012; BDBM50041381; AKOS005101332; MCULE-9582396226; 7P-339S; 1-(2-Benzothiazolyl)-4-benzylpiperazine; SMR000550026; 2-(4-benzylpiperazin-1-yl)benzo[d]thiazole; Z86230191
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| [3H]granisetron | Investigative | [32] | ||
| Synonyms |
[3H]-granisetron; [3H]-BRL-43694
Click to Show/Hide
|
|||
| MOA | Antagonist | |||
| Activity | Ki = 1.45 nM | |||
| External Link | ||||
| meta-chlorphenylbiguanide | Investigative | [33] | ||
| Synonyms |
m-Chlorophenylbiguanide; mCPBG; 1-(3-Chlorophenyl)biguanide; 1-(m-Chlorophenyl)biguanide; N-(3-Chlorophenyl)imidodicarbonimidic diamide; 48144-44-1; Imidodicarbonimidic diamide, N-(3-chlorophenyl)-; UNII-910A4X901V; M-Chlorophenylbiguanidine; 3-Chloro-Phenyl biguanide; 2-(3-chlorophenyl)-1-(diaminomethylidene)guanidine; CHEMBL13790; CHEBI:32347; N-(3-chlorophenyl)-N'-(diaminomethylene)guanidine; 910A4X901V; C8H10ClN5; Imidodicarbonimidicdiamide, N-(3-chlorophenyl)-; 1-carbamimidamido-N-(3-chlorophenyl)methanimidamide; 3-chlorophenyl-biguanide; [3H]meta-chlorophenylbiguanide
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | EC50 = 3800 nM | |||
| External Link | ||||
| 2-(1H-Imidazol-4-ylmethyl)-4-phenyl-thiazole | Investigative | [34] | ||
| Synonyms |
CHEMBL289060; BDBM50014174
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| CP-810123 | Investigative | [19] | ||
| Synonyms |
UNII-E6G4550EC4; CHEMBL604798; E6G4550EC4; BSNKYWSMUAGMDO-UHFFFAOYSA-N; 439608-12-5; SCHEMBL1459339; BDBM50309861; 1,4-Diazabicyclo(3.2.2)nonane, 4-(5-methyloxazolo(4,5-b)pyridin-2-yl)-; 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]nonane; 4-(5-Methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diazabicyclo[3.2.2]-nonane; 4-(5-methyloxazolo[4,5-b]pyridin-2-yl)-1,4-diaza-bicyclo[3.2.2]nonane
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | IC50 = 5 nM | |||
| External Link | ||||
| bilobalide | Investigative | [35] | ||
| Synonyms |
33570-04-6; Bilobalid; (-)-Bilobalide; UNII-M81D2O8H7U; CHEBI:3103; M81D2O8H7U; Bilobalide A; 4H,5aH,9H-Furo(2,3-b)furo(3',2':2,3)cyclopenta(1,2-c)furan-2,4,7(3H,8H)-trione, 9-(1,1-dimethylethyl)-10,10a-dihydro-8,9-dihydroxy-, (5aR-(3aS*,5aalpha,8beta,8aS*,9alpha,10aalpha))-; (3aS,8R,8aS,9R,10aS)-9-tert-butyl-8,9-dihydroxydihydro-9H-furo[2,3-b]furo[3',2':2,3]cyclopenta[1,2-c]furan-2,4,7(3H,8H)-trione; tert-butyl(dihydroxy)[ ]trione; C15H18O8; Bilobalide;; Bilobalide A;; ( )-Bilobalide; AC1L2K4G; MLS000563448
Click to Show/Hide
|
|||
| MOA | Blocker (channel blocker) | |||
| External Link | ||||
| 2-(4-Methyl-piperazin-1-yl)-quinoline | Investigative | [25] | ||
| Synonyms |
N-methylquipazine; 2-(4-methylpiperazin-1-yl)quinoline; UNII-0YV1ZIR6S0; 0YV1ZIR6S0; CHEMBL288591; CHEBI:64164; quinoline, 2-(4-methyl-1-piperazinyl)-; Tocris-0566; Lopac-Q-107; Biomol-NT_000084; AC1L1JF3; Oprea1_654246; Lopac0_001000; SCHEMBL606721; BPBio1_001081; DTXSID8043731; CTK6I3065; HOMWNUXPSJQSSU-UHFFFAOYSA-N; MolPort-006-384-975; ZINC403653; 2-(4-Methylpiperazinyl)-quinoline; 1-(2-Quinolyl)-4-methylpiperazine; STK362919; BDBM50053631; AKOS005453926; MCULE-4786527390; CCG-205080
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 0.4571 nM | |||
| External Link | ||||
| 4-((naphthalen-2-yloxy)methyl)piperidine | Investigative | [36] | ||
| Synonyms |
4-[(2-Naphthyloxy)methyl]piperidine; CHEMBL453996; 946680-75-7; 4-[(naphthalen-2-yloxy)methyl]piperidine; CTK7D1529; ZINC14631494; BDBM50278526; AKOS000172158
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 311 nM | |||
| External Link | ||||
| MESULERGINE | Investigative | [37] | ||
| Synonyms |
Mesulerginum; Mesulergina; Mesulergine [INN]; Mesulerginum [INN-Latin]; UNII-SML95FK06I; Mesulergina [INN-Spanish]; 64795-35-3; N'-(1,6-Dimethylergolin-8alpha-yl)-N,N-dimethylsulfamide; SML95FK06I; CQ 32085; CHEMBL12314; C18H26N4O2S; CHEBI:73378; 3-(1,6-Dimethyl-8alpha-ergolinyl)-1,1-dimethylsulfamid; NCGC00163168-01; DSSTox_RID_81540; DSSTox_CID_26324; DSSTox_GSID_46324; CU-32085; N'-[(8alpha)-1,6-dimethylergolin-8-yl]-N,N-dimethylsulfuric diamide; CAS-64795-35-3; AC1L2AKM; Biomol-NT_000077; AC1Q6V4R; GTPL206
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 7-(piperidin-4-ylmethoxy)-2-naphthonitrile | Investigative | [36] | ||
| Synonyms |
CHEMBL444985
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 404 nM | |||
| External Link | ||||
| QUIPAZINE | Investigative | [38] | ||
| Synonyms |
4774-24-7; 2-(piperazin-1-yl)quinoline; 2-Piperazin-1-yl-quinoline; 2-Piperazin-1-ylquinoline; Quinoline, 2-(1-piperazinyl)-; 2-(1-Piperazinyl)quinoline; Quipazine [INN]; 1-(2-Quinolinyl)piperazine; Quipazinum [INN-Latin]; Quipazina [INN-Spanish]; UNII-4WCY05C0SJ; 1-(2-Quinolyl)piperazine; 2-(1-Piperazinyl)chinolin; BRN 0196945; 4WCY05C0SJ; CHEMBL18772; 2-quinolylpiperazine; F3306-0004; Quipazinum; Quipazina; TPC-A004; MA-1291; Spectrum_001733; Tocris-0629; piperazin-1-yl-quinoline; AC1L1JEX; AC1Q4WAV; Spectrum4_001259
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 3.7 nM | |||
| External Link | ||||
| 5-chloro-3,4-dihydroquinazolin-2-amine | Investigative | [39] | ||
| Synonyms |
CHEMBL401541; 2-Amino-5-chlor-3,4-dihydrochinazolin; 109319-86-0
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 1051 nM | |||
| External Link | ||||
| 2-methyl-5-HT | Investigative | [40] | ||
| Synonyms |
2-methyl-5-hydroxytryptamine; 2-Me-5-HT; 2-methylserotonin
Click to Show/Hide
|
|||
| MOA | Agonist | |||
| Activity | Ki = 1200 nM | |||
| External Link | ||||
| 5,6-dichloro-3,4-dihydroquinazolin-2-amine | Investigative | [39] | ||
| Synonyms |
Anagrelide impurity 5; 2-Amino-5,6-dichloro-3,4-dihydroquinazoline; 444904-63-6; CHEMBL1548; 2-Quinazolinamine, 5,6-dichloro-1,4-dihydro-; W-202785; SCHEMBL1569300; CTK1D2410; DTXSID80432648; VBKOTIVQMCTTAQ-UHFFFAOYSA-N; ZINC29130869; BDBM50371434; AKOS030254941; 5,6-Dichloro-1,4-dihydro-2-quinazolinamine; 2-amino-5,6-dichloro-3,4dihydroquinazoline; FT-0722369
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki = 172 nM | |||
| External Link | ||||
| SEROTONIN | Investigative | [25] | ||
| Synonyms |
5-HYDROXYTRYPTAMINE; 3-(2-Aminoethyl)-1H-indol-5-ol; 50-67-9; Enteramine; 5-HT; Serotonine; Thrombotonin; Thrombocytin; Antemovis; Ds substance; Hippophain; Antemoqua; Substance DS; Substanz DS; 1H-Indol-5-ol, 3-(2-aminoethyl)-; 5-Hta; Tryptamine, 5-hydroxy-; 3-(2-Aminoethyl)indol-5-ol; Enteramin; UNII-333DO1RDJY; Indol-5-ol, 3-(2-aminoethyl)-; 5-Hydroxy-3-(beta-aminoethyl)indole; 3-(beta-Aminoethyl)-5-hydroxyindole; EINECS 200-058-9; 3-(2-Amino-ethyl)-1H-indol-5-ol; BRN 0143524; 333DO1RDJY; CHEBI:28790
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | EC50 = 141 nM | |||
| External Link | ||||
| A-987306 | Investigative | [41] | ||
| Synonyms |
SCHEMBL604437; CHEMBL519240; BDBM26226; MolPort-023-276-880; DJKJVWJQAVGLHJ-YPMHNXCESA-N; ZINC42887577; AKOS024457726; NCGC00370852-01; UNII-6BVK16R925 component DJKJVWJQAVGLHJ-YPMHNXCESA-N; (12S,17S)-6-(piperazin-1-yl)-11-oxa-3,5-diazatetracyclo[8.7.0.0^{2,7}.0^{12,17}]heptadeca-1(10),2(7),3,5-tetraen-4-amine; (-)-(7aS*,11aS*)-4-piperazin-1-yl-5,6,7a,8,9,10,11,11a-octahydro[1]benzofuro[2,3h]quinazolin-2-amine; (+/-)-(7aR*,11aR*)-5,6,7a,8,9,10,11,11a-Octahydro-4-(1-piperazinyl)-benzofuran[2,3-h]quinazolin-2-amine
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| External Link | ||||
| 4-(4-butylpiperidin-1-yl)-1-o-tolylbutan-1-one | Investigative | [42] | ||
| Synonyms |
AC-42; CHEMBL1242950; AC42; GTPL289; SCHEMBL4504348; ANTKBACNWQHQJE-UHFFFAOYSA-N; ZINC2022527; BDBM50326219; AKOS030284249; NCGC00485453-01; gamma-(4-Butylpiperidino)-2'-methylbutyrophenone; L019209; 4-(4-butylpiperdin-1-yl)-1-(2-methylphenyl)butan-1-one; 4-(4-butylpiperidin-1-yl)-1-(2-methylphenyl)butan-1-one
Click to Show/Hide
|
|||
| MOA | Inhibitor | |||
| Activity | Ki >= 1000 nM | |||
| External Link | ||||
| 2B70: Esophageal cancer | 15 Compound(s) Regulating the Disease | Click to Show/Hide the Full List | ||
| Pembrolizumab | Approved | [43] | ||
| External Link | ||||
| Nivolumab | Approved | [43] | ||
| External Link | ||||
| Claudiximab | Phase 3 | [44] | ||
| Synonyms |
IMAB-362; Anti-GC182 mAbs (cancer), Ganymed; Anti-GC182 monoclonal antibodies (cancer), Ganymed; Anti-CLDN18-2 mAbs (cancer), Ganymed; Anti-CLDN18-2 monoclonal antibodies (cancer), Ganymed
Click to Show/Hide
|
|||
| External Link | ||||
| Golnerminogene pradenovac | Phase 3 | [45] | ||
| Synonyms |
TNFerade (TN)
Click to Show/Hide
|
|||
| External Link | ||||
| DKN-01 | Phase 2 | [46] | ||
| External Link | ||||
| Pegamotecan | Phase 2 | [47] | ||
| Synonyms |
Prothecan; EZ-246; PEG-camptothecin; PEG-camptothecin, Enzon; Polyethylene glycol-camptothecin, Enzon
Click to Show/Hide
|
|||
| External Link | ||||
| APR-246 | Phase 2 | [43] | ||
| Synonyms |
Eprenetapopt
Click to Show/Hide
|
|||
| External Link | ||||
| Opdivo + Yervoy | Phase 3 | [43] | ||
| External Link | ||||
| Anti-NY-ESO-1 CAR-T cells | Phase 1/2 | [48] | ||
| External Link | ||||
| Anti-MUC1 CAR-T cells | Phase 1/2 | [49] | ||
| External Link | ||||
| CAR-T Cells targeting EpCAM | Phase 1/2 | [50] | ||
| External Link | ||||
| PCA062 | Phase 1 | [43] | ||
| External Link | ||||
| Cellspan esophageal implant | Clinical trial | [43] | ||
| External Link | ||||
| PKI166 | Discontinued in Phase 2 | [51] | ||
| Synonyms |
PKI-166; CGP-75166; 187724-61-4; NVP-PKI166; CHEMBL1914653; AC1OCFE0; UNII-9RIE5HW38P; 9RIE5HW38P; SCHEMBL177814; GTPL7642; CHEMBL1963502; ZINC23255; AOB1619; PKI-75166; BDBM50358046; NCGC00387215-02; AS-16676; KB-275097; PKI-166, > 4-[4-[[(1R)-1-phenylethyl]amino]-7H-pyrrolo[4,5-e]pyrimidin-6-yl]phenol
Click to Show/Hide
|
|||
| External Link | ||||
| Ramorelix | Discontinued in Phase 1 | [52] | ||
| Synonyms |
Hoe-013
Click to Show/Hide
|
|||
| External Link | ||||
References